Antiproliferative Activities of Water Infusions from Leaves of Five Cornus L. Species
Abstract
:1. Introduction
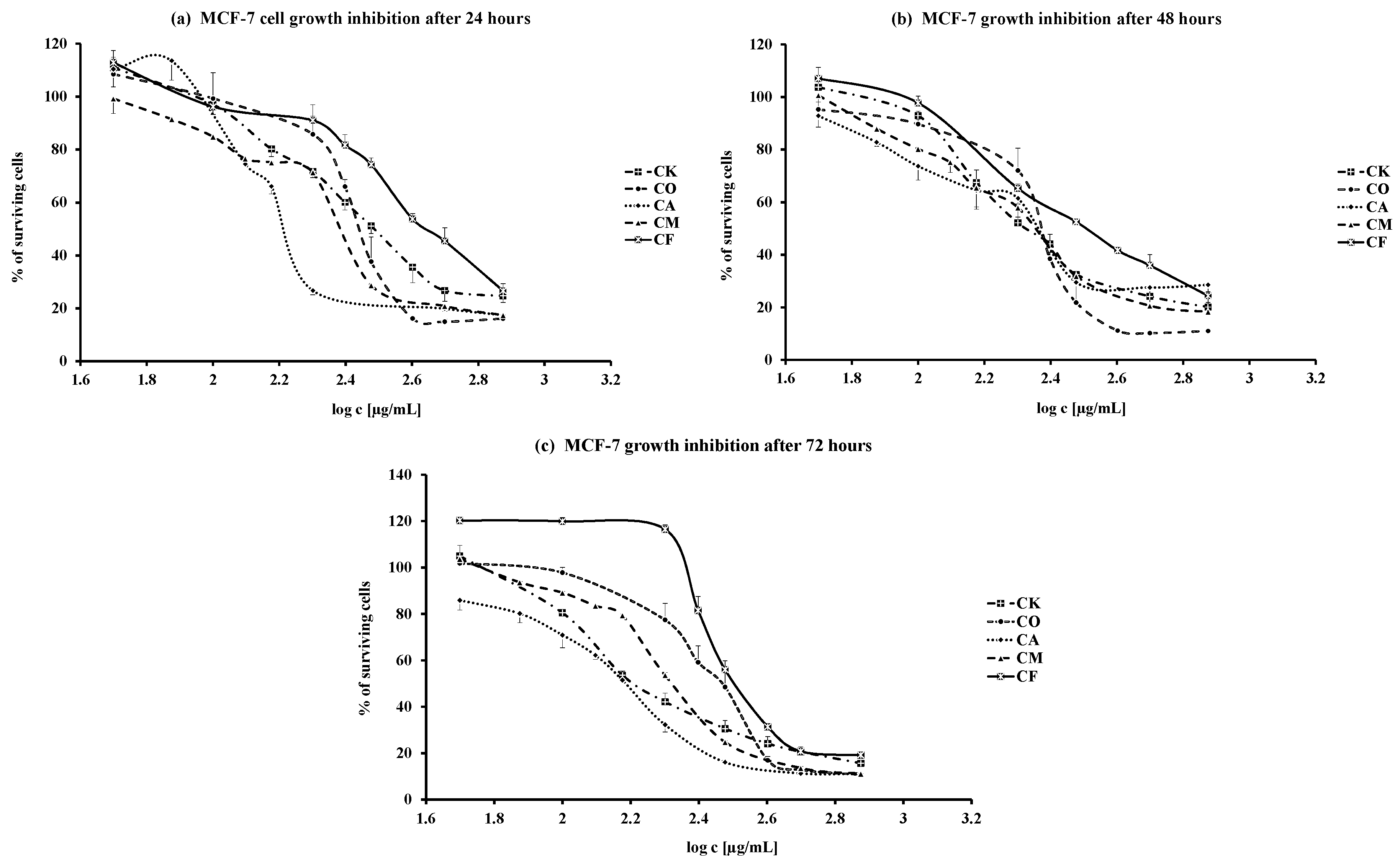
2. Results and Discussion
| Sample | 24 h | 48 h | 72 h |
|---|---|---|---|
| CA | 17.5 ± 0.3 a,b,d | 18.9 ± 0.1 | 11.2 ± 0.2 |
| CO | 16.1 ± 0.4 c,e | 10.4 ± 0.3 1,2 | 10.3 ± 0.5 |
| CK | 24.7 ± 2.4 a,c,* | 20.1 ± 1.8 1 | 15.6 ± 0.8 * |
| CM | 17.5 ± 0.1 | 18.3 ± 0.4 | 11.1 ± 0.3 |
| CF | 26.6 ± 2.7 b,d,e | 24.4 ± 1.6 2 | 19.2 ± 0.3 |
| Sample | Flavonoids | THD | Total Polyphenols | Tannins |
|---|---|---|---|---|
| CA | 0.28 ± 0.04 d | 1.1 ± 0.04 | 8.1 ± 0.13 c | 4.8 ± 0.12 b,d |
| CO | 0.37 ± 0.03 b,* | 1.4 ± 0.10 | 10.1 ± 0.82 b | 7.4 ± 0.76 b,c |
| CK | 0.36 ± 0.006 a | 1.3 ± 0.02 | 9.6 ± 0.80 a | 7.2 ± 0.67 a,d |
| CM | 0.21 ± 0.01 c,* | 1.2 ± 0.01 | 8.3 ± 0.11 d | 5.8 ± 0.10 e |
| CF | 0.72 ± 0.04 a,b,c,d | 1.5 ± 0.10 | 6.1 ± 0.20 a,b,c,d | 3.5 ± 0.14 a,c,e |
| Secondary Metabolite Content | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|
| R | p< | R | p< | R | p< | |
| Flavonoids | 0.9921 | 0.01 | 0.9999 | 0.001 | 0.9928 | 0.01 |
| THD | 0.9704 | 0.05 | 0.9894 | 0.02 | 0.9266 | 0.05 |
| Tannins | −0.9537 | 0.05 | −0.9874 | 0.02 | −0.9311 | NS |
| Polyphenols | −0.9072 | 0.05 | −0.9916 | 0.01 | −0.9998 | 0.001 |
3. Experimental Section
3.1. Plant Material
3.2. Extract Preparation
3.3. Secondary Metabolites Quantification
3.3.1. Total Polyphenols and Tannins Spectrophotometric Assay
3.3.2. Flavonoids Spectrophotometric Assay
3.3.3. Hydroxycinnamic Derivatives Spectrophotometric Assay
3.4. Cell Culture and Treatment
3.5. Cell Proliferation Test
3.6. Calculations and Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiang, J.; Boufford, D.E. Cornaceae. Flora China 2005, 14, 206–221. [Google Scholar]
- Popovic, B.M.; Stajner, D.; Slavko, K.; Sandra, B. Antioxidant capacity of cornelian cherry (Cornus mas L.)—Comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Polat, R.; Cakilcioglu, U.; Satil, F. Traditional uses of medicinal plants in Solhan (Bingöl-Turkey). J. Ethnopharmacol. 2013, 148, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bertová, L. Cornales Drieňotvaré. In Flóra Slovenska, 1st ed.; Bertová, L., Ed.; VEDA: Bratislava, Slovakia, 1984; Volume 4/1, pp. 389–415. [Google Scholar]
- Graziose, R.; Rojas-Silva, P.; Rathinasabapathy, T.; Dekock, C.; Grace, M.H.; Poulev, A.; Ann Lila, M.; Smith, P.; Raskin, I. Antiparasitic compounds from Cornus florida L. with activities against Plasmodium falciparum and Leishmania tarentolae. J. Ethnopharmacol. 2012, 142, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, L.; Jean-Gilles, D.; Seeram, N.P. Identification of bioactive compounds from New England plants. In Proceedings of the 238th ACS National Meeting, Washington, DC, USA, 16–20 August 2009.
- Kwon, S.H.; Kwon, S.J.; Kim, J.Y.; Kang, K.S.; Shim, K.H.; Lee, M.K. Antitumor activity of Corni fructus ethanol extract in sarcoma-180 cancer cells. J. Korean Soc. Food Sci. Nutr. 2010, 39, 960–965. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor‑positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Xu, D.G.; Yu, X.Y.; Zhao, F.M.; Xu, D.Q.; Zhang, X.; Cai, B.C.; Wang, M.Y. Discrepancy between the effects of morroniside on apoptosis in human embryonic lung fibroblast cells and lung cancer A549 cells. Oncol. Lett. 2014, 7, 927–932. [Google Scholar] [PubMed]
- Vareed, S.K.; Reddy, M.K.; Schutzki, R.E.; Nair, M.G. Anthocyanins in Cornus alternifolia, and Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006, 78, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Yoo, K.H.; Chung, I.S.; Kim, J.Y.; Chung, D.K.; Kim, D.K.; Kim, S.H.; Baek, N.I. A new lignan glycoside from the fruits of Cornus kousa Burg. Arch. Pharm. Res. 2008, 31, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Bhakuni, R.S.; Shukla, Y.N.; Tripathi, A.K.; Prajapati, V.; Kumar, S. Insect growth inhibitor activity of arjunolic acid isolated from Cornus capitata. Phytother. Res. 2002, 16 (Suppl. 1), S68–S70. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, S.J.; Lee, S.H.; Ro, J.; Lee, K.; Kinghorn, A.D. Phenolic compounds from the leaves of Cornus controversa. Phytochemistry 2000, 53, 405–407. [Google Scholar] [CrossRef]
- Gűlçin, İ.; Beydemir, Ş.; Şat, I.G.; Kűfrevioğlu, Ő.İ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment. 2005, 34, 193–202. [Google Scholar]
- Gao, D.; Li, Q.; Gao, Z.; Wang, L. Antidiabetic effects of Corni fructus extract in streptozotocin-induced diabetic rats. Yonsei Med. J. 2012, 53, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Krol, W. Natural polyphenols target the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signalling pathway for cancer chemoprevention. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults, 1st ed.; Watson, R.R., Ed.; Academic Press: Waltham, MA, USA, 2015; pp. 119–134. [Google Scholar]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar]
- Kwon, S.H.; Yang, H.S.; Kim, J.Y.; Park, K.W.; Shon, M.Y.; Kang, K.S.; Shim, K.H.; Seo, K.I. Biological activities of ethanol extract from Corni fructus. J. Korean Soc. Food Sci. Nutr. 2009, 38, 287–291. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission. Particle-size distribution estimation by analytical sieving. In European Pharmacopoeia, 8th ed.; EDQM: Strasbourg, France, 2014; pp. 351–353. [Google Scholar]
- Czech and Slovak Pharmacopoea Commissions. Decocta. Infusa. In Pharmacopoea Bohemoslovaca, 4th ed.; Avicenum: Prague, Czech Republic, 1987; pp. 43–44. [Google Scholar]
- Forman, V.; Haladová, M.; Grančai, D. Quantification of some secondary metabolites in selected Cornaceae species. Acta Fac. Pharm. Univ. Comen. 2015, 62, S8–S11. [Google Scholar]
- European Pharmacopoeia Commission. Hamamelidis folium. In European Pharmacopoeia, 8th ed.; EDQM: Strasbourg, France, 2014; pp. 1270–1271. [Google Scholar]
- European Pharmacopoeia Commission. Betulae folium. In European Pharmacopoeia, 8th ed.; EDQM: Strasbourg, France, 2014; pp. 1173–1175. [Google Scholar]
- European Pharmacopoeia Commission. Rosmarini folium. In European Pharmacopoeia, 8th ed.; EDQM: Strasbourg, France, 2014; pp. 1369–1370. [Google Scholar]
- Sample Availability: Samples of the compounds (extracts) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forman, V.; Haladová, M.; Grančai, D.; Ficková, M. Antiproliferative Activities of Water Infusions from Leaves of Five Cornus L. Species. Molecules 2015, 20, 22546-22552. https://doi.org/10.3390/molecules201219786
Forman V, Haladová M, Grančai D, Ficková M. Antiproliferative Activities of Water Infusions from Leaves of Five Cornus L. Species. Molecules. 2015; 20(12):22546-22552. https://doi.org/10.3390/molecules201219786
Chicago/Turabian StyleForman, Vladimír, Mária Haladová, Daniel Grančai, and Mária Ficková. 2015. "Antiproliferative Activities of Water Infusions from Leaves of Five Cornus L. Species" Molecules 20, no. 12: 22546-22552. https://doi.org/10.3390/molecules201219786






