Synthesis of a Stable Primary-Alkyl-Substituted Selenenyl Iodide and Its Hydrolytic Conversion to the Corresponding Selenenic Acid
Abstract
:1. Introduction

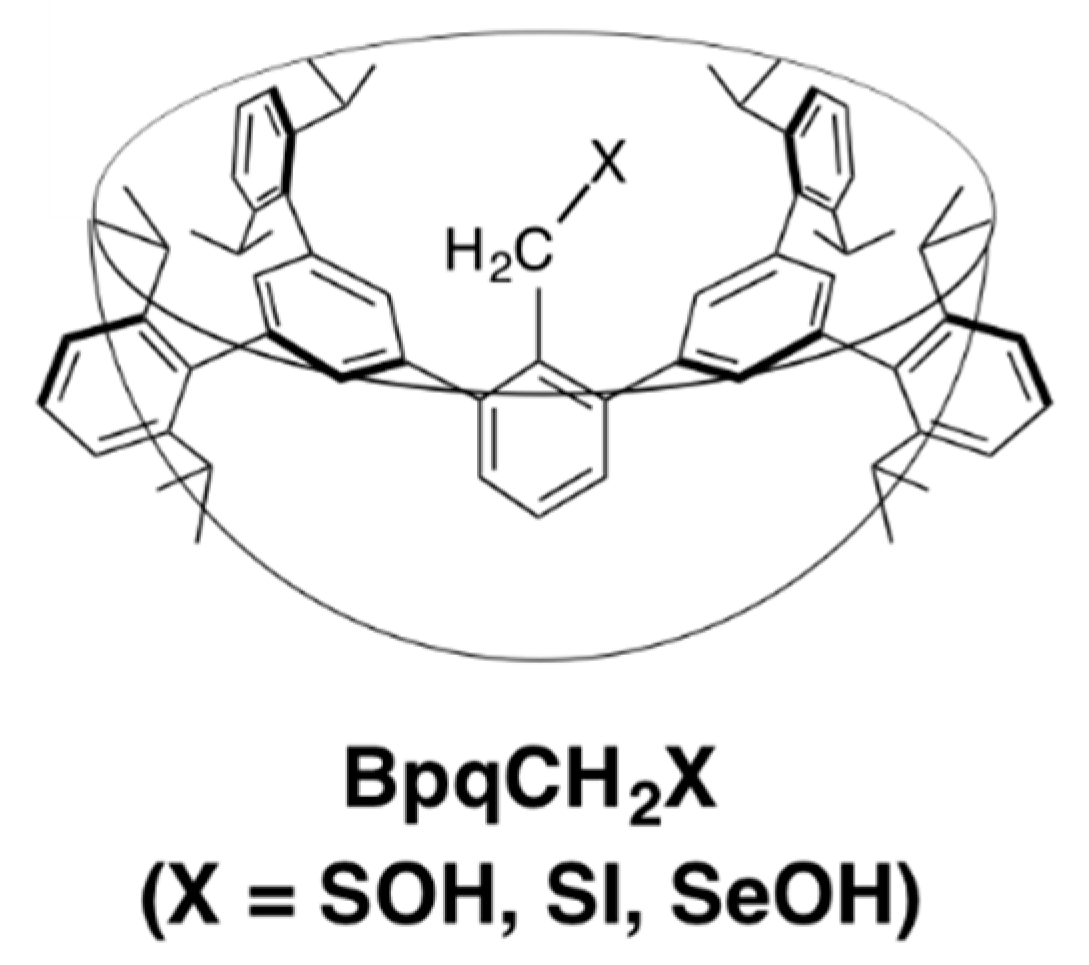
2. Results and Discussion

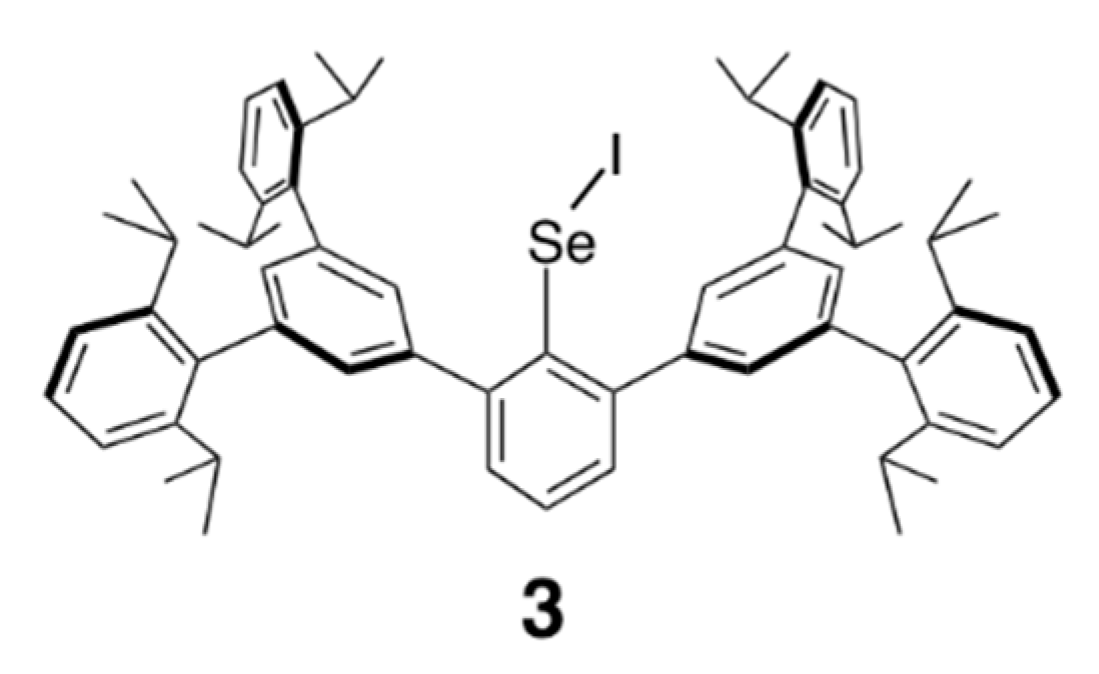

3. Experimental Section
3.1. Synthesis of BpqCH2SeI (2)
3.2. Heating of 2 in [D8]toluene
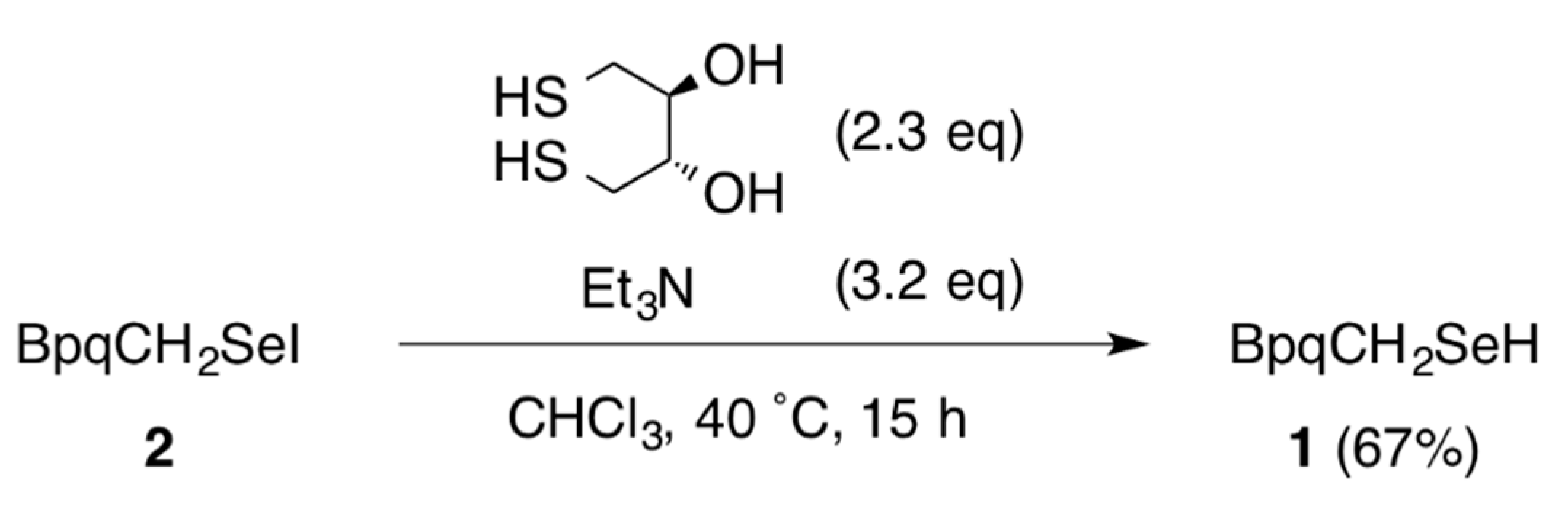
3.3. Reduction of 2 with Dithiothreitol
3.4. Hydrolysis of 2
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Iodothyronine deiodinases. Methods Enzymol. 2002, 347, 125–167. [Google Scholar] [PubMed]
- Du Mont, W.-W.; Martens-von Salzen, A.; Ruthe, F.; Seppala, E.; Mugesh, G.; Devillanova, F.A.; Lippolis, V.; Kuhn, N. Tuning selenium-iodine contacts: From secondary soft-soft interactions to covalent bonds. J. Organomet. Chem. 2001, 623, 14–28. [Google Scholar] [CrossRef]
- Du Mont, W.-W.; Martens, A.; Pohl, S.; Saak, W. Reversible dismutation and coordination of bis(2,4,6-triisopropylphenyl) diselenide with iodine—A model study that relates to iodine interaction between selenium chains. Inorg. Chem. 1990, 29, 4847–4848. [Google Scholar] [CrossRef]
- Du Mont, W.-W.; Kubiniok, S.; Peters, K.; von Schnering, H.G. Synthesis and structure of a stable iodoselane. Angew. Chem. Int. Ed. Engl. 1987, 26, 780–781. [Google Scholar] [CrossRef]
- Panda, A.; Mugesh, G.; Singh, H.B.; Butcher, R.J. Synthesis, structure, and reactivity of organochalcogen (Se, Te) compounds derived from 1-(N,N-dimethylamino)naphthalene and N,N-dimethylbenzylamine. Organometallics 1999, 18, 1986–1993. [Google Scholar] [CrossRef]
- Goto, K.; Sonoda, D.; Shimada, K.; Sase, S.; Kawashima, T. Modeling of the 5′-deiodination of thyroxine by iodothyronine deiodinase: Chemical corroboration of a selenenyl iodide intermediate. Angew. Chem. Int. Ed. 2010, 49, 545–547. [Google Scholar] [CrossRef] [PubMed]
- A selected reference for model studies on iodothyronine deiodinases: Manna, D.; Mugesh, G. A chemical model for the inner-ring deiodination of thyroxine by iodothyronine deiodinase. Angew. Chem. Int. Ed. 2010, 49, 9246–9249. [Google Scholar] See also references 9 and 10.
- Manna, D.; Mugesh, G. Regioselective deiodination of thyroxine by iodothyronine deiodinase mimics: An unusual mechanistic pathway involving cooperative chalcogen and halogen bonding. J. Am. Chem. Soc. 2012, 134, 4269–4279. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Mugesh, G. Remarkable effect of chalcogen substitution on an enzyme mimetic for deiodination of thyroid hormones. Angew. Chem. Int. Ed. 2015, 54, 7674–7678. [Google Scholar] [CrossRef] [PubMed]
- Sase, S.; Aoki, Y.; Abe, N.; Goto, K. Stable sulfenyl iodide bearing a primary alkyl steric protection group with a cavity-shaped framework. Chem. Lett. 2009, 38, 1188–1189. [Google Scholar] [CrossRef]
- Ishihara, M.; Abe, N.; Sase, S.; Goto, K. Synthesis, structure, and reactivities of a stable primary-alkyl-substituted sulfenic acid. Chem. Lett. 2015, 44, 615–617. [Google Scholar] [CrossRef]
- Sase, S.; Kakimoto, R.; Goto, K. Synthesis of a stable selenoaldehyde by self-catalyzed thermal dehydration of a primary-alkyl-substituted selenenic acid. Angew. Chem. Int. Ed. 2015, 54, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Schlicker, C.; Braun, D.; Köhrle, J.; Steegborn, C. Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 10526–10531. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Karplus, P.A.; Claiborne, A. Protein sulfenic acids in redox signaling. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 2010, 5, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Gunzler, W.A.; Schock, H.H. Glutathione peroxidase- selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Flohé, L. Free Radicals in Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1982; Volume V, Chapter 7; p. 223. [Google Scholar]
- Goto, K.; Shimada, K.; Furukawa, S.; Miyasaka, S.; Takahashi, Y.; Kawashima, T. Formation of a stable sulfenic acid by hydrolysis of a thionitrate and a sulfenyl bromide. Chem. Lett. 2006, 35, 862–863. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Zhang, J.; Stamler, J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 8306–8311. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta 2014, 1840, 847–875. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sase, S.; Kakimoto, R.; Kimura, R.; Goto, K. Synthesis of a Stable Primary-Alkyl-Substituted Selenenyl Iodide and Its Hydrolytic Conversion to the Corresponding Selenenic Acid. Molecules 2015, 20, 21415-21420. https://doi.org/10.3390/molecules201219773
Sase S, Kakimoto R, Kimura R, Goto K. Synthesis of a Stable Primary-Alkyl-Substituted Selenenyl Iodide and Its Hydrolytic Conversion to the Corresponding Selenenic Acid. Molecules. 2015; 20(12):21415-21420. https://doi.org/10.3390/molecules201219773
Chicago/Turabian StyleSase, Shohei, Ryo Kakimoto, Ryutaro Kimura, and Kei Goto. 2015. "Synthesis of a Stable Primary-Alkyl-Substituted Selenenyl Iodide and Its Hydrolytic Conversion to the Corresponding Selenenic Acid" Molecules 20, no. 12: 21415-21420. https://doi.org/10.3390/molecules201219773





