1. Introduction
One of the main disadvantages for the general use of anthocyanins as food colorants is their low stability. In fact, the color stability of anthocyanins depends on a combination of several factors, which include their main structures, concentration, pH, temperature, the presence of complexing agents (phenols, metal ions), oxygen, light, enzymes, and interaction with other food components, among others [
1,
2,
3,
4]. It has been proved by several authors that anthocyanins have radical scavenging capacity, which confers them antioxidant properties [
5,
6,
7].
Processing of fresh fruits is always accompanied by a consequent cellular stress and breakdown by tissue damage. Therefore the activity of many enzymes increases as a result of increased permeability and mixing of enzymes and substrates that, in intact tissues, are entrapped into vacuoles and other cellular compartments [
8]. Polyphenol oxidase (PPO) acts on anthocyanins in the presence of
o-diphenols, via a coupled oxidation mechanism [
2], and can catalyze various enzymatic browning reactions affecting the quality and acceptability of processed products. In many fruits, including strawberry, PPO activity is responsible for red color losses due to degradation of anthocyanins [
9].
It is well known nowadays that the colorless polyphenols (flavones, flavonols, tannins esters of cinnamic and benzoic acids,) are capable of forming complex non-covalent molecular adducts with the flavylium ion nuclei of anthocyanins, which is flat and rich in π electrons; an interaction that increases color intensity due to the formation of colorful aggregates [
10,
11]. These copigments include a variety of structurally related and unrelated compounds, such as flavonoids, amino acids, non-flavonoid phenolics and organic acids [
12].
A wide range of phenolic extracts of vegetable origin contain flavonoids as their main constituents in the forms of aglycones, such as quercetin, myricetin, kaempferol and their glycosides [
13]. Some species such as thyme (
Thymus vulgaris) and rosemary (
Rosmarinus officinalis) are widely known to have high antioxidant capacity, and some methylated flavones with potent antioxidant activity have been isolated from thyme. Although some phenolic compounds are not directly involved in the enzymatic browning, they can act as polyphenol oxidase synergists. Shannon and Pratt reported that
p-coumaric acid is an uncompetitive inhibitor of apple PPO, and that it catalyzes the degradation of chlorogenic acid [
14,
15].
The formation of complexes between anthocyanins and copigments, under acidic conditions, improves the visual quality and color stability of foods that contain them. The stability of copigmented anthocyanins in the presence of ascorbic acid is known to have a chemoprotective effect during food processing [
16,
17]. Several substances such as quercetin-5-sulfonate, chlorogenic acid, morin, rutin, quercetin,
etc., have been identified as good copigments [
18]. However, several of these compounds, mainly phenolic acids such as caffeic, chlorogenic and protocatechuic acids, also act as polyphenol oxidase substrates [
19].
Several strategies have been devised to avoid the accumulation of specific phenolic compounds. Some involve the use of 2-aminoindane-2-phosphonic acid to inhibit phenylpropanoid biosynthesis in some tissues, however, even when this results effective in decreasing enzymatic browning, it also decreases the antioxidant properties of the plants [
20]. In other studies, Guillen
et al., reported a fractionation process for wine phenolics using two solid-phase extraction cartridges; the first one was a C18 resin, followed by an anion exchange resin [
21]. Although organic solvents can be used for elution of adsorbed polyphenols from C18 cartridges, the selectivity of such solvents is not enough to achieve molecular separation based only on minor structural differences in polarity. The use of ionic resins for fractionation of phenolic extracts in food applications has been previously reported as an analytical sample preparation stage and for targeted fractionation [
21,
22,
23]. Similarly, a study by Petelnic
et al. showed that fractionation of phenolic extracts has an effect on the antioxidant capacity when testing the uptake of specific compounds in yeast cells [
24].
The use of natural phenolic additives in food processing has been suggested as an alternative to chemical additives due to their properties as antioxidants and copigments for certain food matrices [
5,
16,
17]. In the present work, the use of treatment steps for extracts with anion exchange chromatography were explored in an attempt to fractionate thyme phenolics, without affecting their ability to stabilize anthocyanins through copigmentation. Potential uses of extracts modified by anion exchange chromatography as anthocyanins copigments, were also validated at equivalent phenolic levels. Phenolic profile analyses of modified extracts and their ability to serve as enzymatic substrates were explored as well in the present work, to verify that the desired functional modifications of the extracts were attained.
3. Experimental Section
3.1. Materials
HPLC calibration standards, Folin-Ciocalteau reagent and polyphenol oxidase (E.C. 1.14.18.1) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) and Fluka Chemie GmbH (Buchs, Switzerland). Pectic enzymes mixture was purchased from Novozymes (Krogshoejvej, Denmark). The acids and buffers used were obtained from Products Químicos Monterrey S.A. de C.V. (Monterrey, N.L., Mexico), Merck Co. (Darmstadt, Germany) and Desarrollo de Especialidades Químicas, S.A. de C.V. (San Nicolás de los Garza, N.L., Mexico). HPLC-grade solvents were purchased from Fisher Scientific Int. (Winnipeg, MB, Canada). The Sep-Pak C-18 cartridges were from Waters Co. (Milford, MA, USA). The two anion exchange resins Diaion® WA30 and Diaion® PA308 were obtained from Supelco (Bellefonte, PA, USA).
3.2. Preparation of Water-Soluble Extracts from Thyme
The preparation of water-soluble thyme extract (unprocessed, UNP) was carried out according to the procedure described by Bailey [
25]. Briefly, an infusion of dried thyme leaves (60 g/L) (obtained from a local supermarket) was acidified after boiling and filtered to remove solids. Supernatant was subjected to solid phase extraction to concentrate phenolic compounds using previously activated Sep-Pak C18 cartridges. Phenolic compounds were eluted with acidified methanol (0.01%
v/
v HCl). Methanol was removed using evaporation under reduced pressure at 45 °C and −25 mm Hg. A stock solution with 5 g of thyme extract in 50 mL of phosphate buffer pH 6.5, I = 0.05 (identified as extract UNP) was prepared and passed through polypropylene columns loaded with an anion exchanger (BioRad, Hercules, CA, USA). The anion resins used were Diaion
® WA30 (WAE) with alkylamine type RxNH
3OH functional groups and the Diaion
® resin PA308 (SAE) with benzyltrimethylamine type RxNR
3OH functional groups. A flow rate of 4 mL/min was used with 4.6 mL of extract per g. resin. The concentration of total phenolics in thyme extract was determined by the Folin-Ciocalteu method reported by Vinson
et al. and expressed as gallic acid equivalents (EAG) [
43].
3.3. Copigmentation Assays
Enzymatically clarified strawberry juice was used for copigmentation assays according to the procedure previously reported by Del Pozo-Insfran, using copigment:pigment molar ratios of 0, 25, 50, 75 and 100 for the two types of processed (WAE, SAE) and unprocessed (UNP) phenolic extracts [
16]. These relationships were established based on total concentration of monomeric anthocyanins in strawberry juice measured by a differential pH method [
44]. Copigmentation systems were formulated with 3 mL of strawberry juice and copigment volume such as to provide the aforementioned molar ratio according to the concentration of total phenolics in the extract. Systems were completed to 10 mL with 0.02 M pH 3.5 citrate buffer.
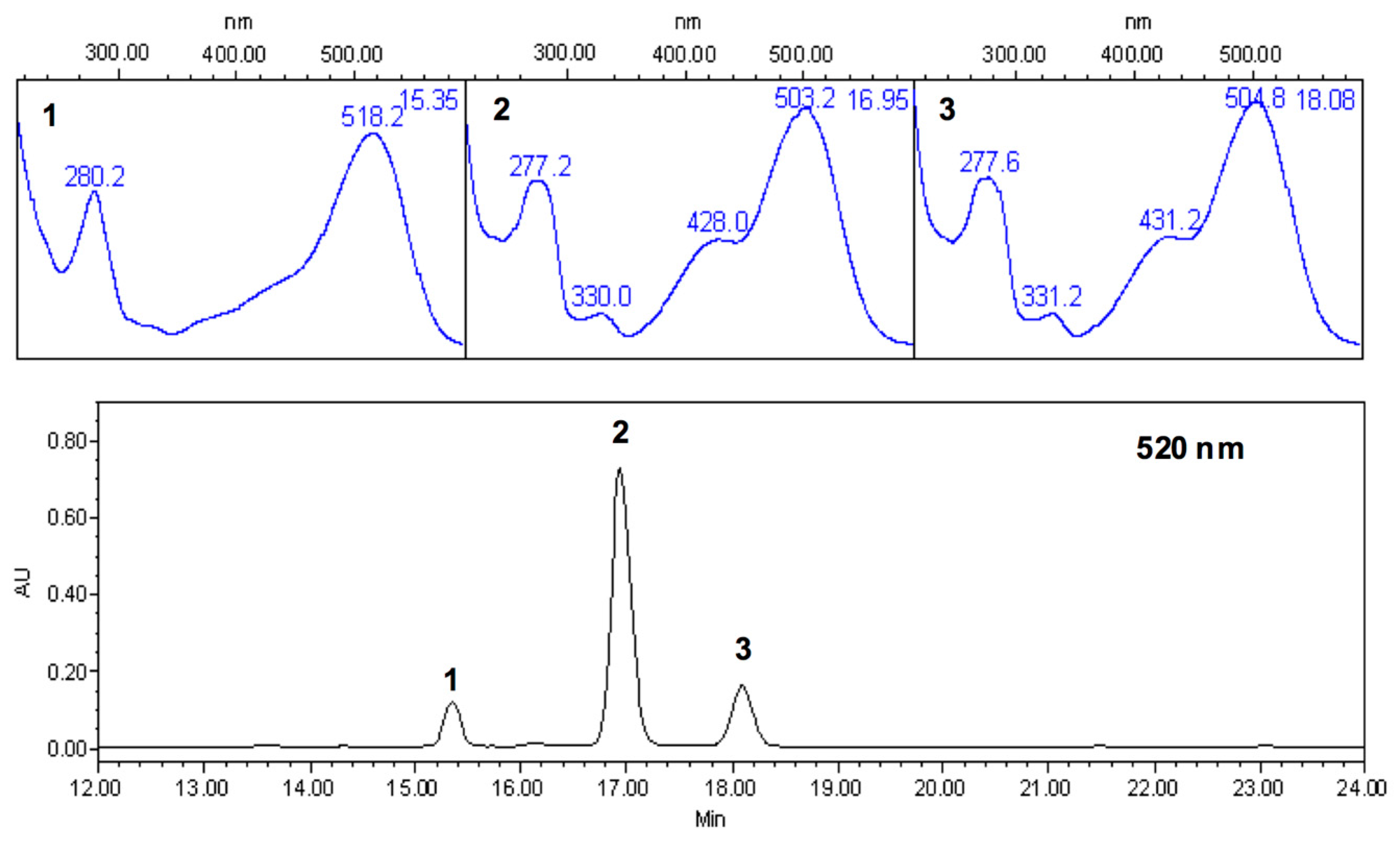
Physicochemical changes occurring following the addition of copigments were evaluated as a function of the phenolic extract added to the system and its concentration. The spectral changes were monitored by the increase in absorbance (% hyperchromic shift) and the displacement of the wavelength (λ) of maximum absorbance (bathochromic shift) ranging from 420 to 700 nm [
10]. Instrumental CIE color features including lightness (L*), chroma (C*) and hue angle (h*) were measured using a Minolta Chroma Meter CR-300 Series (Minolta Co. Ltd., Osaka, Japan) with a D65 light source and a 10° standard observer. Additionally, the percentage of monomeric/polymeric anthocyanins was determined based on color retention in the presence of sodium metabisulfite, while color density was determined by spectral measurements at 420, 520, and 700 nm according to the method reported by Rodriguez-Saôna
et al. for all copigment:pigment relations [
45].
3.4. Polyphenol Oxidase Assay
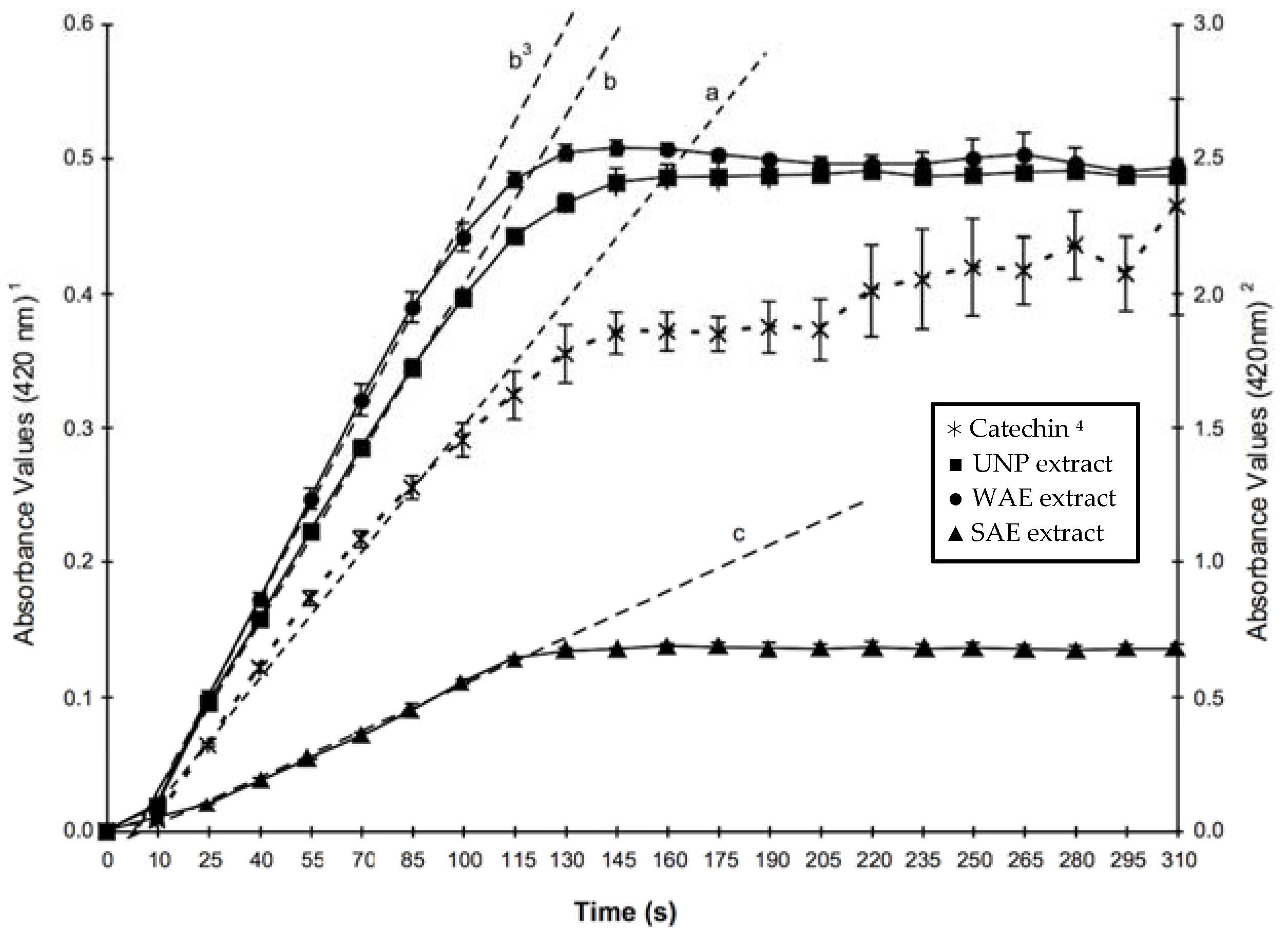
The spectrophotometric quantification of polyphenol oxidase was made modifying the methods described by Stauffer [
19] and Worthington [
46]. Briefly, 0.1 mL of copigment were mixed with 2.8 mL of phosphate buffer, pH 6.5, I = 0.05 in a temperature-controlled cell at 30 °C. After temperature equilibration, an aliquot of 0.1 mL of polyphenol oxidase with 300 U/mL was added and the absorbance was monitored at 420 nm in a Model DU 650 spectrophotometer (Beckman
®, Fullerton, CA, USA). The initial reaction rates were determined considering the molar absorptivity of quinones in phosphate buffer as 5 × 10
3 M
−1·cm
−1 [
47].
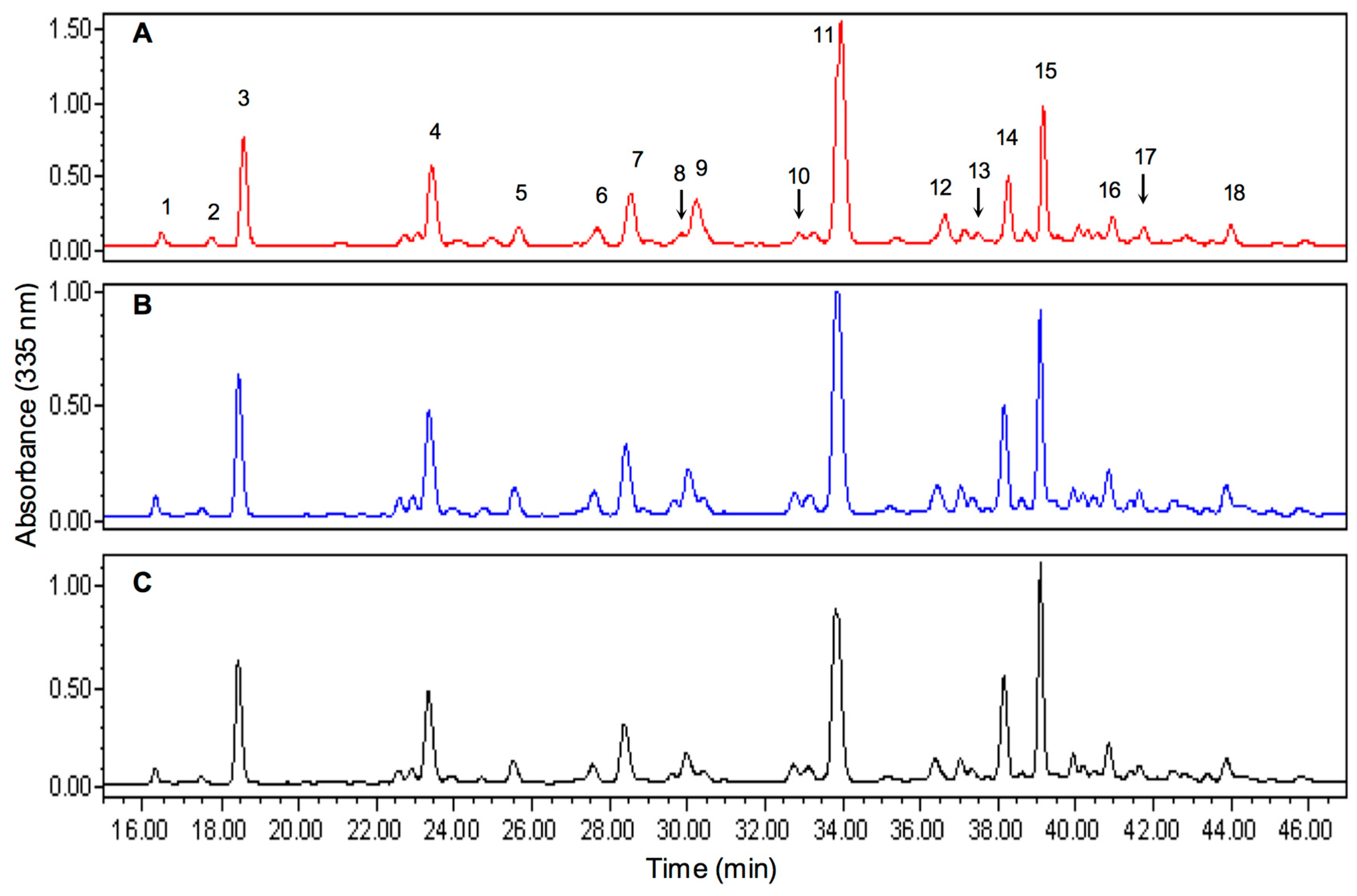
3.5. Chromatographic Profile of Thyme Phenolic Extracts
The determination of the phenolic profile of thyme extracts was performed by RP-HPLC-PDA using the conditions reported by Del Pozo-Insfran
et al. Two mobile phases consisting of water (phase A) and 60% methanol (phase B) adjusted to pH 2.4 with orthophosphoric acid were used. The separation was performed on a Symmetry C18 column 4.6 mm × 250 mm equipped with a Novapak C18 5 mm guard column (Waters Co.). Samples were diluted 1:1 with 1.2 M HCl solution in methanol HPLC grade and filtered through 0.45 μm PTFE membranes (Gelman, Ann Arbor, MI, USA). The elution gradient consisted of phase B from 0%–30% in 3 min, 30%–50% in 5 min, 50%–70% in 17 min, 70%–80% in 5 min, 80%–100% in 5 min and maintained for 10 min, with a constant flow of 0.8 mL/min [
35]. The identification of each peak was performed by comparison with authentic standards (Method A), spectral interpretation (Method B) and retention times (Method C). The chromatograms obtained were analyzed in three wavelengths selected according to the chemical nature: 280 nm for phenolic acids, 335 nm for flavones and 380 for flavonols.
3.6. Statistical Analysis
The copigmentation study was designed as a full 3 × 5 factorial including three different extracts (UNP, WAE and SAE) and five molar relations copigment:pigment (0, 25, 50, 75 y 100). Data represent the mean of three replicates. Regression analyses, Pearson correlation coefficients, and analysis of variance (ANOVA) were conducted using JMP software Version 5.0.1.2 (SAS Institute, Cary, NC, USA), with mean separation performed by the least significant difference (LSD) test (p < 0.05).