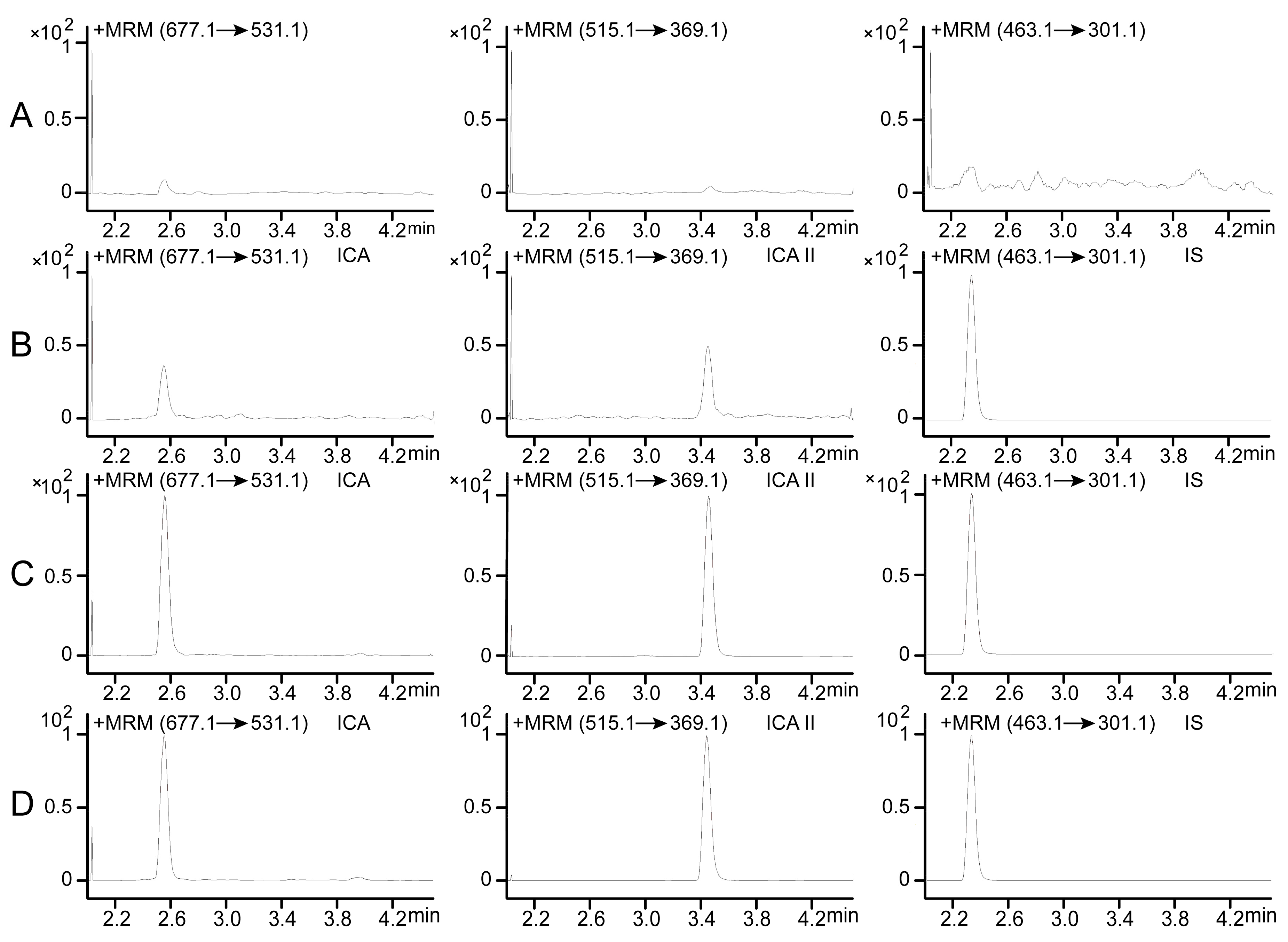
2.1. Optimization of UPLC-MS/MS Conditions
For detecting ICA and ICA II in rat plasma and investigating their pharmacokinetics, a UPLC-MS/MS method was developed and validated. To increase the sensitivity of ion response and obtain the baseline separation of ICA and ICA II, chromatographic conditions and MS parameters were optimized. MS determination of ICA, ICA II, and diosmetin-7-
O-β-
d-glucopyranoside (IS) standard solutions in both positive and negative ionization modes was attempted during method development. In the full-scan mode, electrospray ionization (ESI) source in the positive mode had a higher quality resolution and higher intensity to permit quantitative measurement than did the negative mode for both ICA and ICA II. To ascertain the precursor ions and determine product ions for use, the product ions and multiple reaction monitoring (MRM) mode were selected. MS parameters, such as fragmentor energies and collision energy, were optimized. Quantification was performed using the MRM mode with transitions
m/
z 677.1/531.1 for ICA, 515.1/369.1 for ICA II, and 463.1/301.1 for IS (
Figure 1).
Figure 1.
Mass spectra of ICA, ICA II and IS. (A) ICA; (B) ICA II; (C) IS.
Figure 1.
Mass spectra of ICA, ICA II and IS. (A) ICA; (B) ICA II; (C) IS.
To improve sensitivity in the positive ion mode of LC-MS/MS analysis, a small amount of 0.5% acetic acid was added into the mobile phase to help achieve high-quality resolution and the optimum peak shape in chromatograms. The optimum mobile phase conditions consisted of acetonitrile (A)–0.5% formic acid water (B) and gradient elution program was employed in the UPLC conditions: 0–1 min, 15% B; 1–2 min, 15%–40% B; 2–4 min, 40%–70% B; 4–5 min, 70%–90% B; 5–7 min, 90% B; 7–7.01 min, 90%–15% B; 7.01–8 min, 15% B.
2.4. Pharmacokinetic Study
The LC-MS/MS method has been successfully used in pharmacokinetic studies in rat plasma after ICA or ICA II administration. ICA and ICA II concentrations could be detected in all rat plasma samples for up to 12 h after administration.
Table 5 and
Table 6 list the main pharmacokinetic parameters of ICA and ICA II; these values are not in line with those reported in the literature [
26,
27,
28]. This dissimilarity is due to rats not being administered pure ICA but EF or its preparation in most pharmacokinetic studies [
26]. Only one study reported the administration of pure ICA to rats; however, the reported
Cmax and AUC
0–∞ (ng × min/mL) for ICA and ICA II differ partly from those in our study [
28]. Firstly, we predicted different solvent used to dissolve ICA in reference [
28] and our study would affect the pharmacokinetic action of ICA. Because ICA is insoluble in water and ethanol, we used a mixed solution consisted of solutol HS 15, PEG 400 and water (15:15:70) as it would not induce hemolysis after intravenous administration. Moreover, Xu [
28] used PEG 400 to dissolve ICA; however, PEG 400 cannot be used in an intravenous administration study because it may induce hemolysis. Therefore, we compared the pharmacokinetic properties of ICA dissolved in these two solvents after oral administration. The mean plasma concentration–time profiles of ICA are plotted in
Figure S1. A pharmacokinetic analysis of ICA (
Table S1) showed that all major pharmacokinetic parameters, including the
Tmax,
Cmax, MRT
0–t, and AUC
0-t, of ICA dissolved in the mixed HS 15 solution were nonsignificantly different from those of ICA dissolved in PEG 400, indicating that different solvents have minimal effects in the pharmacokinetic properties of ICA. On the basis of the hypothesis that intestinal flora are responsible for the intestinal absorption and metabolism of Epimedium flavonoids [
29], we suggest that the amount of intestinal flora and activity of enzymes in rats from different sources used in Xu’s and our study affect the intestinal absorption and hydrolysis of ICA. These findings warrant further research for exploring whether the absorption and hydrolysis of ICA vary among rats from different sources or among animal models with various diseases.
Table 5.
Pharmacokinetic parameters of ICA and ICA II following a single oral dose of 30 mg/kg (Mean ± SD, n = 5).
Table 5.
Pharmacokinetic parameters of ICA and ICA II following a single oral dose of 30 mg/kg (Mean ± SD, n = 5).
| Parameters | Oral Administration of ICA | Oral Administration of ICA II |
|---|
| ICA | ICA II | ICA II |
|---|
| Tmax (min) | 15.0 ± 0.0 | 147.0 ± 17.1 | 24.0 ± 13.4 |
| Cmax (ng/mL) | 27.2 ± 5.4 | 29.6 ± 6.8 | 103.6 ± 29.3 |
| t1/2z (min) | 73.9 ± 107.4 | 261.7 ± 185.9 | 135.0 ± 127.4 |
| CL (L/min/kg) | 46.2 ± 6.0 | 4.98 ± 1.76 | 3.68 ± 1.02 |
| MRT0-t (min) | 67.9 ± 54.0 | 271.3 ± 43.4 | 153.1 ± 68.6 |
| MRT0-∞ (min) | 83.1 ± 60.8 | 330.5 ± 107.1 | 189.4 ± 99.8 |
| AUC0-t (ng/mL × min) | 642.7 ± 83.2 | 6403 ± 2146 | 8387 ± 2539 |
| AUC0-∞ (ng/mL × min) | 657.8 ± 80.4 | 6601 ± 2067 | 8711 ± 2579 |
Table 6.
Pharmacokinetic parameters of ICA and ICA II following intravenous administration at 30 mg/kg, respectively ((Mean ± SD, n = 5).
Table 6.
Pharmacokinetic parameters of ICA and ICA II following intravenous administration at 30 mg/kg, respectively ((Mean ± SD, n = 5).
| Parameters | Intravenous Injection of ICA | Intravenous Injection of ICA II |
|---|
| ICA | ICA II | ICA II |
|---|
| Tmax (min) | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 |
| Cmax (ng/mL) | 3.543 × 104. ± 1.026 × 104 | 79.8 ± 22.4 | 4292 ± 1364 |
| T1/2z (min) | 319.8 ± 339.0 | 118.1 ± 57.3 | 281.9 ± 322.0 |
| MRT0-t (min) | 27.4 ± 5.9 | 87.3 ± 47.8 | 98.4 ± 42.1 |
| MRT0-∞ (min) | 33.0 ± 14.3 | 91.5 ± 47.8 | 230.8 ± 314.3 |
| AUC0-t (ng/mL × min) | 6.942 × 105 ± 1.700 × 105 | 2997 ± 1000 | 1.962 × 105 ± 6.328 × 105 |
| AUC0-∞ (ng/mL × min) | 6.954 × 105 ± 1.687 × 105 | 3013 ± 1002 | 2.111 × 105 ± 4.906 × 104 |
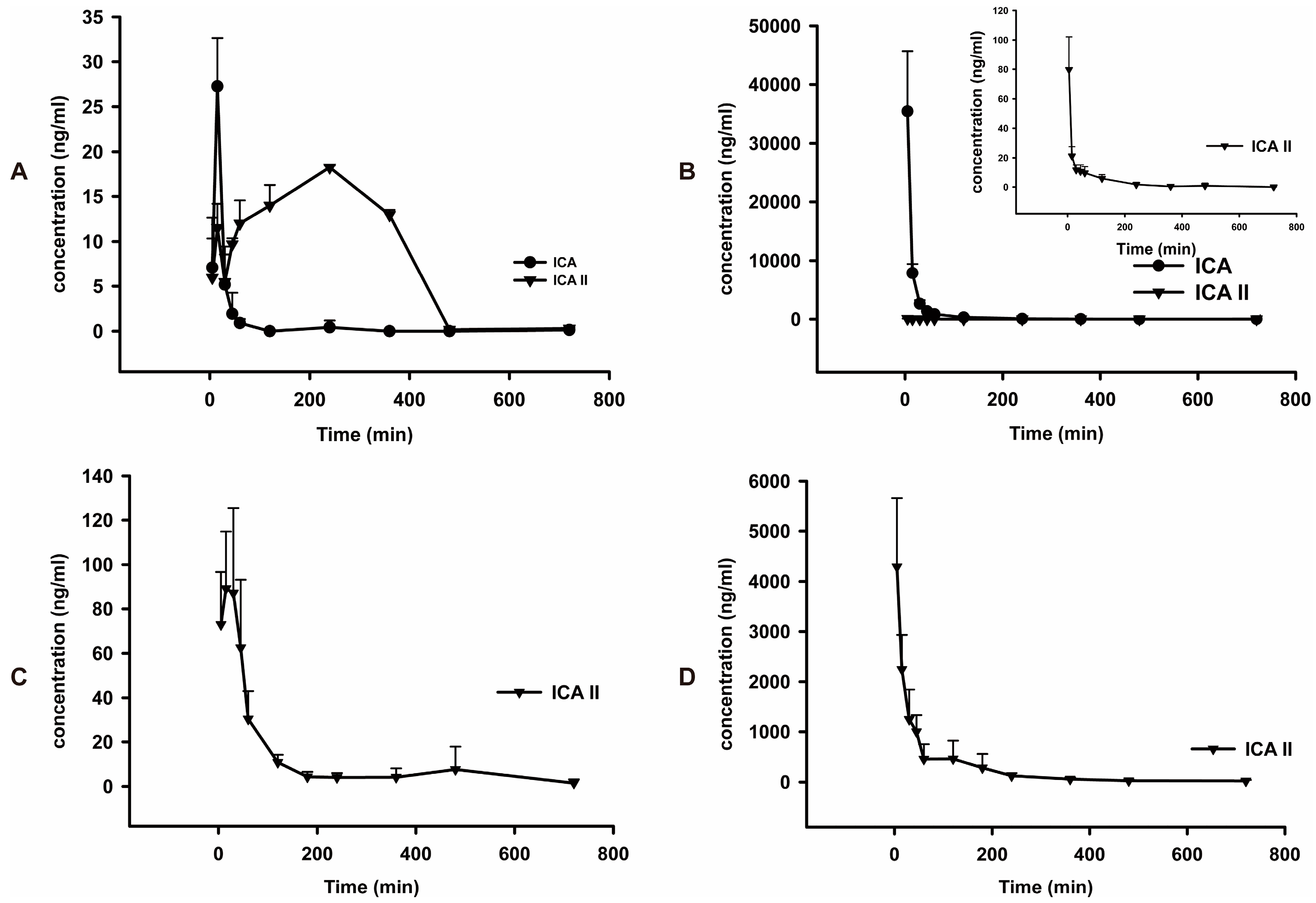
The mean plasma concentration-time profiles of ICA and ICA II after oral and intravenous administration of pure ICA and ICA II are shown in
Figure 3. A pharmacokinetic analysis of ICA (
Table 5) shows orally administrated ICA is rapidly absorbed in rats and is efficiently metabolized into its major metabolite, ICA II, which was detected in plasma at 5 min postdose. The time to reach the
Tmax of ICA was 15.0 ± 0.0 min. The mean AUC
0-t of ICA II (6403 ± 2146) was significantly higher than that of its parent drug, ICA (642.7 ± 83.2).After oral administration, 91.2% of ICA was transformed into ICA II. The MRT
0-t (min) of ICA II was longer than that of ICA. By comparing the pharmacokinetic parameters of ICA and ICA II (
Table 6), we found that the
Cmax of ICA and ICA II was higher in rats administered ICA intravenously than in rats administered ICA orally; however, the AUC
0–t of ICA II (2997 ± 1000) was significantly lower in rats administered ICA intravenously than that in rats administered ICA orally (6403 ± 2146). Only 0.4% ICA was transformed into ICA II after intravenous administration. The main form of ICA in blood after oral administration was ICA II, whereas ICA remains unchanged in blood after intravenous administration. Thus, these findings suggest that the phaemacodynamic actions of ICA differ depending on the mode of administration. However, additional studies are required to verify these results. By individually comparing the pharmacokinetic parameters of ICA and ICA II after oral administration, we observed that the
Cmax and AUC
0–t of ICA II were 3.8 and 13 times higher, respectively, than those of ICA, indicating that ICA II is absorbed faster than ICA and metabolized slower than ICA. Therefore, the
in vivo hydrolysis of ICA into ICA II facilitates ICA absorption. This result was consistent with that of the intestinal permeability test of ICA and ICA II [
30]. A similar trend was observed in MRT
0–t in ICA and ICA II in rats. MRT
0-t of ICA II was almost twice that of ICA. Moreover, the absolute bioavailability of ICA II is approximately 4.1% ± 1.4%, which is significantly higher than that of ICA (0.1% ± 0.0%). This result indicates that ICA II has a higher systemic exposure than ICA does, which may be used to elucidate the differential effects of ICA and ICA II on the same disease.
Figure 3.
Mean plasma concentration-time curves of ICA and ICA II in normal rats (n = 5). (A) Intragastric administration of ICA at a dose of 30 mg/kg; (B) Intravenous injection of ICA at a dose of 30 mg/kg; (C) Intragastric administration of ICA II at a dose of 30 mg/kg; (D) Intravenous injection of ICA II at a dose of 30 mg/kg.
Figure 3.
Mean plasma concentration-time curves of ICA and ICA II in normal rats (n = 5). (A) Intragastric administration of ICA at a dose of 30 mg/kg; (B) Intravenous injection of ICA at a dose of 30 mg/kg; (C) Intragastric administration of ICA II at a dose of 30 mg/kg; (D) Intravenous injection of ICA II at a dose of 30 mg/kg.
The pharmacokinetic parameters of ICA II after intravenous administration was compared with those of ICA. After intravenous administration, the
Cmax and AUC
0–t of ICA were significantly higher than those of ICA II; these results differ from those obtained in rats after oral administration, suggesting that the site and pathway that metabolize ICA and ICA II in rats are completely different. ICA is a dual glucoside and is considered to be metabolized to ICA II after the removal of 7-
O-glucose; subsequently, ICA II is metabolized to produce ICT after the further removal of 3-
O-rhamnose. However, the microbial flora in the colon is apparently crucial for metabolizing ICA. After intravenous administration, ICA cannot be hydrolyzed without the aid of microbial flora. The
Cmax and AUC
0–t of ICA II in rats after intravenous administration were approximately 12.1% and 4.2% of those of ICA in rats after intravenous administration. ICA II appears to be metabolized faster than ICA is after intravenous administration. We hypothesized that ICA II metabolizes into other forms after intravenous administration. Thus, an LC-MS/MS method for quantifying ICA, ICA II, and ICT simultaneously in rat plasma was developed. However, only ICA II, as a metabolite of ICA, was detected in rat plasma after administering ICA and ICA II both orally and intravenously. ICT appears to not be produced during ICA II metabolism. Studies have shown that ICT is an intermediate product of ICA II, which is transformed into its sulfated and glucuronidated conjugated form in the liver [
31]. Moreover, we found sulfated and glucuronidated conjugates of ICT in rat plasma after intravenous administration, indicating that ICA II in rats can be metabolized without the aid of microbial flora in the colon. Sulfated and glucuronidated conjugates of ICT can be quantified using the hydrolysis method with β-glucuronidase and sulfatase [
32]; we will present this research in a future paper.