Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoemulsion Formulations
2.2. Preliminary Stability Tests
| Parameters | FX | FXE |
|---|---|---|
| After 24 h | ||
| Centrifugation | N | N |
| pH value | 5.62 ± 0.04 | 5.53 ± 0.02 |
| Electrical conductivity (µS/cm) | 676.0 ± 3.0 * | 689.4 ± 4.7 * |
| Droplet size (nm) | 142.6 ± 2.3 * | 139.1 ± 2.8 * |
| PDI | 0.26 ± 0.01 | 0.23 ± 0.01 |
| Zeta potential (mV) | −38.10 ± 1.69 * | −42.98 ± 0.88 |
| After TS | ||
| pH value | N/A | N/A |
| Electrical conductivity (µS/cm) | N/A | N/A |
| Droplet size (nm) | N/A | N/A |
| PDI | N/A | N/A |
| Zeta potential (mV) | N/A | N/A |
| After FDC | ||
| pH value | 5.55 ± 0.05 | 5.49 ± 0.01 |
| Electrical conductivity (µS/cm) | 457.7 ± 33.6 * | 510.7 ± 3.1 * |
| Droplet size (nm) | 160.6 ± 3.3 * | 148.8 ± 1.6 * |
| PDI | 0.21 ± 0.02 | 0.26 ± 0.01 |
| Zeta potential (mV) | −47.01 ± 1.64 * | −42.58 ± 0.99 |
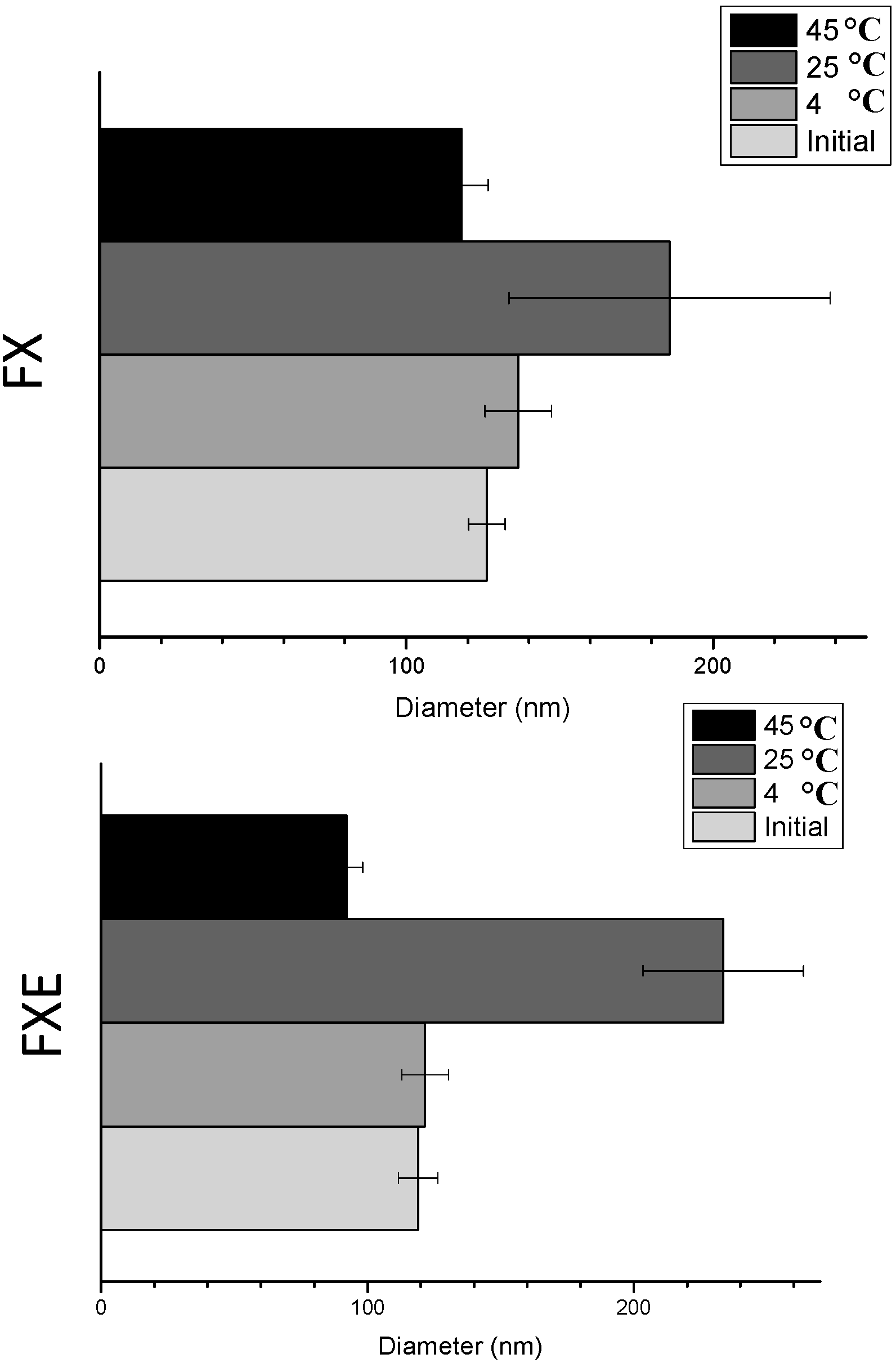
2.3. Accelerated Stability Tests
| FX | FXE | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial 24 h | After 60 Days | Initial 24 h | After 60 Days | |||||
| 4 °C | 25 °C | 45 °C | 4 °C | 25 °C | 45 °C | |||
| Macroscopic characteristics | N | N | N | N | N | N | N | N |
| pH | 5.67 ± 0.03 | 5.68 ± 0.03 | 5.62 ± 0.03 * | 5.42 ± 0.02 * | 5.62 ± 0.02 | 5.63 ± 0.02 | 5.56 ± 0.02 * | 5.32 ± 0.21 * |
| Particle size (nm) | 126.3 ± 5.9 | 136.5 ± 10.8 | 185.9 ± 52.4 * | 117.9 ± 8.8 | 119.0 ± 7.4 | 121.6 ± 8.8 | 233.6 ± 30.1 * | 92.17 ± 6.1 * |
| PDI | 0.264 ± 0.030 | 0.231 ± 0.071 | 0.349 ± 0.052 | 0.260 ± 0.020 | 0.257 ± 0.038 | 0.251 ± 0.020 | 0.305 ± 0.126 | 0.228 ± 0.044 |
| Zeta potential (mV) | −28.60 ± 10.33 | −44.35 ± 4.07 * | −36.53 ± 12.94 | −43.28 ± 4.72 * | −26.71 ± 6.67 | −41.71 ± 4.55 * | −39.77 ± 5.99 * | −41.21 ± 6.02 * |


2.4. Evaluation of in Vivo Moisturizing Properties

| C1 | C2 | C3 | C4 | |
|---|---|---|---|---|
| BASAL | 6.06 ± 1.71 | 6.40 ± 1.60 | 6.91 ± 1.09 | 9.06 ± 2.24 |
| T1 | 6.47 ± 1.70 | 6.49 ±1.88 | 7.57 ± 1.67 | 8.13 ± 1.78 |
| T2 | 6.95 ± 2.21 | 6.74 ± 1.34 | 6.66 ± 1.88 | 7.66 ± 2.04 |
| T3 | 7.10 ± 1.47 | 6.87 ± 1.36 | 7.48 ± 1.60 | 8.31 ± 2.34 |
| T4 | 7.00 ± 1.80 | 7.15 ± 1.53 | 6.85 ± 1.85 | 7.74 ± 2.06 |
| T5 | 7.37 ± 2.36 | 7.16 ± 2.21 | 7.55 ± 2.32 | 8.38 ± 2.57 |
3. Experimental Section
3.1. Materials
3.2. Preparation of Opuntia ficus-indica (L.) Mill Extract
3.3. Preparation and Formulations
| COMPONENTS | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | FX | FXE |
|---|---|---|---|---|---|---|---|---|---|---|
| BHT | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Caprylic/Capric Triglyceride | 2.0 | 4.0 | 8.0 | 1.0 | 1.0 | 2.0 | 3.0 | 1.0 | 2.0 | 2.0 |
| Ethylhexyl Palmitate | 2.0 | 4.0 | 8.0 | 1.0 | 1.0 | 2.0 | 3.0 | 1.0 | 2.0 | 2.0 |
| C12–15 Alkyl Benzoate | 1.0 | 2.0 | 4.0 | 0.5 | 0.5 | 1.0 | 1.5 | 0.5 | 1.0 | 1.0 |
| Paraffinum Liquidum | 5.0 | 10.0 | 20.0 | 2.5 | 2.5 | 5.0 | 7.5 | 2.5 | 5.0 | 5.0 |
| Phenoxyethanol (and) Caprylic Glycol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Sorbitan Oleate | 4.7 | 4.7 | 4.7 | 4.7 | 2.4 | 2.4 | 2.4 | 1.4 | 4.7 | 4.7 |
| Polysorbate 80 | 5.3 | 5.3 | 5.3 | 5.3 | 2.6 | 2.6 | 2.6 | 1.6 | 5.3 | 5.3 |
| Disodium EDTA | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Xanthan Gum | --- | --- | --- | --- | --- | --- | --- | --- | 0.5 | 0.5 |
| Opuntia ficus-indica (L.) Mill extract | --- | --- | --- | --- | --- | --- | --- | --- | --- | 1.0 |
| Distilled Water | 78.8 | 68.8 | 48.8 | 83.8 | 88.8 | 83.8 | 78.8 | 90.8 | 78.3 | 77.3 |
3.4. Preliminary Stability Tests
3.5. Accelerated Stability Tests
3.6. Evaluation of Mosturizing Eficacy in Vivo
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breuer, M.M. Cosmetics emulsions. In Encyclopedia of Emulsions Technology; Becher, P., Ed.; Marcel Dekker: New York, NY, USA, 1985; Volume 2, pp. 385–424. [Google Scholar]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.J.; Azemar, N.; Solans, C. Formation and stability of nano-emulsions prepared using the phase inversion temperature method. Langmuir 2001, 18, 26–30. [Google Scholar] [CrossRef]
- Mcclements, D.J. Nanoemulsions versus microemulsions: Terminology, differences and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Kabri, T.-H.; Arab-Tehrany, E.; Belhaj, N.; Linder, M. Physico-chemical characterization of nano-emulsions in cosmetic matrix enriched on omega-3. J. Nanobiotechnol. 2011, 9, 41. [Google Scholar] [CrossRef]
- Chebil, A.; Desbrières, J.; Nouvel, C.; Six, J.-L.; Durand, A. Ostwald ripening of nanoemulsions stopped by combined interfacial adsorptions of molecular and macromolecular nonionic stabilizers. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 24–30. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gianeti, M.D.; Wagemaker, T.A.L.; Seixas, V.C.; Maia Campos, P.M.B.G. The use of nanotechnology in cosmetic formulations: The influence of vehicle in the vitamin a skin penetration. Curr. Nanosci. 2011, 8, 526–534. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Whey protein peptides as components of nanoemulsions: A review of emulsifying and biological functionalities. J. Food Eng. 2014, 122, 15–27. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure and physical properties. J. Phys. Condens. Matter. 2006, 18, 635–666. [Google Scholar] [CrossRef]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Piorkowski, D.; Mcclements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2013, 42, 5–41. [Google Scholar] [CrossRef]
- Peshkovsky, A.S.; Peshkovsky, S.L.; Bystryak, S. Scalable high-power ultrasonic technology for the production of translucent nanoemulsions. Chem. Eng. Process. 2013, 69, 77–82. [Google Scholar] [CrossRef]
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. CCID 2010, 3, 5–13. [Google Scholar] [CrossRef]
- Mancini, A.J. Skin. Pediatrics 2004, 113, 1114–1119. [Google Scholar] [PubMed]
- Barco, D.; Giménez-Arnau, A. Xerosis: Una disfunción de la barrera epidérmica. Actas Dermo-Sifiliogr. 2008, 99, 671–682. [Google Scholar] [CrossRef]
- Capitani, R.D.; Mercúrio, D.G.; Camargo Júnior, F.B.; Maia Campos, P.M.B.G. Stability and clinical efficacy of moisturizing cosmetics formulations containing vitamins C and E. Biomed. Biopharm. Res. 2012, 9, 215–224. [Google Scholar]
- Dal’Belo, S.E.; Rigo Gaspar, L.; Maia Campos, P.M.B.G. Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res. Technol. 2006, 12, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bonté, F. Skin moisturization mechanisms: New data. Ann. Pharm. Fr. 2011, 69, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C. Cosmetologia Aplicada a Dermoestética, 2nd ed.; Pharmabook: São Paulo, SP, Brasil, 2010. [Google Scholar]
- Lee, D.-H.; Seo, E.-S.; Hong, J.-T.; Lee, G.-T.; You, Y.-K.; Lee, K.-K.; Jo, G.-W.; Kim, N.-K. The efficacy and safety of proposed herbal moisturizing cream for dry skin and itch relief: A randomized, doubled-blind, placebo-controlled treial-study protocol. BMC Complement. Altern. Med. 2013, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.A.R.; Costa, C.A.G.; de Araújo, J.C. Effective root depth of the Caatinga biome. J. Arid Environ. 2013, 89, 1–4. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.E.F.; Nascimento, L.C.; Araújo, E.; Lopes, E.B.; Souto, F.M. Ocorrência e identificação dos agentes etiológicos de doenças em Palma Forrageira (Opuntia ficus-indica Mill.) no Semi-Árido paraibano. Biotemas 2010, 23, 11–20. [Google Scholar]
- Damasceno, G.A.B. Obtenção de Extratos da Opuntia ficus-indica (L.) Mill e suas Aplicações em Formulações Cosméticas: Avaliação in vivo do Sensorial e da Eficácia Hidratante. Master Thesis, Universidade Federal do Rio Grande do Norte, Natal, RN, Brazil, 28 March 2014. [Google Scholar]
- Habibi, Y.; Heyraud, A.; Mahrouz, M.; Vignon, M.R. Structural features of pectic polysaccharides from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1119–11127. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-K.; Jin, X.; Lai, F.-Y.; Lin, Q.-S.; Jiang, J.-G. Chemical analysis and antioxidant activities in vitro of polysaccharide extracted from Opuntia ficus-indica Mill cultivated in China. Carbohydr. Polym. 2010, 82, 722–727. [Google Scholar] [CrossRef]
- Kamershwarl, V.; Mistry, N.D. Propriedades sensoriais dos emolientes. Cosmet. Toilet. 2001, 13, 52–59. [Google Scholar]
- Ševčíková, P.; Vltavská, P.; Kašpárková, V.; Krejčí, J. Formation, Characterization and Stability of Nanoemulsions Prepared by Phase Inversion. In Proceedings of the 13th WSEAS International Conference on Mathematical and Computational Methods in Science and Engineering, Catania, Sicily, Italy, 3 November 2011; Demiralp, M., Bojkovic, Z., Repanovici, A., Eds.; World Scientific and Engineering Academy and Society (WSEAS): Zografou, Athens, Greece, 2011. [Google Scholar]
- Anton, N.; Vandamme, T. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oilnanoemulsions. Colloids Surf. B Biointerfaces 2010, 75, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.; Basri, M.; Zakaria, M.; Salleh, A.; Rahman, R.; Rahman, M. A potential tocopherol acetate loaded palm oil esters-in-water nanoemulsions for nanocosmeceuticals. J. Nanobiotechnol. 2010, 8, 4. [Google Scholar] [CrossRef]
- Sevcíková, P.; Kaspárková, V.; Vltavská, P.; Krejcí, J. On the preparation and characterization of nanoemulsions produced by phase inversion emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 130–135. [Google Scholar] [CrossRef]
- Lima, C.G.; Vilela, A.F.G.; Silva, A.A.S.; Piannovski, A.R.; Silva, K.K.; Carvalho, V.F.M.; de Musis, C.R.; Machado, S.R.P.; Ferrari, M. Desenvolvimento e avaliação da estabilidade física de emulsões O/A contendo óleo de babaçu (Orbignya oleifera). Rev. Bras. Farm. 2008, 89, 239–245. [Google Scholar]
- Rieger, M.M. Teste de estabilidade para macroemulsões. Cosmet. Toilet. 1996, 8, 47–53. [Google Scholar]
- Oliveira, E.C.V.; Boock, K.P.; Maruno, M.; Rocha-Filho, P.A. Accelerated stability and moisturizing capacity of emulsions presenting lamellar gel phase obtained from brazilian natural raw material. J. Dispers. Sci. Technol. 2011, 32, 1135–1139. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Souto, E.B.; Boonme, P.; Müller, R.H. Q10-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation. Int. J. Pharm. 2009, 377, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nanoemulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária—Anvisa. Guia de Estabilidade de Produtos Cosméticos; Anvisa: Brasília, DF, Brazil, 2005; Volume 1. [Google Scholar]
- Casteli, V.C.; Mendonça, C.C.; Maia Campos, P.M.B.G.; Ferrari, M.; Machado, S.R.P. Desenvolvimento e estudos de estabilidade preliminares de emulsoes O/A contendo Cetoconazol 2,0%. Acta Sci. Health Sci. (UEM) 2008, 30, 121–128. [Google Scholar]
- Komaiko, J.; McClements, D.J. Optimization of isothermal low-energy nanoemulsion formation: Hydrocarbon oil, non-ionic surfactant, and water systems. J. Colloid Interface Sci. 2014, 425, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.; Idson, B. Skin lipids. Int. J. Cosmet. Sci. 1991, 13, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mei, Z.; Xu, J.; Sun, D. Effect of inorganic electrolytes on the formation and the stability of water-in-oil (W/O) emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 82–90. [Google Scholar] [CrossRef]
- Bernardi, D.; Pereira, T.; Maciel, N.; Bortoloto, J.; Viera, G.; Oliveira, G.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar]
- Kong, M.; Park, H.J. Stability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrier. Carbohydr. Polym. 2011, 83, 1303–1310. [Google Scholar] [CrossRef]
- Wiącek, A.; Chibowski, E. Zeta potential, effective diameter and multimodal size distribution in oil/water emulsion. Colloids Surf. A Physicochem. Eng. Asp. 1999, 159, 253–261. [Google Scholar] [CrossRef]
- Tang, S.Y.; Manickam, S.; Wei, T.K.; Nashiru, B. Formulation development and optimization of a novel Cremophore EL-based nanoemulsion using ultrasound cavitation. Ultrason. Sonochem. 2012, 19, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Ashokkumar, M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems—Physicochemical characterization. Food Hydrocoll. 2014, 39, 151–162. [Google Scholar] [CrossRef]
- Ferrari, M.; Rocha-filho, P.A. Multiple emulsions containing amazon oil: Açaí oil (Euterpe oleracea). Rev. Bras. Farmacogn. 2011, 21, 737–743. [Google Scholar] [CrossRef]
- Maruno, M.; Rocha-Filho, P.A. O/W nanoemulsion after 15 years of preparation: A suitable vehicle for pharmaceutical and cosmetic applications. J. Dispers. Sci. Technol. 2010, 31, 17–22. [Google Scholar] [CrossRef]
- Moulai Mostefa, N.; Hadj Sadok, A.; Sabri, N.; Hadji, A. Determination of optimal cream formulation from long-term stability investigation using a surface response modelling. Int. J. Cosmet. Sci. 2006, 28, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Borade, G.; Rasve, G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine 2014, 21, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Kulbacka, J.; Wilk, K.A. Dicephalic ionic surfactants in fabrication of biocompatible nanoemulsions: Factors influencing droplet size and stability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 312–320. [Google Scholar] [CrossRef]
- Leite e Silva, V.R.; Schulman, M.A.; Ferelli, C.; Gimenis, J.M.; Ruas, G.W.; Baby, A.R.; Velasco, M.V.R.; Taqueda, M.E.; Kaneko, T.M. Hydrating effects of moisturizer active compounds incorporated into hydrogels: In vivo assessment and comparison between devices. J. Cosmet. Dermatol. 2009, 8, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Courage Khazaka. Available online: http://courage-khazaka.de/index.php/en/products/scientific (accessed on 1 August 2014).
- Gaspar, L.R.; Camargo, F.B., Jr.; Gianeti, M.D.; Maia Campos, P.M.B.G. Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem. Toxicol. 2008, 46, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.B.G.; Gianeti, M.D.; Camargo, F.B., Jr.; Gaspar, L.R. Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. Eur. J. Pharm. Biopharm. 2012, 82, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Alexandrino, F.; Verissimo, L.M.; Agnez-Lima, L.F.; Egito, L.C.M.; de Oliveira, A.G.; Do Egito, E.S.T. Physical factors affecting plasmid DNA compaction in stearylamine-containing nanoemulsions intended for gene delivery. Pharmaceuticals 2012, 5, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Faria, W.C.S.; Damasceno, G.A.B.; Ferrari, M. Moisturizing effect of a cosmetic formulation containing pequi oil (Caryocar brasiliense) from the Brazilian cerrado biome. Braz. J. Pharm. Sci. 2014, 50, 131–136. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the nanoemulsions are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.C.d.A.; Barreto, S.M.A.G.; Ostrosky, E.A.; Rocha-Filho, P.A.d.; Veríssimo, L.M.; Ferrari, M. Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent. Molecules 2015, 20, 2492-2509. https://doi.org/10.3390/molecules20022492
Ribeiro RCdA, Barreto SMAG, Ostrosky EA, Rocha-Filho PAd, Veríssimo LM, Ferrari M. Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent. Molecules. 2015; 20(2):2492-2509. https://doi.org/10.3390/molecules20022492
Chicago/Turabian StyleRibeiro, Renato Cesar de Azevedo, Stella Maria Andrade Gomes Barreto, Elissa Arantes Ostrosky, Pedro Alves da Rocha-Filho, Lourena Mafra Veríssimo, and Márcio Ferrari. 2015. "Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent" Molecules 20, no. 2: 2492-2509. https://doi.org/10.3390/molecules20022492






