Phenolic Assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, Stem, Bark and Wood Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Yield and Total Phenolic Content in U. tomentosa Extracts
| Location | Sample | Extraction Yield (%) 1 | Total Phenolic Content (mg/g) 2 |
|---|---|---|---|
| Asomat (AS) | Leaves (AS-H) | 20.6 | 356.3 ± 38.3 |
| Stems (AS-T) | 3 | 460.0 ± 36.7 | |
| Bark (AS-C) | 3.2 | 315.6 ± 3.1 | |
| Wood (AS-M) | 2.3 | 95.7 ± 4.3 | |
| Los Chiles 1 (L1) | Leaves (L1-H) | 10.4 | 431.7 ± 11.5 |
| Stems (L1-T) | 4.4 | 247.7 ± 20.5 | |
| Los Chiles 2 (L2) | Leaves (L2-H) | 5.7 | 426.3 ± 5.3 |
| Palacios (P1) | Leaves (P1-H) | 14.9 | 371.8 ± 0.7 |
| Stems (P1-T) | 3 | 283.3 ± 3.3 | |
| Bark (P1-C) | 5.2 | 282.7 ± 13.5 | |
| Wood (P1-M) | 4.6 | 80.4 ± 4.3 | |
| Fortuna 1 (F1) | Leaves (F1-H) | 7.7 | 336.4 ± 23.4 |
| Stems (F1-T) | 2.2 | 236.4 ± 22.7 | |
| Wood (F1-M) | 3.7 | 40.5 ± 2.7 | |
| Fortuna 2 (F2) | Leaves (F2-H) | 9.3 | 401.1 ± 24.7 |
| Stems (F2-T) | 0.8 | 225.0 ± 12.5 | |
| Gema (GE) | Leaves (GE-H) | 7.7 | 480.5 ± 5.2 |
| Stems (GE-T) | 4.8 | 391.7 ± 4.2 | |
| Sarapiquí (S1) | Leaves (S1-H) | 11.1 | 364.0 ± 6.3 |
| Stems (S1-T) | 1.8 | 366.7 ± 22.2 | |
| Bark (S1-C) | 10.6 | 494.3 ± 2.8 | |
| Wood (S1-M) | 2.1 | 166.7 ± 9.5 |
2.2. Phenolic Profile of U. tomentosa Extracts Determined by UPLC/TQ-ESI-MS
| Compounds | Bark Extracts | Leaves Extracts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS | P1 | S1 | AS | F1 | F2 | GE | L1 | L2 | P1 | S1 | |
| Concentration (µg/g Extract) | |||||||||||
| Hydroxybenzoic acids | |||||||||||
| Benzoic acid | 83.5 ± 3.7 | 1747.0 ± 20.4 | 134.5 ± 0.2 | 55.8 ± 5.3 | 20.6 ± 0.9 | 71.0 ± 3.1 | nd | 57.9 ± 5.6 | 44.1 ± 4.4 | 34.9 ± 3.5 | 50.2 ± 5 |
| Salicylic acid | 1.1 ± 0.1 | 78.7 ± 6.5 | 14.0 ± 0.6 | 12.0 ± 0.2 | 13.1 ± 0.2 | 21.7 ± 0.3 | 0.9 ± 0 | 11.9 ± 0.7 | 9.8 ± 0.5 | 21.7 ± 0.6 | 31.2 ± 3.1 |
| 4-Hydroxybenzoic acid | 25.1 ± 1.2 | 74.1 ± 4.1 | 55.4 ± 2.9 | 38.2 ± 1.3 | 158.4 ± 3.5 | 430.3 ± 0.1 | 18.2 ± 1.3 | 15.8 ± 0.7 | 22.5 ± 1 | 47.3 ± 0.5 | 12.3 ± 0 |
| Protocatechuic acid | 168.0 ± 2.3 | 331.9 ± 24.1 | 79.4 ± 8.5 | 68.0 ± 1 | 254.1 ± 0.6 | 684.5 ± 15.7 | 18.1 ± 0.9 | 31.5 ± 0.9 | 32.6 ± 1.6 | 78.5 ± 0.2 | 21.5 ± 0.9 |
| Gallic acid | 9.4 ± 0.6 | 22.8 ± 1.1 | 14.5 ± 0.8 | 4.5 ± 0.3 | 15.5 ± 1.6 | 24.6 ± 1 | 6.7 ± 0.4 | 22.4 ± 0.7 | 3.4 ± 0 | 18.7 ± 1.2 | 9.0 ± 0.9 |
| Vanillic acid | 17.5 ± 1.7 | 104.1 ± 8.9 | 62.1 ± 3.3 | nd | 6.7 ± 0.7 | 10.5 ± 1.1 | nd | 3.8 ± 0.2 | 4.3 ± 0.4 | 5.1 ± 0.5 | 3.7 ± 0.37 |
| Syringic acid | 3.4 ± 0.2 | 24.6 ± 1.4 | 23.2 ± 1 | nd | 5.9 ± 0.6 | 6.9 ± 0.7 | nd | 3.4 ± 0.3 | 4.1 ± 0.3 | 2.8 ± 0.1 | nd |
| ∑ Hydroxybenzoic acids | 309.8 | 2383.2 | 383.1 | 178.5 | 474.3 | 1249.5 | 43.9 | 146.4 | 120.8 | 209 | 127.9 |
| Hydroxycinnamic acids | |||||||||||
| p-Coumaric acid | 0.9 ± 0.1 | 4.2 ± 0.4 | 9.5 ± 0.5 | 31.5 ± 1.4 | 37.1 ± 0.2 | 209.4 ± 1.7 | 11.2 ± 1 | 4.5 ± 0.4 | 5.1 ± 0.1 | 9.4 ± 0.1 | 3.5 ± 0.3 |
| Caffeic acid | 2.1 ± 0.1 | 12.5 ± 0.8 | 9.7 ± 1 | 6.2 ± 0 | 14.6 ± 0.2 | 35.4 ± 0.4 | 8.0 ± 0.3 | 5.5 ± 0.3 | 6.7 ± 0.3 | 4.1 ± 0.4 | 2.4 ± 0 |
| Ferulic acid | nd | 5.6 ± 0.4 | 6.6 ± 0.7 | 30.7 ± 1.5 | 29.9 ± 1 | 56.8 ± 2.7 | 13.5 ± 0.8 | 7.8 ± 0.5 | 15.5 ± 0.5 | 16.2 ± 1.4 | 11.5 ± 1.6 |
| Isoferulic acid | nd | 5.7 ± 0.4 | 2.2 ± 0.2 | nd | 10.5 ± 0.8 | 6.8 ± 0.7 | nd | 15.0 ± 0.9 | nd | 10.9 ± 0.2 | 13.1 ± 1.3 |
| ∑ Hydroxycinnamic acids | 3.0 | 28.0 | 28.0 | 68.4 | 92.1 | 308.4 | 32.7 | 32.8 | 27.3 | 40.6 | 29.5 |
| Flavan-3-ols: monomers | |||||||||||
| (+)-Catechin | 29.9 ± 2.8 | 238.4 ± 3.2 | 93.1 ± 9.3 | 569.1 ± 28.4 | 428.1 ± 3.1 | 2387.9 ± 152.3 | 202.6 ± 6.5 | 738.3 ± 36.4 | 203.4 ± 3 | 754.4 ± 0.4 | 1689.1 ± 48.8 |
| (−)-Epicatechin | 419.6 ± 9.6 | 1581.8 ± 87.2 | 678.2 ± 49.5 | 2062.3 ± 59.2 | 613.5 ± 17.2 | 2518.8 ± 34.7 | 189.9 ± 7.5 | 1054.7 ± 15 | 735.9 ± 23.9 | 963.9 ± 81.8 | 2128.0 ± 22.1 |
| ∑ Monomers | 449.5 | 1820.2 | 771.3 | 2631.4 | 1041.6 | 4906.7 | 392.5 | 1793.0 | 939.3 | 1718.3 | 3817.1 |
| Flavan-3-ols: procyanidin dimers | |||||||||||
| Procyanidin B1 | 39.1 ± 1.7 | 170.6 ± 17.1 | 306.7 ± 30.7 | 105.9 ± 5.5 | 717.2 ± 11.2 | 454.6 ± 7.3 | 93.1 ± 6.1 | 1092.1 ± 83.7 | 474.4 ± 15.1 | 684.9 ± 15.2 | 612.1 ± 34.3 |
| Procyanidin B2 | 827.7 ± 16.1 | 1651.8 ± 151.4 | 2184.9 ± 218.5 | 343.9 ± 1.4 | 887.3 ± 22 | 748.6 ± 0 | 128.2 ± 4.9 | 1254.9 ± 83.6 | 857.3 ± 44.7 | 930.2 ± 2.2 | 892.1 ± 48.9 |
| Procyanidin B3 | 44.1 ± 4.1 | 128.3 ± 12.8 | 372.6 ± 37.3 | 96.3 ± 1.7 | 1518.3 ± 53.1 | 1562.3 ± 82.1 | 307.0 ± 6.6 | 2569.3 ± 142.5 | 623.9 ± 20.8 | 1686.6 ± 36.2 | 1869.2 ± 18.1 |
| Procyanidin B4 | 661.6 ± 8.9 | 2175.1 ± 217.5 | 3962.1 ± 396.2 | 544.2 ± 28.8 | 2061.3 ± 66.9 | 2318.3 ± 64.4 | 602.7 ± 6.7 | 3089.5 ± 108 | 1852.6 ± 30.2 | 2630.7 ± 113.7 | 2593.2 ± 175.6 |
| Procyanidin B5 | 130.4 ± 10.6 | 282.3 ± 26.8 | 563.4 ± 9.1 | 77.5 ± 5.6 | 180.2 ± 4.1 | 226.0 ± 15.9 | 0.0 ± 0 | 260.1 ± 26 | 143.5 ± 12 | 232.7 ± 7.8 | 351.9 ± 3.4 |
| Procyanidin B7 | nd | 75.3 ± 7.5 | 108.4 ± 8.2 | 25.0 ± 0.4 | 185.4 ± 6.4 | 110.5 ± 11.1 | 45.0 ± 0.1 | 278.3 ± 3.6 | 99.8 ± 6 | 162.6 ± 9.5 | 140.3 ± 5.8 |
| Procyanidin B (5.47 min) | 101.0 ± 10.1 | 199.9 ± 14 | 28.4 ± 0.3 | 70.3 ± 2.4 | 94.7 ± 5.8 | 41.8 ± 2.1 | 157.8 ± 10.7 | 136.2 ± 5.3 | 106.8 ± 6.9 | 129.7 ± 12.4 | |
| Procyanidin B (9.27 min) | 16.7 ± 0.5 | 48.1 ± 4.8 | 71.1 ± 3.9 | 40.0 ± 0.2 | 70.5 ± 6.4 | 32.7 ± 1.8 | 37.3 ± 3.7 | 26.5 ± 2.4 | 99.8 ± 0.7 | 227.7 ± 7.7 | |
| ∑ Procyanidin dimers | 1702.9 | 4601.1 | 7746.1 | 1292.3 | 5660.0 | 3272.6 | 1250.5 | 8739.3 | 4214.2 | 6534.3 | 6816.2 |
| Flavan-3-ols: properlargonidin dimers | |||||||||||
| Propelargonidin dimer (4.43 min) | 23.4 ± 1 | 85.5 ± 3.7 | 308.9 ± 30.9 | 1181.4 ± 15.2 | 4235.8 ± 58.3 | 3691.4 ± 29.8 | 2767.6 ± 47.2 | 3598.9 ± 106.9 | 1688.7 ± 48.1 | 5021.9 ± 263.7 | 3669.9 ± 276.6 |
| Propelargonidin dimer (5.01 min) | 141.3 ± 11.7 | 319.4 ± 31.9 | 1493.6 ± 149.4 | 1442.5 ± 18.2 | 2498.9 ± 45.6 | 2675.8 ± 35.3 | 2227.9 ± 46.6 | 2421.8 ± 116.5 | 1328.6 ± 54.1 | 3147.7 ± 111.7 | 2633.1 ± 103.4 |
| Propelargonidin dimer (5.65 min) | 168.0 ± 4.7 | 0.1 ± 0.1 | 80.3 ± 5.2 | 1570.9 ± 46.7 | 428.3 ± 16.2 | 627.3 ± 2.4 | 995.7 ± 12.8 | 437.8 ± 2.8 | 1652.8 ± 16.2 | 654.9 ± 57.9 | 789.4 ± 25.8 |
| Propelargonidin dimer (9.27 min) | 23.8 ± 0.7 | 96.4 ± 9.6 | 375.8 ± 37.6 | 270.6 ± 18.1 | 662.1 ± 15.4 | 555.2 ± 5.6 | 252.1 ± 5.6 | 729.1 ± 54.2 | 283.9 ± 4.1 | 897.9 ± 15.5 | 695.5 ± 31.6 |
| ∑ Properlargonidin dimers | 365.5 | 501.4 | 2258.6 | 4465.4 | 7825.1 | 7549.7 | 6243.3 | 4036.7 | 4954 | 9722.4 | 7787.9 |
| Flavan-3-ols: procianidin trimers | |||||||||||
| Trimer T2 | nd | 0.9 ± 1.3 | nd | nd | nd | nd | nd | 8.1 ± 8.8 | nd | nd | 5.9 ± 8.4 |
| Procyanidin C1 | 436.9 ± 45.5 | 133.7 ± 8.7 | 211.3 ± 21.1 | nd | 51.3 ± 5.1 | 29.7 ± 3.0 | 63.1 ± 6.3 | 46.5 ± 4.6 | 139.5 ± 2.6 | 22.2 ± 1.4 | 16.0 ± 0.9 |
| Trimer B (4.62 min) | nd | 23.2 ± 0.9 | 83.3 ± 34.3 | nd | 56.0 ± 3.8 | 63.7 ± 6.4 | 84.6 ± 5.3 | 101.6 ± 8.1 | 273.3 ± 8.6 | 53.7 ± 0.1 | 62.3 ± 6.2 |
| ∑ Procyanidin trimers | 436.9 | 157.8 | 294.6 | nd | 107.3 | 93.4 | 147.7 | 156.2 | 412.8 | 75.9 | 84.2 |
| Flavalignans | |||||||||||
| Cinchonain (7.37 min) | 149.7 ± 4.4 | 584.1 ± 53.8 | 937.8 ± 40.9 | 582.0 ± 2.1 | 709.5 ± 10.6 | 422.3 ± 18.8 | 177.1 ± 3.7 | 544.8 ± 41.3 | 537.3 ± 15.7 | 84.1 ± 8.4 | 1009.2 ± 95.9 |
| Cinchonain (9.05 min) | 12.5 ± 0.8 | 49.8 ± 5 | 129.1 ± 0 | 517.2 ± 23.1 | 1145.9 ± 53.3 | 896.3 ± 25.1 | 437.6 ± 9.8 | 973.8 ± 39.5 | 771.1 ± 26.1 | 108.0 ± 1 | 1939.9 ± 89.1 |
| Cinchonain (9.30 min) | 13.1 ± 0.9 | 50.0 ± 5 | 129.7 ± 11.4 | 843.0 ± 2.3 | 1152.2 ± 15.8 | 1040.2 ± 54 | 645.3 ± 13.5 | 1215.4 ± 73.4 | 915.3 ± 28.9 | 103.6 ± 7.9 | 2276.7 ± 82.9 |
| Cinchonain (12.27 min) | 189.9 ± 4.5 | 541.3 ± 90.1 | 904.1 ± 154.1 | 770.0 ± 25.8 | 545.0 ± 6.5 | 542.4 ± 3.5 | 255.8 ± 14 | 480.4 ± 0.8 | 540.5 ± 23.6 | 59.1 ± 6.1 | 1076.0 ± 5.8 |
| ∑ Flavalignans | 365.2 | 1225.2 | 2100.7 | 2712.2 | 3552.6 | 2901.2 | 1515.8 | 3214.4 | 2764.2 | 354.8 | 6301.8 |
| Compounds | Stems | Wood | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS | F1 | F2 | GE | L1 | P1 | S1 | AS | F1 | P1 | S1 | |
| Concentration (µg/g Extract) | |||||||||||
| Hydroxybenzoic acids | |||||||||||
| Benzoic acid | 130.6 ± 4.8 | 989.1 ± 51.3 | 498.2 ± 38.3 | 14.0 ± 1 | 342.4 ± 12 | 506.0 ± 2.1 | 365.6 ± 21.5 | 1188.3 ± 87.8 | 23.1 ± 2.3 | 1718.5 ± 43.8 | 1285.7 ± 119.9 |
| Salicylic acid | 47.1 ± 1.4 | 75.5 ± 1.5 | 185.3 ± 2.4 | 39.1 ± 1.5 | 70.5 ± 4 | 95.2 ± 0.2 | 101.5 ± 0.9 | 37.8 ± 2.6 | 34.5 ± 2.3 | 53.1 ± 0.1 | 58.2 ± 1.4 |
| 4-hydroxybenzoic acid | 19.2 ± 0.9 | 160.2 ± 3.3 | 292.6 ± 15.4 | 23.1 ± 0.8 | 46.0 ± 2 | 81.5 ± 2 | 74.2 ± 5.7 | 115.6 ± 3.6 | 42.3 ± 0.7 | 38.0 ± 0.9 | 139.2 ± 2.4 |
| Protocatechuic acid | 126.6 ± 2.8 | 777.5 ± 15.3 | 1313.7 ± 54.6 | 114.2 ± 7.3 | 249.8 ± 11.5 | 276.0 ± 1.7 | 148.6 ± 7.6 | 181.2 ± 3.8 | 464.4 ± 10.1 | 107.2 ± 1.9 | 120.2 ± 0.8 |
| Gallic acid | 25.6 ± 1.6 | 33.5 ± 0.2 | 64.2 ± 1 | 29.1 ± 3 | 37.5 ± 0.8 | 47.3 ± 1.5 | 30.9 ± 1.2 | 17.1 ± 1.5 | 27.7 ± 0.2 | 27.2 ± 0.7 | 22.3 ± 2 |
| Vanillic acid | 85.5 ± 4.4 | 467.7 ± 2.8 | 676.1 ± 52.7 | 104.9 ± 10.4 | 241.8 ± 11.5 | 334.6 ± 16.9 | 238.4 ± 1.9 | 214.0 ± 5.8 | 77.6 ± 1.2 | 152.3 ± 0.5 | 322.7 ± 3.4 |
| Syringic acid | 25.8 ± 2.3 | 157.2 ± 11 | 190.8 ± 19.1 | 22.8 ± 1.6 | 65.3 ± 0.4 | 78.2 ± 6.8 | 79.8 ± 4 | 76.5 ± 7.8 | 39.9 ± 0.8 | 69.9 ± 3.3 | 160.3 ± 5.7 |
| ∑ Hydroxybenzoic acids | 4482.5 | 1999.2 | 2276.1 | 4322.7 | 1122.7 | 1669.6 | 3246.1 | 1326.6 | 747.9 | 1296.7 | 1073.1 |
| Hydroxycinnamic acids | |||||||||||
| p-Coumaric acid | 7.0 ± 0.3 | 17.1 ± 1.5 | 107.4 ± 1.6 | 10.6 ± 0.7 | 7.8 ± 0.5 | 11.3 ± 0.3 | 17.9 ± 1.5 | 22.6 ± 1.9 | 11.3 ± 1.1 | 6.6 ± 0.6 | 31.7 ± 2 |
| Caffeic acid | 10.4 ± 0.5 | 29.7 ± 1.9 | 57.3 ± 1 | 8.5 ± 0.6 | 12.3 ± 0.4 | 18.7 ± 0.2 | 18.4 ± 1 | 42.2 ± 0.7 | 122.8 ± 9.6 | 34.3 ± 3 | 44.4 ± 0.3 |
| Ferulic acid | 12.5 ± 1.3 | 43.9 ± 4 | 148.4 ± 14.8 | 21.5 ± 1.4 | 18.1 ± 0.5 | 24.2 ± 0.1 | 27.8 ± 1.2 | 45.0 ± 3.3 | 14.4 ± 1.4 | 30.3 ± 3 | 28.2 ± 2.3 |
| Isoferulic acid | nd | 17.9 ± 1.8 | 24.5 ± 1.9 | 11.6 ± 1 | 25.0 ± 2.4 | 20.1 ± 2 | 29.3 ± 2.9 | 39.3 ± 2.4 | 12.6 ± 1.3 | 16.1 ± 1.6 | 21.7 ± 2.2 |
| ∑Hydroxycinnamic acids | 29.9 | 108.6 | 337.6 | 51.6 | 63.2 | 74.3 | 93.4 | 149.1 | 161.1 | 87.3 | 126.0 |
| Flavan-3-ols: monomers | |||||||||||
| (+)-Catechin | 124.7 ± 11.7 | 92.1 ± 5.8 | 394.7 ± 7.3 | 623.7 ± 17.7 | 211.3 ± 4.5 | 219.2 ± 11.6 | 201.2 ± 0.9 | nd | 57.3 ± 5.7 | 9.8 ± 0.7 | 20.5 ± 3.9 |
| (−)-Epicatechin | 1106.7 ± 35 | 337.2 ± 1.7 | 758.4 ± 56.3 | 1964.8 ± 148.4 | 847.6 ± 0.2 | 815.3 ± 1.1 | 1000.5 ± 6.5 | 19.5 ± 0.4 | 167.1 ± 5.7 | 62.1 ± 3.7 | 127.4 ± 2 |
| ∑ Monomers | 1231.4 | 429.3 | 1153.1 | 2588.5 | 1058.9 | 1034.5 | 1201.7 | 19.5 | 224.4 | 71.9 | 147.9 |
| Flavan-3-ols: procyanidin dimers | |||||||||||
| Procyanidin B1 | 317.3 ± 6.6 | 116.0 ± 11.1 | 21.6 ± 1.7 | 433.7 ± 31.3 | 243.8 ± 6 | 343.3 ± 11.2 | 404.2 ± 11.8 | nd | nd | nd | 21.1 ± 0.6 |
| Procyanidin B2 | 2049.6 ± 20.6 | 629.7 ± 14.1 | 157.4 ± 7.8 | 1644.7 ± 141.4 | 949.5 ± 95 | 1166.9 ± 1.3 | 1867.7 ± 96.1 | nd | 14.8 ± 1.5 | 19.7 ± 1.1 | 363.9 ± 31.9 |
| Procyanidin B3 | 842.3 ± 4.6 | 440.9 ± 4.8 | 226.3 ± 1.3 | 1746.8 ± 2.4 | 658.1 ± 11.3 | 1145.1 ± 12.8 | 1080.0 ± 4.1 | nd | 2.4 ± 0.2 | 0.1 ± 0 | 55.3 ± 5.5 |
| Procyanidin B4 | 5500.0 ± 45.6 | 1861.2 ± 68.4 | 1062.9 ± 106.3 | 5443.4 ± 338.9 | 2360.8 ± 192.1 | 3569.1 ± 92.1 | 4152.2 ± 117.7 | nd | 35.9 ± 1.5 | 29.7 ± 0.3 | 554.2 ± 18.2 |
| Procyanidin B5 | 446.9 ± 12.5 | 93.9 ± 5.1 | 45.8 ± 4.6 | 382.2 ± 37 | 177.8 ± 17.8 | 202.8 ± 18.1 | 522.9 ± 52.3 | nd | 4.1 ± 0.4 | 5.5 ± 0.5 | 54.7 ± 4.7 |
| Procyanidin B7 | 158.4 ± 6.5 | 48.8 ± 0.9 | 20.0 ± 2 | 204.7 ± 1.4 | 80.9 ± 3.4 | 122.7 ± 0.2 | 163.2 ± 16.3 | nd | nd | nd | 15.7 ± 1.2 |
| Procyanidin B (5.47 min) | 195.0 ± 15.9 | 34.9 ± 3.1 | 17.4 ± 1.7 | 200.1 ± 15.6 | 81.2 ± 8.1 | 113.4 ± 8.2 | 178.6 ± 17.9 | nd | nd | nd | 15.9 ± 1.5 |
| Procyanidin B (9.27 min) | 31.6 ± 2.3 | nd | nd | 55.6 ± 1.7 | 13.3 ± 1.2 | nd | 40.9 ± 4.1 | nd | nd | nd | 5.6 ± 0.6 |
| ∑ Procyanidin dimers | 9541.1 | 3225.4 | 1551.4 | 10111.2 | 4565.4 | 6663.3 | 8409.7 | 57.2 | 55.0 | 722.5 | |
| Flavan-3-ols: propelargonidin dimers | |||||||||||
| Propelargonidin dimer (4.43 min) | 416.2 ± 11.8 | 462.0 ± 4.5 | 127.1 ± 8.5 | 724.6 ± 50.9 | 250.9 ± 17.6 | 773.3 ± 25.5 | 610.1 ± 4.2 | nd | nd | 0.1 ± 0 | 21.3 ± 2.1 |
| Propelargonidin dimer (5.01 min) | 1880.3 ± 31.3 | 939.5 ± 16.3 | 452.9 ± 45.3 | 1577.4 ± 117.7 | 637.1 ± 63.7 | 1799.6 ± 76.6 | 1892.5 ± 100.4 | nd | 13.0 ± 0.5 | 7.0 ± 0.7 | 110.0 ± 10.2 |
| Propelargonidin dimer (5.65 min) | 963.0 ± 21.5 | 45.9 ± 4.6 | 26.4 ± 2.6 | 737.1 ± 71.4 | 43.6 ± 4.4 | 104.5 ± 9 | 115.7 ± 5.2 | nd | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 |
| Propelargonidin dimer (9.27 min) | 208.8 ± 2.3 | 90.9 ± 9.1 | 49.2 ± 4.9 | 158.7 ± 15.7 | 69.2 ± 6.9 | 209.4 ± 20.9 | 285.7 ± 14.7 | nd | nd | 3.8 ± 0.4 | 24.4 ± 2.4 |
| ∑ Propelargonidin dimers | 3468.3 | 1538.3 | 655.6 | 3197.8 | 1000.8 | 2886.8 | 2904.0 | 13.1 | 11.0 | 155.8 | |
| Flavan-3-ols: procyanidin trimers | |||||||||||
| Trimer T2 | 104.3 ± 10 | nd | nd | 108.7 ± 9.1 | 5.4 ± 5.1 | 4.6 ± 4.1 | 13.9 ± 0.8 | nd | nd | nd | nd |
| Procyanidin C1 | 1095.0 ± 41.2 | 60.2 ± 6 | nd | 884.7 ± 61.6 | 116.4 ± 11.6 | 83.6 ± 4.4 | 226.4 ± 22.6 | nd | nd | nd | 33.8 ± 3.4 |
| Trimer B (4.62 min) | 987.0 ± 39.7 | 38.6 ± 3.9 | 1.7 ± 0.1 | 1000.7 ± 58.6 | 134.8 ± 13.5 | 113.4 ± 7.5 | 221.2 ± 9.6 | nd | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0 |
| ∑ Procyanidin trimers | 2186.3 | 98.8 | 1.7 | 1994.1 | 256.6 | 201.6 | 461.5 | 1.8 | 1.7 | 35.6 | |
| Flavalignans | |||||||||||
| Cinchonain (7.37 min) | 1122.9 ± 17.8 | 591.4 ± 21.7 | 385.7 ± 38.6 | 608.7 ± 66.1 | 315.1 ± 30.7 | 483.6 ± 2.4 | 830.5 ± 25.4 | 394.4 ± 3.6 | 220.9 ± 6.5 | 400.9 ± 6 | 339.5 ± 4.2 |
| Cinchonain (9.05 min) | 339.8 ± 4 | 150.9 ± 0.9 | 281.7 ± 27.3 | 667.0 ± 62.8 | 93.6 ± 7.4 | 139.3 ± 13 | 278.2 ± 27.8 | 18.2 ± 0.4 | 29.4 ± 2.9 | 30.7 ± 3.1 | 20.6 ± 1.1 |
| Cinchonain (9.30 min) | 329.7 ± 6.6 | 130.4 ± 4.2 | 270.4 ± 8.8 | 632.5 ± 56.2 | 88.4 ± 7.6 | 114.4 ± 4.2 | 224.9 ± 6.8 | 27.6 ± 1.5 | 22.8 ± 0.9 | 26.8 ± 0.1 | 25.0 ± 2.5 |
| Cinchonain (12.27 min) | 1010.3 ± 7.1 | 422.6 ± 7.9 | 393.1 ± 19.5 | 557.5 ± 29 | 221.8 ± 17 | 339.3 ± 7.6 | 704.7 ± 27.4 | 420.3 ± 14.4 | 211.3 ± 0.3 | 390.4 ± 20.7 | 321.2 ± 11.3 |
| ∑ Flavalignans | 2802.7 | 1295.3 | 1330.9 | 2465.7 | 718.9 | 1076.6 | 2038.3 | 860.5 | 484.4 | 848.8 | 706.3 |
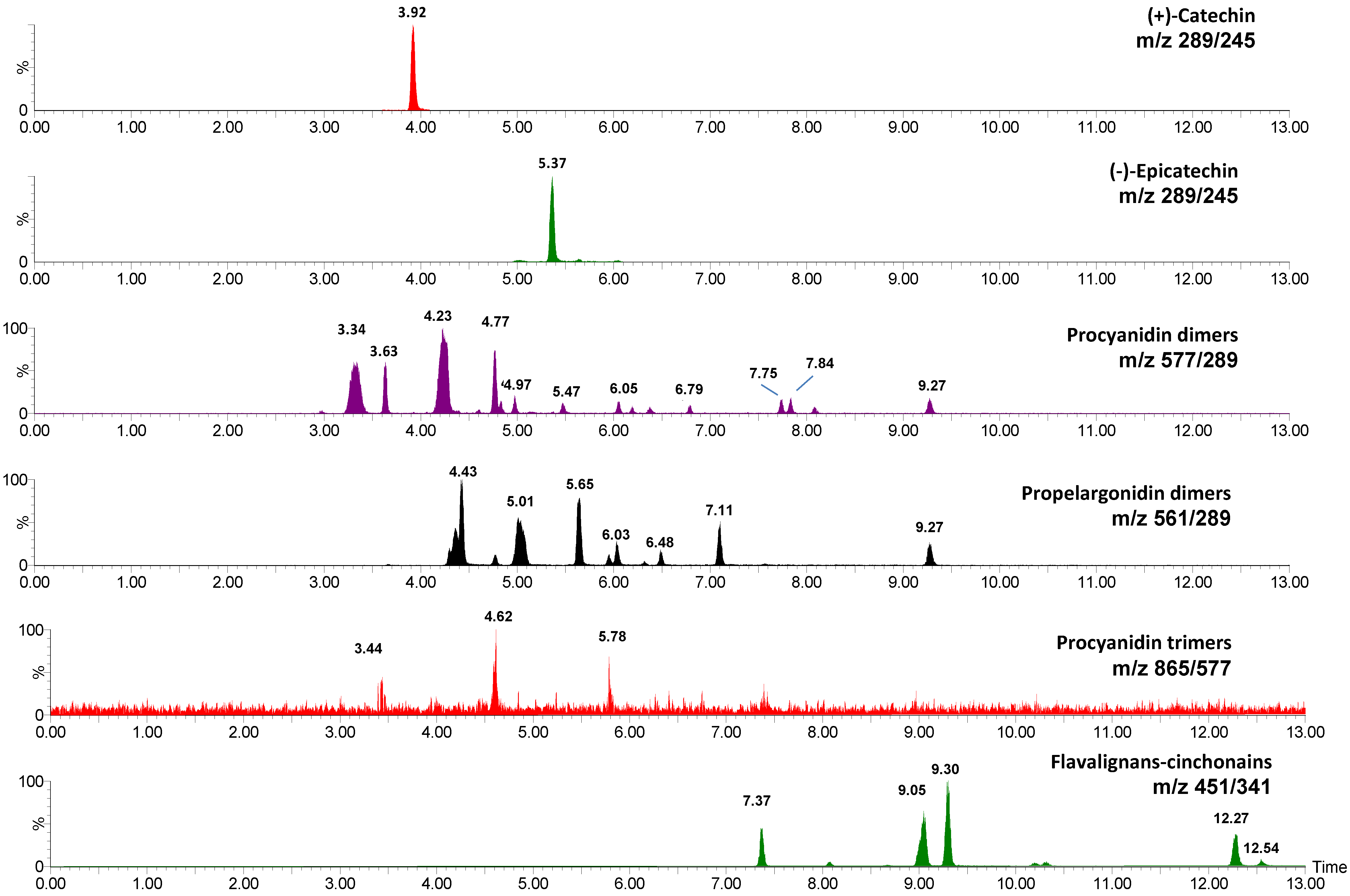
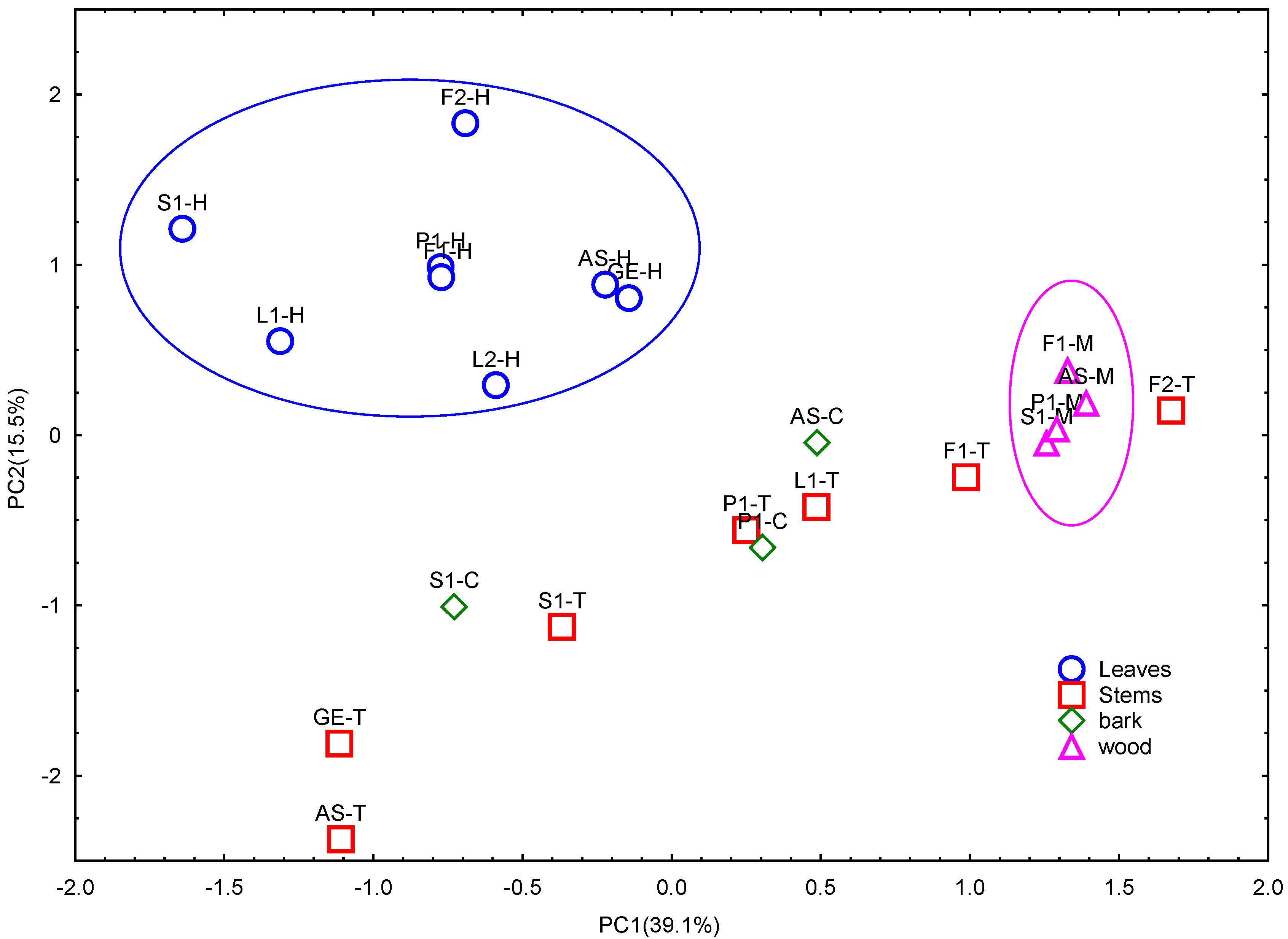
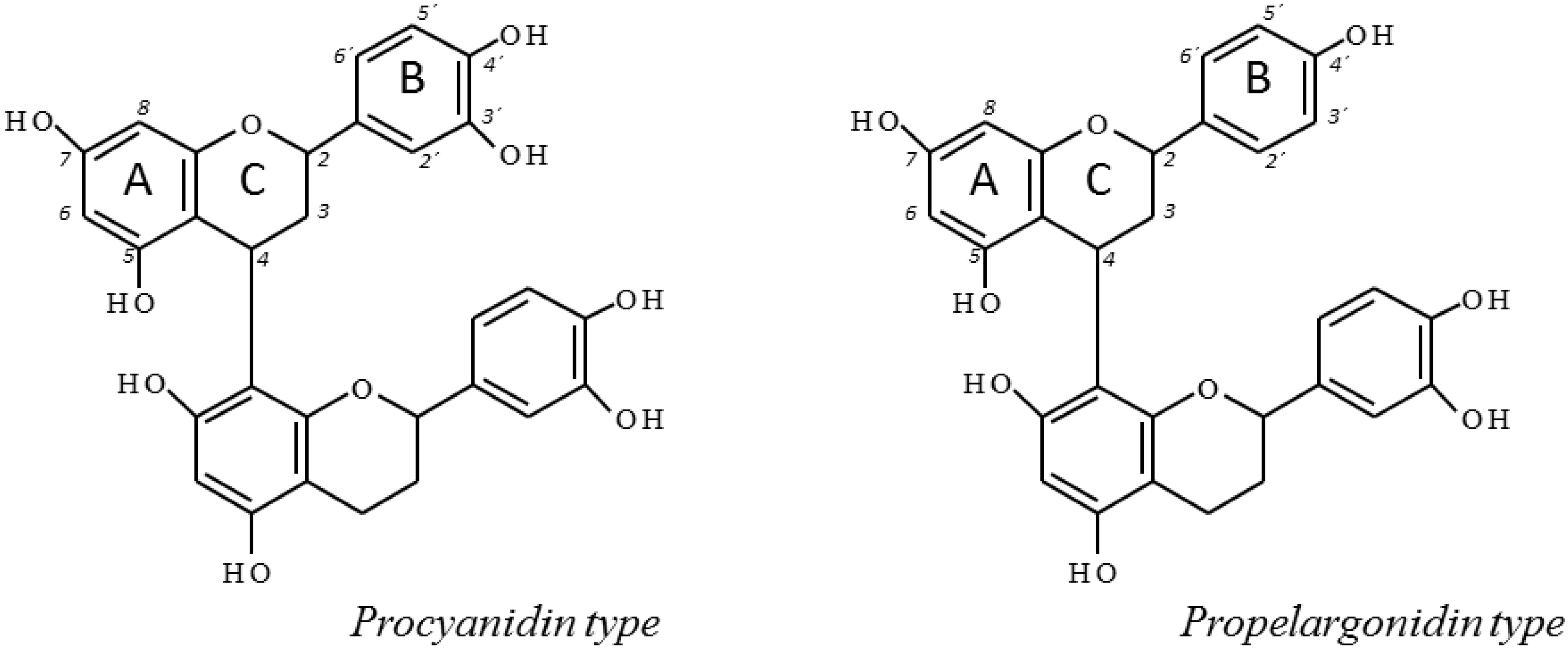
2.3. 13C-NMR Analysis of U. tomentosa Extracts

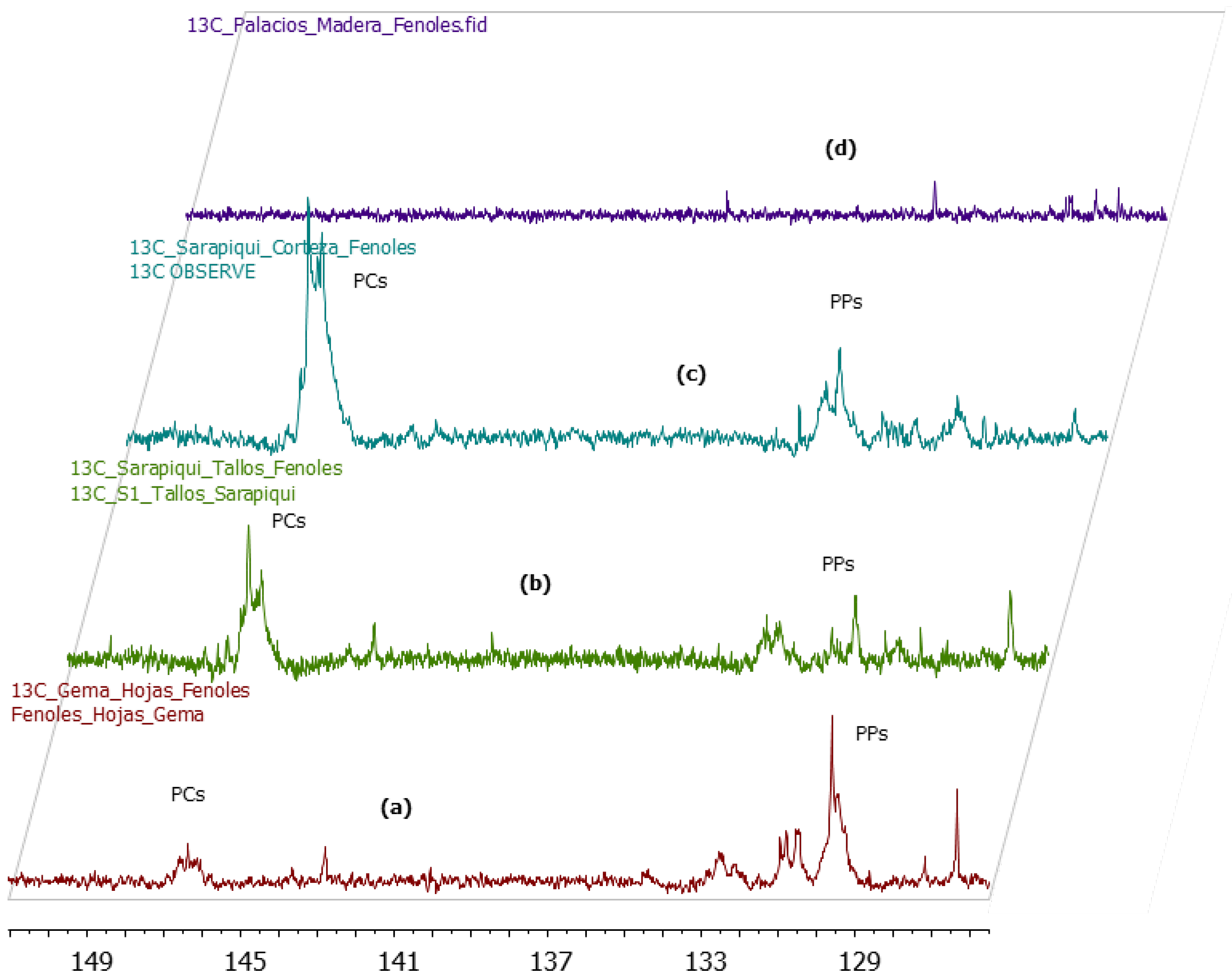
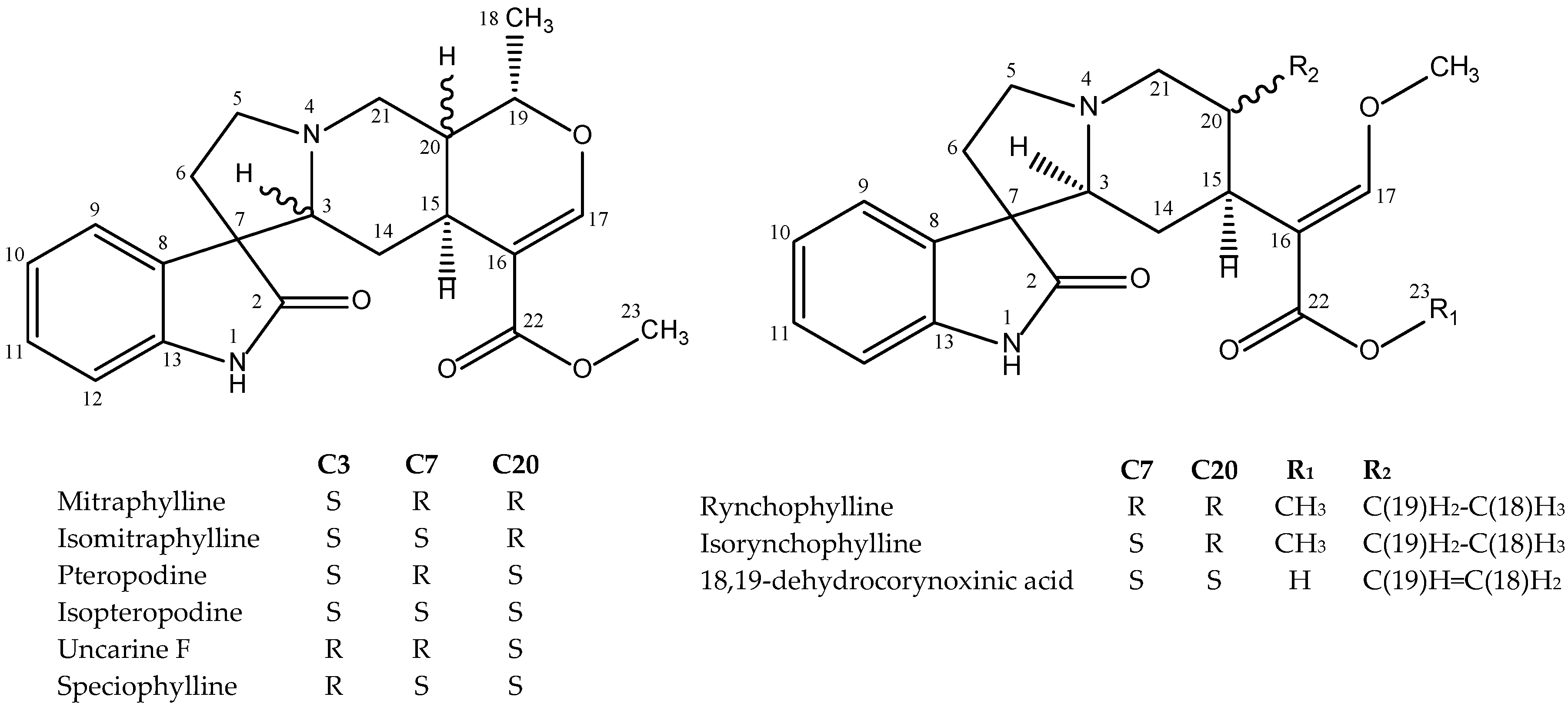
2.4. Alkaloids Contents of U. tomentosa Extracts
3. Experimental Section
3.1. Plant Material
| Sample | Extraction Yield (%) 1 | Alkaloid Content (mg/100 g Dry Plant Material) 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | R | M | UF | IR | IM | P | IP | ∑ Alkaloids | ||
| AS-C | 0.20 | 16.8 ± 0.2 | nd | 13.6 ± 0.1 a | 10.1 ± 0.1 | nd | 7.92 ± 0.15 a | 50.2 ± 0.4 a | 36.0 ± 0.3 a | 135 |
| AS-H | 0.44 | nd | 85.1 ± 3.9 a | nd | nd | 185 ± 3 a | nd | 64.3 ± 1.3 b | 68.9 ± 1.4 b | 403 |
| F1-H | 0.81 | nd | 221 ± 2 b | nd | nd | 483 ± 5 b | nd | nd | nd | 704 |
| GE-H | 0.79 | nd | 195 ± 1 c | nd | nd | 350 ± 2 c | nd | nd | nd | 545 |
| L1-C | 0.32 | nd | nd | 9.22 ± 0.15 b | nd | nd | 26.9 ± 0.1 b | 54.9 ± 0.1 c | 55.3 ± 0.1 c | 146 |
| L1-H | 0.78 | nd | 16.6 ± 0.3 d | 135 ± 1c | nd | nd | 179 ± 1 c | nd | nd | 330 |
| P1-C | 0.23 | nd | 27.3 ± 0.4 e | nd | nd | 57.4 ± 0.1 d | nd | 4.72 ± 0.25 d | 5.65 ± 0.29 d | 95.0 |
| P1-H | 0.72 | nd | 190 ± 1 c | nd | nd | 509 ± 1 e | nd | nd | nd | 699 |
| S1-C | 0.35 | nd | 77.5 ± 0.1 f | 7.22 ± 0.95 d | nd | 177 ± 1 a | 6.42 ± 0.05 d | nd | 6.95 ± 0.19 d | 275 |
| S1-H | 0.65 | nd | 139 ± 1 g | nd | nd | 413 ± 3 f | nd | nd | nd | 552 |
3.2. Extraction of Phenolic Compounds from the Different Parts of U. tomentosa
3.3. Obtention of Alkaloids Extracts
3.4. Determination of Total Phenolic Content
3.5. Analysis of Phenolic Compounds by UPLC-DAD-ESI-TQ MS
3.6. Analysis by 13C-NMR
3.7. Alkaloid Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinberg, P.N. Cat’s Claw: An herb from the Peruvian Amazon. SIDAhora 1995, 35–36. [Google Scholar]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, Phytochemistry and Pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Falkiewicz, B.; Lukasiak, J. Vilcacora [Uncaria tomentosa (Willd.) DC and Uncaria guianensis (Aublet) Gmell.]—A review of published scientific literature. Case Rep. Clin. Pract. Rev. 2001, 2, 305–316. [Google Scholar]
- Sandoval, M.; Okuhama, N.N.; Zhang, X.-J.; Condezo, L.A.; Lao, J.; Angeles, F.M.; Musah, R.A.; Bobrowski, P.; Miller, M.J.S. Anti-inflammatory and antioxidant activities of cat’s claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine 2002, 9, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, R.; Re, F.; Bianchi, A.; de Feo, V.; De Simone, F.; Bianchi, L.; Stivala, L.A. Mutagenic and antimutagenic activities of uncaria tomentosa and its extracts. J. Ethnopharmacol. 1993, 38, 63–77. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Mongelli, E.; Coussio, J.; Ciccia, G. Evaluation of the in vitro antioxidant activity in extracts of Uncaria tomentosa (willd.) DC. Phytother. Res. 1997, 11, 254–256. [Google Scholar] [CrossRef]
- Choi, H.R.; Choi, J.S.; Han, Y.N.; Bae, S.J.; Chung, H.Y. Peroxynitrite Scavenging Activity of Herb Extracts. Phytother. Res. 2002, 16, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Chan, Y.Y. Constituents of leaves of Uncaria hirsute Haviland. J. Chin. Chem. Soc. (Taipei Taiwan) 1994, 41, 209–212. [Google Scholar] [CrossRef]
- Sekiya, N.; Shimada, Y.; Shibahara, N.; Takagi, S.; Yokoyama, K.; Kasahara, Y.; Sakakibara, I.; Terasawa, K. Inhibitory effects of choto-san (diao-teng-san), and hooks and stems of Uncaria sinensis on free radical-induced lysis of rat red blood cells. Phytomedicine 2002, 9, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Pavei, C.; Kaiser, S.; Borre, G.L.; Ortega, G.G. Validation of a LC Method for Polyphenols Assay in Cat’s Claw (Uncaria tomentosa). J. Liquid Chromatogr. Relat. Technol. 2010, 33, 1551–1561. [Google Scholar] [CrossRef]
- De Matta, M.; Delle Monache, F.; Ferrari, F.; Marini-Bettolo, G.B. Alkaloids and procyanidins in Uncaria sp. from Peru. Farm. Sci. 1976, 31, 527–535. [Google Scholar]
- Gonçalves, C.; Dinis, T.; Batista, M.T. Antioxidant properties of proanthocyanidins of Uncaria tomentosa bark decoction: A mechanism for anti-inflammatory activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.; Wagner, H. Pharmacologically active procyanidines from the bark of Uncaria tomentosa. Phytomedicine 1997, 4, 265–266. [Google Scholar] [CrossRef]
- Ito, H.; Kobayashi, E.; Takamatsu, Y.; Li, S.-H.; Hatano, T.; Sakagami, H.; Kusama, K.; Yoshida, T. Polyphenols from Eriobotrya japonica and Their Cytotoxicity against Human Oral Tumor Cell Lines. Chem. Pharmacol. Bull. 2000, 48, 687–693. [Google Scholar] [CrossRef]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric proanthocyanidins. Stereochemistry, structural units and molecular weight. J. Chem. Soc. Perkin Trans. 1980, 1, 2278–2286. [Google Scholar] [CrossRef]
- Fu, C.; Wang, H.; Ling, W.; Song, L.; Huang, D. Antioxidant Activity and Proanthocyanidin Profile of Selliguea feei Rhizomes. Molecules 2013, 18, 4282–4292. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ma, B.; Wu, C.; Yang, J.; Zhang, L.; Liu, S.; Wu, L.; Kano, Y. Alkaloids from the Leaves of Uncaria rhynchophylla and their Inhibitory Activity on NO Production in Lipopolysaccharide-Activated Microglia. J. Nat. Prod. 2008, 71, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Sánchez-Patán, F.; Quintanilla, J.E.; Lebrón-Aguilar, R.; Bartolomé, B.; Navarro, M. Phenolic Extracts of Uncaria Tomentosa L. (Cat’s Claw) Containing Procyanidins, Propelargonidins and Flavanolignans, Method for the Production Thereof, and Applications of Same. Patent WO2014/096488 A1, 26 June 2014. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Sánchez-Patán, F.; Monagas, M.; Moreno-Arribas, M.V.; Bartolome, B. Determination of microbial phenolic acids in human faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011, 59, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Bartolome, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targetted analysis of precursor compounds, intermediate metabolites and end-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Seki, H.; Takayama, H.; Aimi, N.; Sakai, S.; Ponglux, D. A nuclear Magnetic Resonance Study on the Eleven Stereoisomers of Heteroyohimbine-Type Oxindole Alkaloids. Chem. Pharm. Bull. 1993, 41, 2077–2086. [Google Scholar] [CrossRef]
- Paradowska, K.; Wolniak, M.; Pisklak, M.; Gliński, J.; Davey, M.; Wawer, I. 13C, 15N CPMAS NMR and GIAO DFT calculations of stereoisomeric oxindole alkaloids from Cat’s Claw (Uncaria tomentosa). Solid State Nucl. Magn. Reson. 2008, 34, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Nurmi, T.; Tuomainen, T.-P.; Salonen, J.; Pukkala, E.; Voutilainen, S. Intake of flavonoids and risk of cancer in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Int. J. Cancer 2008, 123, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. A Review. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.; Hutfless, S.; Ding, X.; Girotra, S. Chocolate and Prevention of Cardiovascular Disease: A Systematic Review. Nutr. Metab. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Qa’dan, F.; Verspohl, E.J.; Nahrstedt, A.; Petereit, F.; Matalka, K.Z. Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo. J. Ethnopharmacol. 2009, 124, 224–227. [Google Scholar]
- Sample Availability: Samples of the extracts from Uncaria tomentosa L. are available from the authors at the University of Costa Rica.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro Hoyos, M.; Sánchez-Patán, F.; Murillo Masis, R.; Martín-Álvarez, P.J.; Zamora Ramirez, W.; Monagas, M.J.; Bartolomé, B. Phenolic Assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, Stem, Bark and Wood Extracts. Molecules 2015, 20, 22703-22717. https://doi.org/10.3390/molecules201219875
Navarro Hoyos M, Sánchez-Patán F, Murillo Masis R, Martín-Álvarez PJ, Zamora Ramirez W, Monagas MJ, Bartolomé B. Phenolic Assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, Stem, Bark and Wood Extracts. Molecules. 2015; 20(12):22703-22717. https://doi.org/10.3390/molecules201219875
Chicago/Turabian StyleNavarro Hoyos, Mirtha, Fernando Sánchez-Patán, Renato Murillo Masis, Pedro J. Martín-Álvarez, William Zamora Ramirez, Maria J. Monagas, and Begoña Bartolomé. 2015. "Phenolic Assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, Stem, Bark and Wood Extracts" Molecules 20, no. 12: 22703-22717. https://doi.org/10.3390/molecules201219875