The Synthesis and Antitumor Activity of Twelve Galloyl Glucosides
Abstract
:1. Introduction
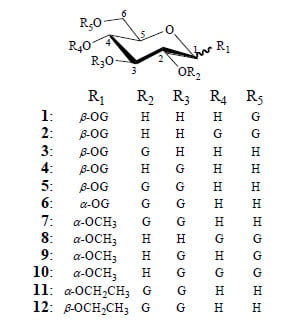
2. Results and Discussion
2.1. Synthesis of 1–12
2.1.1. Synthesis of Esterification Reagent 13 and Intermediates 14–17


2.1.2. Synthesis of 1 and 2 from the Intermediate 15

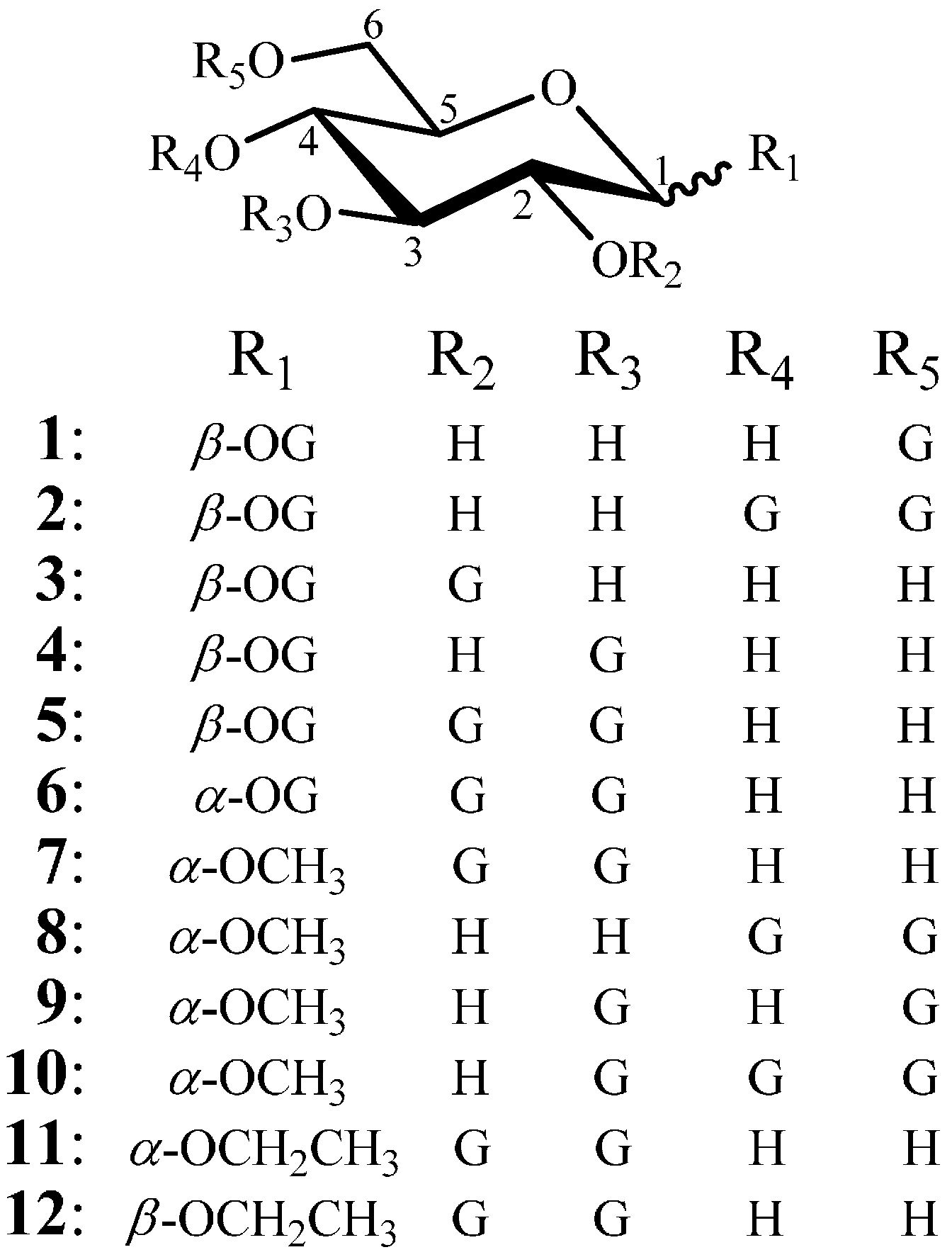
2.1.3. Synthesis of 3–6 from the Intermediate 16
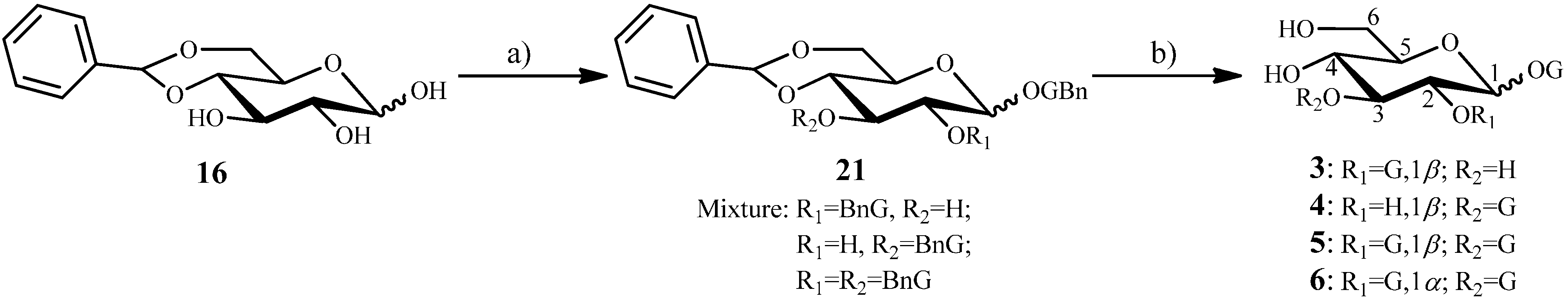
2.1.4. Synthesis of 7 from the Intermediate 14
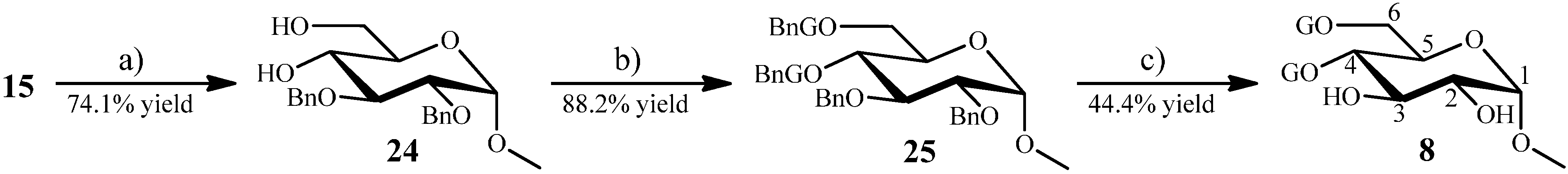
2.1.5. Synthesis of 8 from the Intermediate 15
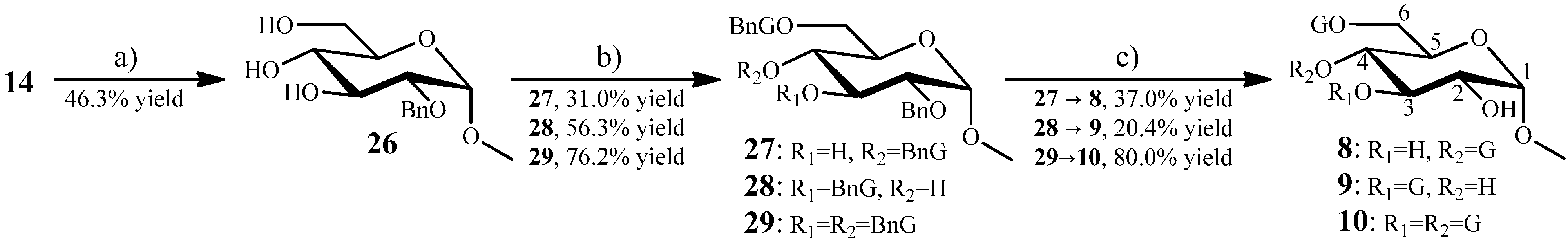
2.1.6. Synthesis of 8–10 from the Intermediate 14
2.1.7. Synthesis of 11 and 12 from the Intermediate 17

2.2. Antitumor Activity Evaluation
2.2.1. The in Vitro Antitumor Activity of 1–12
| Cells | IR% at 100 μg/mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 5-FU | DOC | |
| K562 | 78.5 | 70.5 | 77.0 | 72.9 | 70.9 | 71.5 | 81.6 | 65.5 | 88.1 | 73.3 | 74.9 | 64.2 | 74.1 | 71.8 |
| HeLa | 86.6 | 83.7 | 77.7 | 85.3 | 75.5 | 84.7 | 88.8 | 88.8 | 76.9 | 78.5 | 85.6 | 77.7 | 90.6 | 82.8 |
| HL-60 | 89.4 | 92.1 | 92.9 | 91.8 | 88.0 | 89.0 | 89.0 | 88.9 | 89.3 | 90.6 | 92.0 | 88.5 | 86.3 | 57.1 |
| Cells | IC50 (μM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| K562 | 77.9 | 68.2 | 71.1 | 115.3 | 70.3 | 85.8 | 77.5 | 124.7 | 72.1 | 49.8 | 91.0 | 109.4 |
| HeLa | 61.8 | 44.0 | 93.8 | 63.0 | 52.2 | 45.3 | 71.3 | 66.9 | 77.3 | 49.4 | 68.4 | 81.3 |
| HL-60 | 36.2 | 18.7 | 35.3 | 30.8 | 19.0 | 19.3 | 30.9 | 30.5 | 39.0 | 17.2 | 32.6 | 32.4 |
2.2.2. The in Vivo Antitumor Activity of 1 and 2 in Mice
| Group | Dose (mg/kg) | Body Weight (g) | Tumor Weight (g) | Inhibition Rate (%) |
|---|---|---|---|---|
| Model group | — | 29.54 ± 3.61 | 2.45 ± 0.79 | — |
| Taxol | 20 | 22.28 ± 2.02 * | 1.49 ± 0.46 * | 39.2 |
| 1 | 15 | 28.06 ± 3.09 | 2.10 ± 0.46 | 14.3 |
| 30 | 26.90 ± 2.95 | 1.79 ± 0.45 ** | 26.9 | |
| 2 | 15 | 28.63 ± 3.90 | 2.41 ± 0.72 | 1.6 |
| 30 | 27.22 ± 3.41 | 1.87 ± 0.41 ** | 23.7 |
2.3. Discussion
3. Experimental Section
3.1. General
3.2. Chemical Synthesis
1,6-Di-O-galloyl-β-d-glucopyranose (1)
| Position | δH | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| glucose | ||||||
| 1 | 5.69 (d, J = 6.3 Hz) | 5.79 (d, J = 8.3 Hz) | 5.90 (d, J = 8.6 Hz) | 5.79 (d, J = 8.2 Hz) | 6.06 (d, J = 8.3 Hz) | 6.60 (d, J = 3.5 Hz) |
| 2 | 3.56–3.49 (m) | 3.66 (dd, J = 9.5, 8.3 Hz) | 5.19 (t, J = 8.6 Hz) | 3.79–3.68 (m) | 5.42 (dd, J = 9.7, 8.3 Hz) | 5.29 (dd, J = 10.1, 3.5 Hz) |
| 3 | 3.56–3.49 (m) | 3.85 (t, J = 9.5 Hz) | 3.82–3.70 (m) | 5.25 (t, J = 9.4 Hz) | 5.54 (t, J = 9.7 Hz) | 5.86 (t, J = 10.1 Hz) |
| 4 | 3.56–3.49 (m) | 5.23 (t, J = 9.5 Hz) | 3.58–3.52 (m) | 3.79–3.68 (m) | 3.88 (t, J = 9.7 Hz) | 4.01–3.91 (m) |
| 5 | 3.76–3.67 (m, 1H) | 4.07 (ddd, J = 9.5, 4.6, 2.0 Hz) | 3.58–3.52 (m) | 3.61–3.53 (m) | 3.70 (ddd, J = 9.7, 4.6, 2.2 Hz) | 4.01–3.91 (m) |
| 6 | 4.55 (br d, J = 12.0 Hz) | 4.45 (dd, J = 12.4, 2.0 Hz) | 3.90 (br d, J = 11.9 Hz) | 3.88 (br d, J = 12.1 Hz) | 3.93 (dd, J = 12.3, 2.2 Hz) | 3.90–3.78 (2H, m) |
| 4.40 (dd, J = 12.0, 4.5 Hz) | 4.22 (dd, J = 12.4, 4.7 Hz) | 3.82–3.70 (m) | 3.79–3.68 (m) | 3.81 (dd, J = 12.3, 4.6 Hz) | ||
| galloyl | ||||||
| 2,6 | 7.13 (2H, s) | 7.15 (2H, s) | 7.04 (2H, s) | 7.15 (2H, s) | 7.03 (2H, s) | 7.17 (2H, s) |
| 7.08 (2H, s) | 7.11 (2H, s) | 7.01 (2H, s) | 7.12 (2H, s) | 7.02 (2H, s | 7.06 (2H, s) | |
| 7.07 (2H, s) | 6.92 (2H, s) | 6.91 (2H, s) | ||||
| Position | δH | |||||
| 7 | 8 | 9 | 10 | 11 | 12 | |
| glucose | ||||||
| 1 | 5.05–4.98 (m) | 4.77 (d, J = 3.6 Hz) | 4.77 (d, J = 3.7 Hz) | 4.86 (d, J = 3.7 Hz) | 5.15 (d, J = 3.6 Hz) | 4.73 (d, J = 8.0 Hz) |
| 2 | 5.05–4.98 (m) | 3.58 (dd, J = 9.5, 3.6 Hz) | 3.71 (dd, J = 9.9, 3.7 Hz) | 3.88 (dd, J = 9.9, 3.7 Hz) | 4.99 (dd, J = 10.2, 3.6 Hz) | 5.08 (dd, J = 9.8, 8.0 Hz) |
| 3 | 5.66 (t, J = 8.3 Hz) | 3.93 (t, J = 9.5 Hz) | 5.38 (t, J = 9.9 Hz) | 5.64 (t, J = 9.7 Hz) | 5.68 (dd, J = 10.2, 8.6 Hz) | 5.39 (dd, J = 9.8, 9.6 Hz) |
| 4 | 3.82–3.70 (m) | 5.10 (t, J = 9.5 Hz) | 3.66 (t, J = 9.9 Hz) | 5.35 (t, J = 9.7 Hz) | 3.91–3.70 (m) | 3.74 (t, J = 9.6 Hz) |
| 5 | 3.82–3.70 (m) | 4.11–4.05 (m) | 3.95 (ddd, J = 9.9, 5.8, 2.0 Hz) | 4.27–4.20 (m) | 3.91–3.70 (m) | 3.51 (ddd, J = 9.6, 5.3, 2.2 Hz) |
| 6 | 3.89 (br d, J = 11.6 Hz) | 4.38 (br d, J = 11.8 Hz) | 4.55 (dd, J = 11.9, 2.0 Hz) | 4.43 (br d, J = 10.3 Hz) | 3.91–3.70 (2H, m) | 3.77 (dd, J = 12.1, 5.3 Hz) |
| 3.82–3.70 (m) | 4.19 (dd, J = 11.8, 5.8 Hz) | 4.41 (dd, J = 11.9, 5.8 Hz) | 4.30–4.24 (m) | 3.98–3.89 (m) | ||
| galloyl | ||||||
| 2,6 | 7.03 (2H, s) | 7.09 (2H, s) | 7.13 (2H, s) | 7.08 (2H, s) | 7.03 (2H, s) | 7.00 (2H, s) |
| 6.98 (2H, s) | 7.07 (2H, s) | 7.09 (2H, s) | 6.99 (2H, s) | 6.98 (2H, s) | 6.96 (2H, s) | |
| 6.95 (2H, s) | ||||||
| OCH3 | 3.44 (3H, s) | 3.46 (3H, s) | 3.48 (3H, s) | 3.52 (3H, s) | — | — |
| OCH2CH3 | — | — | — | — | 3.91–3.70 (1H, m) | 3.98–3.89 (1H, m) |
| 3.52 (1H, dq, J = 10.3, 7.1 Hz) | 3.61 (1H, dq, J = 9.7, 7.1 Hz) | |||||
| OCH2CH3 | — | — | — | — | 1.23 (3H, t, J = 7.1 Hz) | 1.12 (3H, t, J = 7.1 Hz) |
| Position | δC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| glucose | ||||||||||||
| 1 | 95.9 | 95.8 | 94.1 | 95.8 | 93.8 | 91.2 | 98.5 | 101.1 | 101.2 | 101.3 | 97.2 | 102.0 |
| 2 | 74.0 | 74.2 | 74.3 | 72.6 | 72.3 | 71.9 | 73.1 | 73.5 | 71.9 | 71.7 | 73.1 | 73.5 |
| 3 | 78.0 | 75.9 | 76.1 | 79.1 | 76.7 | 74.0 | 74.2 | 73.0 | 76.9 | 74.4 | 74.3 | 77.0 |
| 4 | 71.1 | 71.7 | 71.2 | 69.3 | 69.2 | 69.0 | 69.8 | 72.6 | 70.2 | 70.6 | 69.9 | 69.8 |
| 5 | 76.4 | 74.3 | 79.0 | 78.7 | 78.9 | 76.5 | 73.6 | 69.2 | 71.2 | 69.2 | 73.6 | 78.0 |
| 6 | 64.4 | 63.5 | 62.2 | 61.9 | 61.8 | 61.8 | 62.2 | 64.1 | 64.7 | 63.8 | 62.2 | 62.3 |
| galloyl | ||||||||||||
| CO | 168.3 | 168.0 | 167.6 | 168.1 | 167.7 | 168.1 | 168.2 | 168.1 | 168.35 | 168.0 | 168.2 | 167.8 |
| 167.0 | 167.4 | 166.5 | 168.1 | 167.1 | 167.3 | 167.6 | 167.5 | 168.25 | 167.9 | 167.7 | 167.2 | |
| 166.8 | 166.3 | 166.3 | 167.2 | |||||||||
| 1 | 121.2 | 121.1 | 121.1 | 121.6 | 121.0 | 121.1 | 121.3 | 121.2 | 121.7 | 121.1 (2C) | 121.3 | 121.1 |
| 120.5 | 120.9 | 120.1 | 120.5 | 120.4 | 120.4 | 120.5 | 121.0 | 121.3 | 120.4 | 120.6 | 121.0 | |
| 120.4 | 119.9 | 120.2 | ||||||||||
| 2,6 | 110.5 | 110.5 | 110.4 | 110.5 | 110.5 | 110.42 | 110.34 | 110.3 | 110.3 | 110.34 | 110.32 | 110.3 |
| 110.1 | 110.3 | 110.3 | 110.3 | 110.35 | 110.35 | 110.28 | 110.1 | 110.0 | 110.31 | 110.27 | 110.2 | |
| 110.2 | 110.28 | 110.33 | 110.17 | |||||||||
| 3,5 | 146.48 | 146.49 | 146.46 | 146.5 | 146.5 | 146.7 | 146.37 | 146.5 (4C) | 146.5 | 146.5 | 146.4 | 146.33 |
| 146.44 | 146.44 | 146.37 | 146.4 | 146.31 | 146.4 | 146.34 | 146.3 | 146.4 | 146.3 | 146.28 | ||
| 146.37 | 146.29 | 146.3 | 146.3 | |||||||||
| 4 | 140.4 | 140.4 | 140.5 | 140.4 | 140.6 | 140.6 | 140.1 | 140.0 | 139.9 | 140.2 | 140.0 | 139.9 |
| 139.9 | 140.0 | 140.0 | 139.7 | 140.1 | 140.2 | 139.8 | 139.8 | 139.7 | 139.9 | 139.8 | 139.8 | |
| 139.8 | 139.9 | 140.0 | 139.8 | |||||||||
| OCH3 | — | — | — | — | — | — | 55.7 | 55.8 | 55.7 | 56.0 | — | — |
| OCH2CH3 | — | — | — | — | — | — | — | — | — | — | 64.7 | 66.4 |
| OCH2CH3 | — | — | — | — | — | — | — | — | — | — | 15.4 | 15.5 |
1,4,6-Tri-O-galloyl-β-d-glucopyranose (2)
1,2-Di-O-galloyl-β-d-glucopyranose (3), 1,3-di-O-galloyl-β-d-glucopyranose (4), 1,2,3-tri-O-Galloyl-β-d-glucopyranose (5) and 1,2,3-tri-O-galloyl-α-d-glucopyranose (6)
Methyl 2,3-Di-O-galloyl-α-d-glucopyranoside (7)
Methyl 4,6-Di-O-Galloyl-α-d-glucopyranoside (8) from 15
Methyl 3,6-Di-O-Galloyl-α-d-glucopyranoside (9) and Methyl 4,6-di-O-Galloyl-α-d-glucopyranoside (8) from 14
Methyl 3,6-Di-O-galloyl-α-d-glucopyranoside (10)
Ethyl 2,3-Di-O-galloyl-α-d-glucopyranoside (11) and Ethyl 2,3-di-O-galloyl-β-d-glucopyranoside (12)
Tri-O-benzylgalloyl chloride (13)
Methyl 4,6-O-Benzylidene-α-d-glucopyranoside (14)
Methyl 2,3-di-O-Benzyl-4,6-O-benzylidene-α-d-glucopyranoside (15)
4,6-O-Benzylidene-d-glucopyranose (16)
Ethyl 4,6-O-Benzylidene-d-glucopyranoside (17)
3.3. MTT Assay
3.4. The in Vivo Test in Mice for Antitumor Activity of 1 and 2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, C.-M.; Cheng, H.W.; Lin, T.-C.; Chiang, L.-C.; Lin, C.-C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007, 110, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Chauhan, S.M.S. Activity-guided isolation of antioxidants from the leaves of Terminalia arjuna. Nat. Prod. Res. 2014, 28, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Zhang, R.; Ko, D.O.; Wang, Z.H.; Lee, K.H.; Chang, W.Y.; Chae, S.; Jee, Y.; Shin, T.; et al. Antioxidant properties of 1,2,3,4,6-penta-O-galloyl-β-d-glucose from Elaeocarpus sylvestris var. ellipticus. Food Chem. 2009, 115, 412–418. [Google Scholar] [CrossRef]
- Hsu, F.-L.; Huang, W.-J.; Wu, T.-H.; Lee, M.-H.; Chen, L.-C.; Lu, H.-J.; Hou, W.-C.; Lin, M.-H. Evaluation of antioxidant and free radical scavenging capacities of polyphenolics from pods of Caesalpinia pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073–6088. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Nakatsubo, F.; Murakami, K. Quantitative determination of tannin and protein in the precipitates by high-performance liquid chromatography. Phytochemistry 1995, 40, 1503–1505. [Google Scholar] [CrossRef]
- Hatano, T.; Yasuhara, T.; Yoshihara, R.; Agata, I.; Noro, T.; Okuda, T. Effect of interaction of tannins with co-existing substances. VII. Inhibitory effect of tannins and related polyphenols on xanthine oxidase. Chem. Pharm. Bull. 1990, 38, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.; Sancheti, S.; Um, B.-H.; Seo, S.-Y. 1,2,3,4,6-penta-O-galloyl-β-d-glucose: A cholinesterase inhibitor from Terminalia chebula. S. Afr. J. Bot. 2010, 76, 285–288. [Google Scholar] [CrossRef]
- Kang, D.G.; Moon, M.K.; Choi, D.H.; Lee, J.K.; Kwon, T.O.; Lee, H.S. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG) via a nitric oxide–cGMP pathway. Eur. J. Pharmacol. 2005, 524, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-M.; Kim, H.-J.; Oh, G.-S.; Pae, H.-O.; Oh, H.; Jeong, S.; Kwon, T.-O.; Kim, Y.-M.; Chung, H.-T. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose protects rat neuronal cells (Neuro 2A) from hydrogen peroxide-mediated cell death via the induction of heme oxygenase-1. Neurosci. Lett. 2002, 328, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, G.L.; Shylaja, H.; Mohan, C.G. Alleviation of transient global ischemia/reperfusion-induced brain injury in rats with 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose isolated from Mangifera indica. Eur. J. Pharmacol. 2013, 720, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lechtenberg, M.; Sendker, J.; Petereit, F.; Deters, A.; Hensel, A. Wound-healing plants from TCM: In vitro investigations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia 2013, 84, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.G.; Viswanatha, G.L.; Savinay, G.; Rajendra, C.E.; Halemani, P.D. 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose, a bioactivity guided isolated compound from Mangifera indica inhibits 11β-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine 2013, 20, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-G.; Jeong, S.-J.; Kwon, H.-Y.; Lee, H.-J.; Lee, E.-O.; Lee, M.-H.; Choi, S.-H.; Ahn, K.-S.; Kim, S.-H. Penta-O-galloyl-β-d-glucose attenuates cisplatin-induced nephrotoxicity via reactive oxygen species reduction in renal epithelial cells and enhances antitumor activity in Caki-2 renal cancer cells. Toxicol. In Vitro 2012, 26, 206–214. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Hao, W.; Zhao, M.; Peng, S. In vitro inhibition of fatty acid synthase by 1,2,3,4,6-penta-O-galloyl-β-d-glucose plays a vital role in anti-tumour activity. Biochem. Biophys. Res. Commun. 2014, 445, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Pae, H.-O.; Oh, H.; Hong, S.-G.; Kim, I.-K.; Chai, K.-Y.; Yun, Y.-G.; Kwon, T.-O.; Chung, H.-T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-β-d-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. 2001, 174, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Zhang, J.; Pugliese, A.; Kim, S.-H.; Lü, J. Anti-cancer gallotannin penta-O-galloyl-β-d-glucose is a nanomolar inhibitor of select mammalian DNA polymerases. Biochem. Pharmacol. 2010, 80, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Evans, S.C.; Soans, E.; Malki, A.; Liu, Y.; Liu, Y.; Chen, X. Insulin receptor signaling activated by penta-O-galloyl-α-d-glucopyranose induces p53 and apoptosis in cancer cells. Apoptosis 2011, 16, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Lee, H.-J.; Shaik, A.A.; Nkhata, K.; Xing, C.; Zhang, J.; Jeong, S.-J.; Kim, S.-H.; Lü, J. Penta-O-galloyl-β-d-glucose induces G1 arrest and DNA replicative S-phase arrest independently of p21 cyclin-dependent kinase inhibitor 1A, p27 cyclin-dependent kinase inhibitor 1B and p53 in human breast cancer cells and is orally active against triple-negative xenograft growth. Breast Cancer Res. 2012, 12, R67. [Google Scholar] [CrossRef]
- Li, L.; Shaik, A.A.; Zhang, J.; Nhkata, K.; Wang, L.; Zhang, Y.; Xing, C.; Kim, S.-H.; Lü, J. Preparation of penta-O-galloyl-β-d-glucose from tannic acid and plasma pharmacokinetic analyses by liquid-liquid extraction and reverse-phase HPLC. J. Pharm. Biomed. Anal. 2011, 54, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Himmeldirk, K.B.; Qian, Y.; Ren, Y.; Malki, A.; Chen, X. Biological and biomedical functions of penta-O-galloyl-d-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Lin, J.-H.; Lin-Shiau, S.-Y.; Lin, J.-K. Induction of apoptosis by penta-O-galloyl-β-d-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur. J. Pharmacol. 1999, 381, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, H.M.; Ji, S.-T.; Lee, S.-R.; Mar, W.; Gho, Y.S. 1,2,3,4,6-Penta-O-galloyl-β-d-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett. 2004, 208, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lee, H.-J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.-O.; Kim, S.-H.; Lü, J. Penta-1,2,3,4,6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Menichetti, S.; Arapitsas, P.; Nativi, C.; Turchetti, B.; Buzzini, P. O-Methylglucogalloyl esters: Synthesis and evaluation of their antimycotic activity. Bioorg. Med. Chem. Lett. 2005, 15, 4000–4003. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Menichetti, S.; Vincieri, F.F.; Romani, A. Hydrolyzable tannins with the hexahydroxydiphenoyl unit and the m-depsidic link: HPLC-DAD-MS identification and model synthesis. J. Agric. Food Chem. 2007, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, B.; Yao, K.; Luo, Y.; Ma, Z. Synthesis of gallotannins. Carbohydr. Res. 2001, 335, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Kawabata, J.; Kasai, T. Inhibitory effects of eliagi- and gallotannins on rat intestinal α-glucosidase complexes. Biosci. Biotechnol. Biochem. 2001, 65, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaee, K.; Lötzerich, K. Efficient total synthesis of the natural products 2,3,4,6-tetra-O-galloyl-d-glucopyranose, 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose and the unnatural 1,2,3,4,6-penta-O-galloyl-α-d-glucopyranose. Tetrahedron 1997, 53, 10725–10732. [Google Scholar] [CrossRef]
- Chai, Y.-J.; Cui, C.-B.; Li, C.-W.; Wu, C.-J.; Tian, C.-K.; Hua, W. Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 559–582. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-M.; Cui, C.-B.; Li, C.-W.; Wu, C.-J.; Zhang, Z.-J.; Li, L.; Huang, X.-J.; Ye, W.-C. Purpurogemutantin and purpurogemutantidin, new drimenyl cyclohexenone derivatives produced by a mutant obtained by diethyl sulfate mutagenesis of a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, C.-W.; Wu, C.-J.; Hua, W.; Cui, C.-B.; Zhu, T.-J.; Gu, Q.-Q. Metabolites of Paecilomyces lilacinus ZBY-1 from deep-sea water and their antitumor activity. J. Int. Pharm. Res. 2013, 40, 177–186. [Google Scholar]
- Xia, M.-W.; Cui, C.-B.; Li, C.-W.; Wu, C.-J. Three new and eleven known unusual C25 steroids: Activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1545–1568. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Li, C.-W.; Hua, W.; Wu, C.-J.; Cui, C.-B.; Zhu, T.-J.; Gu, Q.-Q. Metabolites of Aspergillus sp. 16-02-1 isolated from a deep sea sediment and preliminary test of their antitumor and antifungal activities. Chin. J. Mar. Drugs 2013, 32, 1–10. [Google Scholar]
- Cui, X.; Li, C.-W.; Wu, C.-J.; Hua, W.; Cui, C.-B.; Zhu, T.-J.; Gu, Q.-Q. Secondary metabolites of Paecilomyces lilacinus ZBY-1 and their activities. J. Int. Pharm. Res. 2013, 40, 765–771. [Google Scholar]
- Fang, S.-M.; Wu, C.-J.; Li, C.-W.; Cui, C.-B. A practical strategy to discover new antitumor compounds by activating silent metabolite production in fungi by diethyl sulphate mutagenesis. Mar. Drugs 2014, 12, 1788–1814. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Li, C.-W.; Cui, C.-B. Seven new and two known lipopeptides as well as five known polyketides: The activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1815–1838. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Li, C.-W.; Cui, C.-B.; Hua, W.; Zhu, T.-J.; Gu, Q.-Q. Nine new and five known polyketides derived from a deep sea-sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, C.-B.; Li, C.-W.; Hua, W.; Wu, C.-J.; Zhu, T.-J.; Gu, Q.-Q. Activation of dormant secondary metabolite production by introducing neomycin resistance into the deep-sea fungus, Aspergillus versicolor ZBY-3. Mar. Drugs 2014, 12, 4326–4352. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cui, C.-B.; Ai, D.Q.; Li, C.-W.; Tao, Z.-W.; Ren, R. Polyanthumin, a novel cyclobutane chalcone trimmer from Memecylon polyanthum. J. Asian Nat. Prod. Res. 2014. [CrossRef]
- Li, C.-W.; Cui, C.-B.; Cai, B.; Han, B.; Dou, D.-Q.; Chen, Y.-J. Aromatic chemical constituents of Choerospondias axillaries and their in vitro antitumor activity. Chin. J. Med. Chem. 2005, 15, 138–141, 147. [Google Scholar]
- Li, C.-W.; Cui, C.-B.; Cai, B.; Han, B.; Li, M.-M.; Fan, M. Flavanoidal constituents of Choerospondias axillaries and their in vitro antitumor and anti-hypoxia activities. Chin. J. Med. Chem. 2009, 19, 48–51, 64. [Google Scholar]
- Li, C.-W.; Cui, C.-B. One new and nine known flavonoids from Choerospondias axillaries and their in vitro antitumor, antihypoxia and antibacterial activities. Molecules 2014, 19, 21363–21377. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Cui, C.-B.; Cai, B.; Han, B.; Li, M.-M.; Fan, M. Galloyl glucosidic constituents of Choerospondias axillaries and their in vitro anti-tumor, anti-hypoxia and anti-bacteria activities. J. Int. Pharm. Res. 2014, 41, 449–455. [Google Scholar]
- Kashiwada, Y.; Nonaka, G.-I.; Nishioka, I.; Yamagishi, T. Galloyl and hydroxycinnamoylglucoses from rhubarb. Phytochemistry 1988, 27, 1473–1477. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Hirose, Y.; Iwata, H.; Sakamota, S.; Tanaka, T.; Kouno, I. Caffeoyl, coumaroyl, galloyl, and hexahydroxydiphenoyl glucoses from Balanophora japonica. Chem. Pharm. Bull. 2001, 49, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, D.S.; Kim, N.H.; Lee, Y.M.; Kim, J.; Kim, J.S. Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo. Biol. Pharm. Bull. 2011, 34, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Nakata, I.; Kishida, A.; Ayer, W.A.; Browne, L. Some biologically active tannins of Nuphar variegatum. Pytochemistry 1990, 29, 2491–2494. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsu, F.-L. Tannin and related compounds from Terminalia catappa and Terminaliaparviflora. J. Chin. Chem. Soc. 1999, 46, 613–618. [Google Scholar]
- Zhang, S.-Q.; Gu, Y.; Tang, L.; Yu, S.-N. Synthesis and the antitumor activity of several phenylpropanoids. Chin. J. Med. Chem. 2004, 14, 80–83. [Google Scholar] [CrossRef]
- Jalsa, N.K.; Singh, G. A unique approach to the synthesis of a dengue vaccine and the novel tetrasaccharide that results. Tetrahedron: Asymmetry 2009, 20, 867–874. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Di, D.; Wu, G.; Guo, H. Chemometric analysis of metabolism disorders in blood plasma of S180 and H22 tumor-bearing mice by high performance liquid chromatography-diode array detection. J. Chemom. 2011, 25, 430–440. [Google Scholar]
- Liao, N.; Ao, M.; Zhang, P.; Yu, L. Extracts of Lycoris aurea induce apoptosis in murine sarcoma S180 cells. Molecules 2012, 17, 3723–3735. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-W.; Dong, H.-J.; Cui, C.-B. The Synthesis and Antitumor Activity of Twelve Galloyl Glucosides. Molecules 2015, 20, 2034-2060. https://doi.org/10.3390/molecules20022034
Li C-W, Dong H-J, Cui C-B. The Synthesis and Antitumor Activity of Twelve Galloyl Glucosides. Molecules. 2015; 20(2):2034-2060. https://doi.org/10.3390/molecules20022034
Chicago/Turabian StyleLi, Chang-Wei, Hua-Jin Dong, and Cheng-Bin Cui. 2015. "The Synthesis and Antitumor Activity of Twelve Galloyl Glucosides" Molecules 20, no. 2: 2034-2060. https://doi.org/10.3390/molecules20022034
APA StyleLi, C.-W., Dong, H.-J., & Cui, C.-B. (2015). The Synthesis and Antitumor Activity of Twelve Galloyl Glucosides. Molecules, 20(2), 2034-2060. https://doi.org/10.3390/molecules20022034