3. Experimental Section
3.1. General
Melting points were determined with an Electro Thermal Mel-Temp II apparatus and are uncorrected. All reactions were monitored by thin layer chromatography (TLC) on pre-coated silica gel plates (0.25 mm, 20 × 20 cm, 60F254, E. Merck KGaA, Konstanz, Germany) with an appropriate solvent system ((A) 1:9 CH3OH–CHCl3; (B) 1:1 tolune–ethylacetate). IR spectra were obtained in the solid state in the form of KBr discs using a Perkin-Elmer Model 1430 spectrometer (Perkin-Elmer, Akron, OH, USA) and carried out in Taif University, Taif, KSA. 1H-NMR spectra were run at 400 MHz and 13C spectra were run at 125 MHz in dimethylsulfoxide (DMSO-d6) and TMS as an internal standard. Mass spectra were recorded on GC Ms-QP 5050A mass spectrometer (Shimadzu Corporation, Tokyo, Japan) at 70 eV and microanalytical data were performed on Elementar Vario EI III CHN analyzer (Elementar, Langenselbold, Germany) at the microanalytical unit, in Regional center for Mycology and Biotechnology, Al-Azhar University, Nasr City, Egypt.
Table 1.
Detection of the antimicrobial activity of some organic compounds by measuring the formed inhibition zone (inhibition zone measured in mm and MIC in µg/mL).
Table 1.
Detection of the antimicrobial activity of some organic compounds by measuring the formed inhibition zone (inhibition zone measured in mm and MIC in µg/mL).
| Compd. No. | Staphylococcus aureus | Escherichia coli | Candida albicans |
|---|
| 6a | 0 | 0 | 0 |
| 6b | 0 | 0 | 0 |
| 6c | 0 | 0 | 0 |
| 6d | 0 | 0 | 0 |
| 6e | 0 | 0 | 0 |
| 6f | 0 | 0 | 0 |
| 6g | 0 | 0 | 0 |
| 7a | 0 | 0 | 0 |
| 7b | 0 | 0 | 8.3 ± 0.881 |
| 7c | 0 | 0 | 0 |
| 7d | 15.3 ± 0.88 * (0.4) | 0 | 12.6 ± 1.1547 * (0.3) |
| 7e | 0 | 0 | 0 |
| 7f | 0 | 0 | 0 |
| 8a | 0 | 0 | 0 |
| 8b | 0 | 0 | 0 |
| 8c | 5.0 ±0.9 * | 0 | 12.3 ± 0.91 * (0.3) |
| 8d | 0 | 0 | 0 |
| 8e | 0 | 0 | 0 |
| 9a | 14.6 ± 1.2 * (0.5) | 0 | 16.0 ± 1.1547 * (0.5) |
| 9b | 0 | 0 | 0 |
| 9c | 0 | 0 | 0 |
| 9d | 0 | 0 | 0 |
| 9e | 0 | 0 | 0 |
| DMSO | 0 | 0 | 0 |
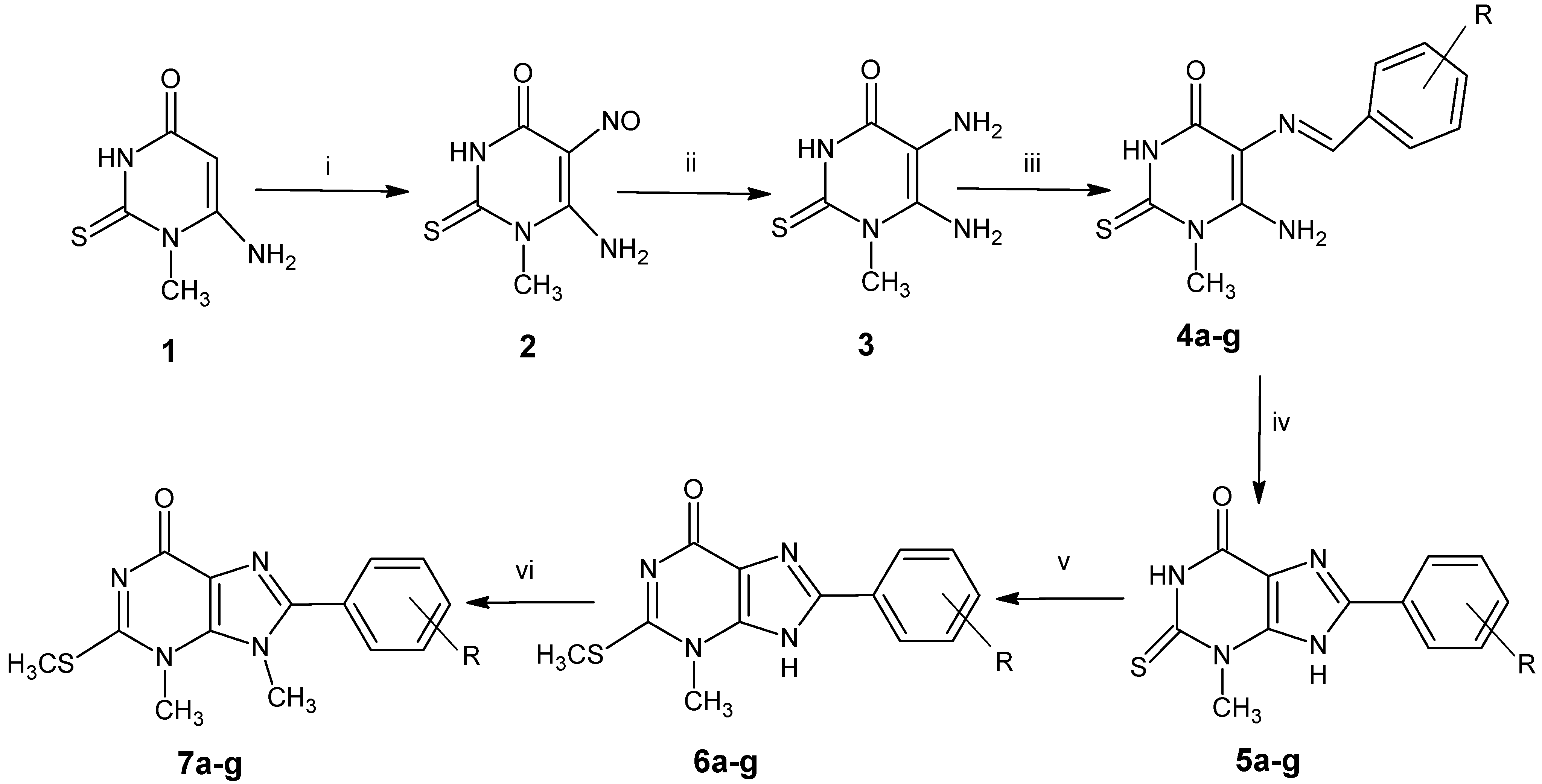
3.2. Synthesis of 8-Aryl-3-methyl-2-methylthio-3,9-dihydro-3,6H-purin-6-one (6a–g)
Dimethyl sulfate (7.0 mL) was added to a mixture of NaOH (12.5 mL, 0.5 N) and 2-thioxanthines 5a–g (5 mmol). Ethanol was added gradually till completely dissolving of 2-thioxanthines. The mixture was stirred for 1 h at room temperature; the formed precipitate was collected by filtration, dried in the oven and crystallized from DMF/ethanol.
3-Methyl-2-(methylthio)-8-phenyl-3,9-dihydro-6H-purin-6-one (6a). Yield: 70%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3425 (NH), 3055 (CH aromatic), 2921 (CH aliphatic), 1688 (C=O), 1617 (C=N), 1290 (NH); 1H-NMR (DMSO-d6): 11.11 (s, 1H, NH), 7.83–7.77 (m, 2H, arom.), 7.58–7.55 (m, 3H, arom.), 4.03 (s, 3H, SCH3), 3.83 (s, 3H, NCH3); 13C-NMR (DMSO-d6): δ = 16.4 (SCH3), 29.28 (NCH3), 108.24, 114.40, 122.78, 128.01, 136.68, 142.92 (C-S), 150.76, 154.15, 154.17 (C=O) ppm; MS: m/z (%) = M+, 272 (7), 256 (9), 213 (16), 82 (100); Anal. calcd. for C13H12N4OS (272.32): C, 57.34; H, 4.44; N, 20.57. Found: C, 57.49; H, 4.51; N, 20.72.
8-(4-Chlorophenyl)-3-methyl-2-(methylthio)-3,9-dihydro-6H-purin-6-one (6b). Yield: 78%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3473 (NH), 3013 (CH arom.), 2955 (CH aliph.),1687 (C=O), 1612 (C=N), 1265 (NH); 1H-NMR (DMSO-d6): 10.31 (s, 1H, NH), 7.84–7.61 (d, 2H, J = 8.7, arom.), 7.60–7.58 (d, 2H, J = 8.4, arom.), 4.54 (s, 3H, SCH3), 3.63 (s, 3H, NCH3); 13C-NMR (DMSO-d6): δ = 16.6 (SCH3), 29.26 (NCH3), 107.21, 113.41, 122.78, 128.01, 136.68, 141.90 (C-S), 149.88, 153.18, 153.89 (C=O) ppm; MS: m/z (%) = M+ +2, 308 (19), M+ + 1, 307 (15), M+, 306 (54), 171 (33), 111 (28), 68 (100). Anal. calcd. for C13H11ClN4OS (306.77): C, 50.90; H, 3.91; N, 18.26. Found: C, 51.08; H, 3.94; N, 18.47.
8-(2-Methoxyphenyl)-3-methyl-2-(methylthio)-3,9-dihydro-6H-purin-6-one (6c). Yield: 67%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3450 (NH), 3076 (CH arom.), 2928 and 2838 (CH aliph.), 1694 (C=O), 1629 (C=N), 1286 (NH); 1H-NMR (DMSO-d6): 10.76 (s, 1H, NH), 8.04–8.01 (d, 1H, J = 1.8, arom.), 7.50–7.50 (m, 1H, J = 1.2, arom.), 7.22–7.19 (d, 1H, J = 8.4, arom.), 7.12–7.07 (m, 1H, J = 7.5, arom.), 4.43(s, 3H, SCH3), 3.93 (s, 3H, OCH3), 3.84 (s, 3H, NCH3). MS: m/z (%) = M+, 302 (95), 257 (51), 134 (65), 68 (100). Anal. calcd. for C14H14N4O2S (302.35): C, 55.61; H, 4.67; N, 18.53. Found: C, 55.74; H, 4.74; N, 18.69.
8-(4-Bromophenyl)-3-methyl-2-(methylthio)-3,9-dihydro-6H-purin-6-one (6d). Yield: 78%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3446 (NH), 3080 (CH arom.), 2924 and 2853 (CH aliph.), 1697 (C=O), 1633 (C=N), 1208 (NH); 1H-NMR (DMSO-d6): 10.88 (s, 1H, NH), 7.89–7.87 (d, 2H, J = 8.4, arom.), 7.59–7.56 (d, 2H, J = 8.1, arom.), 4.43 (s, 3H, SCH3), 3.82 (s, 3H, NCH3).MS: m/z (%) = M+ + 2, 353 (6), M+, 351 (9), 336 (12), 68 (100); Anal. calcd. for C13H11BrN4OS (351.22): C, 44.46; H, 3.16; N, 15.95. Found: C, 44.62; H, 3.12; N, 16.14.
8-(4-Fluorophenyl)-3-methyl-2-(methylthio)-3,9-dihydro-6H-purin-6-one (6e). Yield: 64%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3526 (NH), 3082 (CH arom.), 2933 and 2827 (CH aliph.), 1684 (C=O), 1625 (C=N), 1216 (NH); 1H-NMR (DMSO-d6): 11.02 (s, 1H, NH), 7.69–7.54 (m, 2H, arom.), 7.30–7.25 (m, 2H, arom.), 4.14 (s, 3H, SCH3), 3.72 (s, 3H, NCH3); 13C-NMR (DMSO-d6): δ = 16.3 (SCH3), 29.23 (NCH3), 107.11, 113.42, 122.64, 127.89, 136.57, 141.48 (C-S), 149.83, 153.01, 154.23 (C=O) ppm; MS: m/z (%) = M+ + 1, 291 (16), M+, 290 (27), 122 (32), 68 (100); Anal. calcd. for C13H11FN4OS (290.31): C, 53.78; H, 3.82; N, 19.30. Found: C, 53.87; H, 3.89; N, 19.37.
8-(4-Hydroxyphenyl)-3-methyl-2-(methylthio)-3,9-dihydro-6H-purin-6-one (6f). Yield: 63%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3615 (OH), 3406 (NH), 3071 (CH arom.), 2938 and 2839 (CH aliph.), 1700 (C=O), 1602 (C=N), 1253 (NH); 1H-NMR (DMSO-d6): 13.23 (s, 1H, OH), 10.02 (s, 1H, NH), 7.66–6.64 (d, 2H, J = 4.2, arom.), 6.88–6.86 (d , 2H, J = 6.9, arom.), 4.21 (s, 3H, SCH3), 3.83 (s, 3H, NCH3). MS: m/z (%) = M+, 288 (34), 258 (7), 120 (23), 82 (100), 67 (60); Anal. calcd. for C13H12N4O2S (288.32): C, 54.15; H, 4.20; N, 19.43. Found: C, 54.29; H, 4.27; N, 19.66.
8-(2-Hydroxyphenyl)-3-methyl-2-(methylthio)-3,9-dihydro-6H-purin-6-one (6g). Yield: 58%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3632 (OH), 3400 (NH), 3062 (CH arom.), 2925 and 2829 (CH aliph.), 1688 (C=O), 1624 (C=N), 1251 (NH); 1H-NMR (DMSO-d6): 13.12 (s, 1H, OH), 10.98 (s, 1H, NH), 7.820–7.815 (d, 1H, J = 1.5, arom.), 7.15–7.01 (m, 2H, arom.), 6.87–6.80 (m, 1H, arom.), 4.29 (s, 3H, SCH3), 3.79 (s, 3H, NCH3); 16.21 (SCH3), 28.98 (NCH3), 107.10, 113.41, 122.55, 127.72, 128.17, 136.61, 141.32 (C-S), 149.83, 152.24, 154.13 (C=O) ppm; MS: m/z (%) = M+, 288 (100), 274 (33), 215 (74), 120 (69); Anal. calcd. for C13H12N4O2S (288.32): C, 54.15; H, 4.20; N, 19.43. Found: C, 54.21; H, 4.24; N, 19.62.
3.3. 8-Aryl-3,9-dimethyl-2-methylthio-3,9-dihydro-6H-purin-6-one (7a–g)
Dimethyl sulfate (7.0 mL) was added to a mixture of NaOH (12.5 mL, 0.5 N) and 2-thioxanthines 6a–g (5 mmol), and ethanol was added gradually till 2-thioxanthines were completely dissolved. The mixture was heated under reflux for 1 h with stirring. The reaction mixture was evaporated under reduced pressure, and the residue was collected by filtration and crystallized from ethanol to afford Compounds 7a–g.
3,9-Dimethyl-2-(methylsulfanyl)-8-phenyl-3,9-dihydro-6H-purin-6-one (7a). Yield: 78%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3059 (CH arom.), 2980 (CH aliph.),1687 (C=O), 1609 (C=N); 1H-NMR (DMSO-d6): 7.79–7.76 (m, 2H, arom.), 7.54–7.51 (m, 3H, arom.), 4.00 (s, 3H, SCH3), 3.83 (s, 3H, NCH3), 3.72 (s, 3H, NCH3); 16.18 (SCH3), 29.38 (NCH3), 44.18 (NCH3), 108.13, 113.27, 122.42, 127.89, 136.57, 141.48 (C-S), 149.83, 153.01, 154.21 (C=O) ppm; MS: m/z (%) = M+, 286 (67), 82 (100); Anal. calcd. for C14H14N4OS (286.35): C, 58.72; H, 4.93; N, 19.57. Found: C, 58.89; H, 4.98; N, 19.64.
3,9-Dimethyl-2-(methylsulfanyl)-8-(4-chlorophenyl)-3,9-dihydro-6H-purin-6-one (7b). Yield: 82%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3068 (CH arom.), 2970 (CH aliph.), 1676 (C=O), 1623 (C=N); 1H-NMR (DMSO-d6): 7.62–7.60 (d, 2H, arom.), 7.55–7.52 (d, 2H, arom.), 4.03 (s, 3H, SCH3), 3.80 (s, 3H, NCH3), 3.66 (s, 3H, NCH3); MS: m/z (%) = M+ + 2, 322 (13), M+, 320 (30), 307 (16), 86 (11), 68 (100); Anal. calcd. for C14H13ClN4OS (320.79): C, 52.42; H, 4.08; N, 17.46. Found: C, 52.49; H, 4.13; N, 17.59.
3,9-Dimethyl-2-(methylsulfanyl)-8-(2-methoxyphenyl)-3,9-dihydro-6H-purin-6-one (7c). Yield: 71%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3092 (CH arom.), 2838 (CH aliph.), 1699 (C=O), 1633 (C=N); 1H-NMR (DMSO-d6): 7.46–7.45 (m, 1H, arom.), 7.26–7.23 (m, 2H, arom.), 7.13–7.09 (m, 1H, arom.), 4.13 (s, 3H, SCH3), 3.93 (s, 3H, OCH3), 3.79 (s, 3H, NCH3), 3.70 (s, 3H, NCH3); 16.31 (SCH3), 29.27 (NCH3), 44.16 (NCH3), 52.01 (OCH3), 108.13, 111.29, 113.27, 122.42, 127.89, 128.13, 136.57, 141.48 (C-S), 149.83, 153.01, 154.21 (C=O) ppm; MS: m/z (%) = M+, 316 (21), 302 (100), 287 (13), 243 (12), 119 (8), 70 (11), 64 (12); Anal. calcd. for C15H16N4O2S (316.37): C, 56.94; H, 5.10; N, 17.71. Found: C, 57.21; H, 5.17; N, 17.83.
3,9-Dimethyl-2-(methylsulfanyl)-8-(4-bromophenyl)-3,9-dihydro-6H-purin-6-one (7d). Yield: 79%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3079 (CH arom.), 2953(CH aliph.), 2841 (CH aliph.), 1689 (C=O), 1617 (C=N); 1H-NMR (DMSO-d6): 8.06–8.02 (m, 2H, arom.), 7.69–7.67 (m, 2H, arom.), 4.41 (s, 3H, SCH3), 3.74 (s, 3H, NCH3), 3.39 (s, 3H, NCH3); MS: m/z (%) = M+ + 2, 367 (8), M+, 365 (12), 348 (22), 68 (100); Anal. calcd. for C14H13BrN4OS (365.24): C, 46.04; H, 3.59; N, 15.34. Found: C, 46.19; H, 3.66; N, 15.46.
3,9-Dimethyl-2-(methylsulfanyl)-8-(4-fluorophenyl)-3,9-dihydro-6H-purin-6-one (7e). Yield: 69%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3074 (CH arom.), 2921 (CH aliph.), 2827 (CH aliph.), 1693 (C=O), 1618 (C=N), 1257 (NH); 1H-NMR (DMSO-d6): 7.87–7.84 (m, 2H, arom.), 7.40–7.36 (m, 2H, arom.), 4.03 (s, 3H, SCH3), 3.78 (s, 3H, NCH3), 3.67 (s, 3H, NCH3); MS: m/z (%) = M+ + 1, 305 (6), M+, 304 (18), 171 (33), 122 (54), 68 (100); Anal. calcd. for C14H13FN4OS (304.34): C, 55.25; H, 4.31; N, 18.41. Found: C, 55.34; H, 4.34; N, 18.56.
3,9-Dimethyl-8-(4-methoxyphenyl)-2-(methylsulfanyl)-3,9-dihydro-6H-purin-6-one (7f). Yield: 72%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3654 (OH), 3076 (CH arom.), 2928 (CH aliph.), 2839 (CH aliph.), 1695 (C=O), 1619 (C=N); 1H-NMR (DMSO-d6): 7.97–7.94 (d, 2H, arom.), 6.93–6.91 (d , 2H, arom.), 4.30 (s, 3H, SCH3), 4.05 (s, 3H, OCH3), 3.77 (s, 3H, NCH3), 3.64 (s, 3H, NCH3); MS: m/z (%) = M+, 316 (18), 302 (56), 268 (100), 214 (24); Anal. calcd. for C15H16N4O2S (316.37): C, 56.94; H, 5.10; N, 17.71. Found: C, 57.21; H, 5.07; N, 17.62.
3,9-Dimethyl-8-(2-methoxyphenyl)-2-(methylsulfanyl)-3,9-dihydro-6H-purin-6-one (7g). Yield: 67%; m.p. >300 °C; IR (KBr) νmax (cm−1): 3052 (CH arom.), 2830 (CH aliph.), 1674 (C=O), 1627 (C=N); 1H-NMR (DMSO-d6): 7.43–7.41 (m, 1H, arom.), 7.24–7.21 (m, 2H, arom.), 7.18–7.12 (m, 1H, arom.), 4.22 (s, 3H, SCH3), 3.81 (s, 3H, OCH3), 3.68 (s, 3H, NCH3), 3.69 (s, 3H, NCH3); MS: m/z (%) = M+, 316 (21), 302 (100), 287 (13); Anal. calcd. for C15H16N4O2S (316.37): C, 56.94; H, 5.10; N, 17.71. Found: C, 57.18; H, 5.13; N, 17.85.
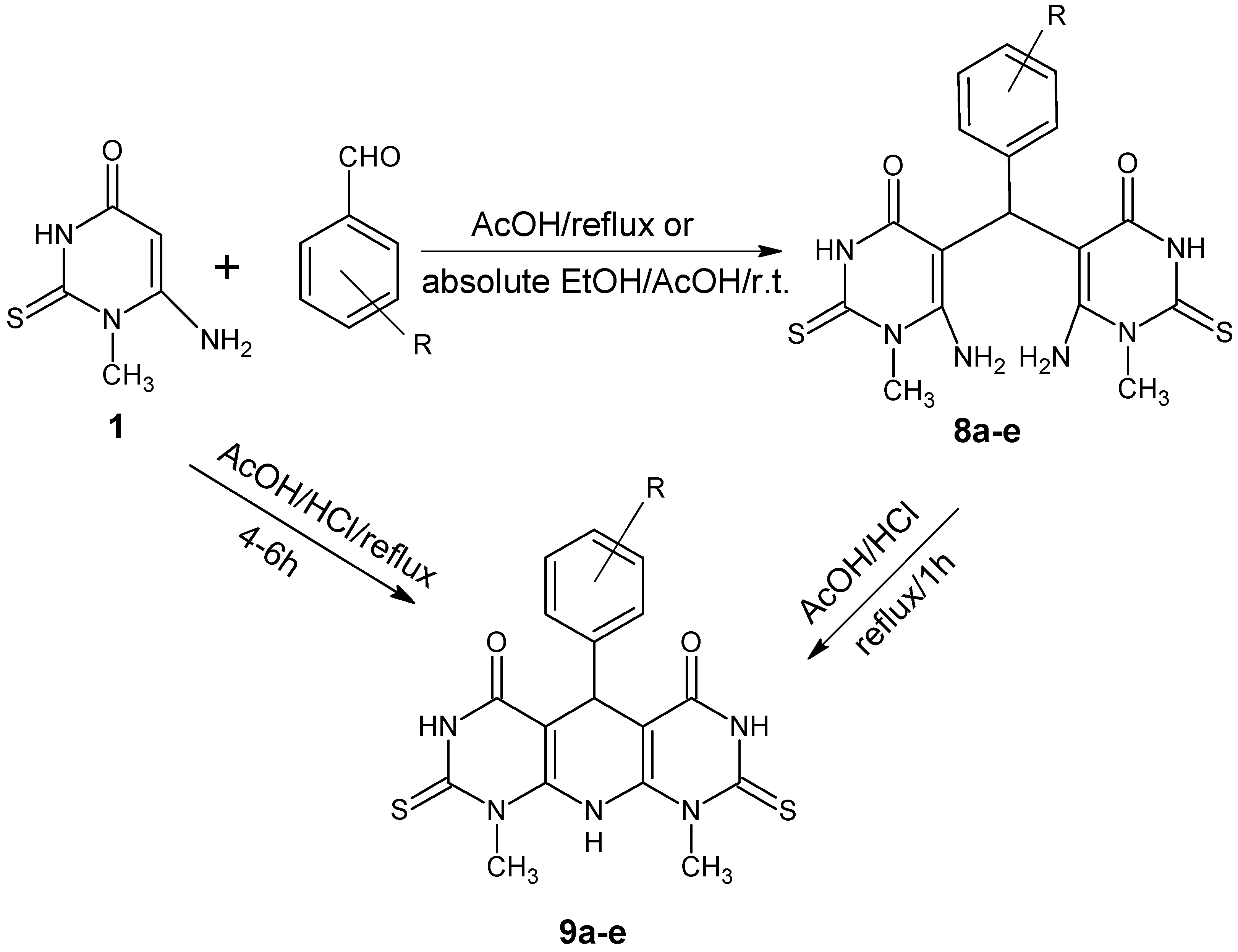
3.4. Synthesis of 5,5′-Arylmethylenebis-(6-Amino-1-methyl-2-thiouracil) (8a–e)
A mixture of 6-amino-1-methyl-2-thiouracil (1) (0.5 g, 2.4 mmol) and appropriate aromatic aldehydes (2.4 mmol) in absolute ethanol (20 mL) in the presence of conc. Hydrochloric acid (1mL) was stirred at room temperature for 1.5 h and/or reflux with glacial acetic acid (5 mL) for 1 h. The formed precipitate was filtered, washed with ethanol and crystallized from DMF/ethanol (3:1) into colorless crystals.
5,5'-[(4-Chlorophenyl)methylene]bis(6-amino-1-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one) (8a). Yield: 95%; m.p. = 240–242 °C; IR (KBr) νmax (cm−1): 3442, 3340 (NH2 and NH), 3064 (CH arom.), 2871 (CH aliph.), 1732, 1662 (C=O), 1598 (C=C), 1086, 1119 (C=S), 747 (C-Cl); 1H-NMR (DMSO-d6): 12.28 (s, 2H, 2NH), 7.59 (bs, 4H, 2NH2), 7.26–7.24 (d, J = 5.9, 2H, arom.), 7.15–7.13 (d, J = 9.2, 2H, arom.), 5.48 (s, 1H, CH-5), 3.77 (s, 6H, 2 CH3); 13C-NMR (DMSO-d6): δ = 28.4 (CH), 38.7 (CH3), 91.2, 111.9, 117.2, 126.1, 129.3, 131.3, 162.2 (C=O), 171.1 (C=S) ppm; MS: m/z (%) = M+ + 2, 439 (8), M+ + 1, 438 (15), M+, 437 (22), 156 (82), 124 (77), 88 (100); Anal. calcd. for C17H17ClN6O2S2 (436.93): C, 46.73; H, 3.92; N, 19.23. Found: C, 46.98; H, 3.98; N, 19.42
5,5'-[(3-Nitrophenyl)methylene]bis(6-amino-1-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one) (8b). Yield: 88%; m.p. = 258–260 °C; IR (KBr) νmax (cm−1): 3406, 3335 (NH2 and NH), 3057 (CH arom.), 2833 (CH aliph.), 1731, 1690 (C=O), 1528 (C=N), 1128, 1081 (C=S); 1H-NMR (DMSO-d6): 12.34 (s, 2H, 2NH), 8.03–8.01 (d, 1H, arom.), 7.9 (d, 1H, arom.), 7.63–7.61 (bs, 4H, 2 NH2), 7.54–7.50 (m, 2H, arom.), 5.60 (s, 1H, CH-5), 3.79 (s, 6H, 2 CH3); MS: m/z (%) = M+, 447 (37), 358 (48), 325 (18), 286 (100); Anal. calcd. for C17H17N7O4S2 (447.49): C, 45.63; H, 3.83; N, 21.91. Found: C, 45.85; H, 3.88; N, 22.14.
5,5'-[(4-Nitrophenyl)methylene]bis(6-amino-1-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one) (8c). Yield: 90%; m.p. = 261–263 °C; IR (KBr) νmax (cm−1): 3450, 3328 (NH2 and NH), 3051 (CH arom.), 2992 (CH aliph.), 1714, 1663 (C=O), 1563 (C=N), 1116, 1086 (C=S); 1H-NMR (DMSO-d6): 12.34 (s, 2H, 2NH), 8.09–8.06 (d, J = 5.8, 2H, arom), 7.61 (bs, 4H, 2 NH2), 7.43–7.41 (d, J = 8.8, 2H, arom.), 5.59 (s, 1H, CH-5), 3.78 (s, 6H, 2 CH3); 13C-NMR (DMSO-d6): δ = 28.6 (CH), 39.2 (CH3), 91.7, 112.1, 117.5, 126.7, 129.6, 131.4, 162.4 (C=O), 171.3 (C=S) ppm; MS: m/z (%) = M+ , 447 (3), 377 (26), 286 (16), 198 (14), 149 (57), 125 (100); Anal. calcd. for C17H17N7O4S2 (447.49): C, 45.63; H, 3.83; N, 21.91. Found: C, 45.91; H, 3.81; N, 22.12.
5,5'-[(4-Bromophenyl)methylene]bis(6-amino-1-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one) (8d). Yield: 93%; m.p. = 224–226 °C; IR (KBr) νmax (cm−1): 3442, 3308 (NH2 and NH), 3060 (CH arom.), 2946 (CH aliph.), 1727 (C=O), 1569 (C=N), 1142, 1077 (C=S); 1H-NMR (DMSO-d6): 12.28 (s, 2H, 2NH), 7.58 (bs, 4H, 2NH2), 7.39–7.37 (d, J = 5.8, 2H, arom.), 7.09–7.07 (d, J = 9.1, 2H, arom.), 5.46 (s, 1H, CH-5), 3.77 (s, 6H, 2 CH3); 13C-NMR (DMSO-d6): δ = 28.3 (CH), 38.7 (CH3), 91.4, 111.8, 117.3, 126.0, 129.2, 131.2, 162.4 (C=O), 171.3 (C=S) ppm; MS: m/z (%) = M+ + 2, 483 (5), M+, 481 (14), 449 (84), 249 (60), 208 (100), 193 (97), 167 (60); Anal. calcd. for C17H17BrN6O2S2 (481.38): C, 42.42; H, 3.56; N, 17.46 Found: C, 42.53; H, 3.58; N, 17.63.
5,5'-[(2-Hydroxyphenyl)methylene]bis(6-amino-1-methyl-2-thioxo-2,3-dihydro-pyrimidin-4(1H)-one) (8e). Yield: 89%; m.p. = 248–250 °C; IR (KBr) νmax (cm−1): 3628 (OH), 3443, 3315 (NH2 and NH), 3028 (CH arom.), 2946 (CH aliph.), 1632 and 1602 (C=O), 1482 (C=N), 1145, 1087 (C=S); 1H-NMR (DMSO-d6): 13.82 (s, 1H, OH), 12.54 (s, 2H, 2NH), 7.29 (bs, 4H, 2NH2), 7.24–7.21 (d, J = 6.0, 1H, arom.), 7.18–7.16 (d, J = 6.0, 1H, arom.), 7.13–7.11 (m, 2H, arom.), 5.62 (s, 1H, CH-5), 3.80 (s, 6H, 2 CH3); 13C-NMR (DMSO-d6): δ = 28.3 (CH), 39.1 (CH3), 91.2, 112.3, 117.6, 126.3, 128.3, 129.7, 131.3, 132.0, 162.2 (C=O), 171.1 (C=S) ppm; MS: m/z (%) = M+, 418 (1), 377 (5), 97 (12), 91 (100); Anal. calcd. for C17H18N6O3S2 (418.49): C, 48.79; H, 4.34; N, 20.08. Found: C, 48.88; H, 4.42; N, 20.32.
3.5. Synthesis of 5-Aryl-1,9-dimethyl-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrimido[5',4':5,6]pyrido [2,3-d]pyrimidine-4,6(1H,7H)-diones (9a–e)
Two methods were used to synthesize the target Compounds
9a–
e:
A mixture of acetic acid/HCl (1:1, 2mL) was added to 6-amino-1-methyl-2-thiouracil (1) (0.26 g, 1.2 mmol) and appropriate aromatic aldehydes (1.2 mmol). The mixture was heated under reflux for 4–6 h. After cooling, the formed precipitate was filtered, washed with ethanol and crystallized from DMF, affording 9a–e.
A mixture of 5,5′-arylmethylene bis(6-amino-1-methyl-2-thiouracil) (8a) (0.1 g, 0.22 mmol) and acetic acid/HCl (1:1, 2 mL) was heated under reflux for 1 h. After cooling, the formed precipitate was filtered, washed with ethanol and crystallized from DMF to afford 9a.
5-(4-Chlorophenyl)-1,9-dimethyl-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrimido [5',4':5,6]pyrido[2,3-d]pyrimidine-4,6(1H,7H)-dione (9a). Yield (method A): 84%, (method B): 67%; m.p. = 298–300 °C; reflux 4 h; IR (KBr) νmax (cm−1): 3219 (NH), 3046 (CH arom.), 2818 (CH aliph.), 1704 (C=O), 1561 (C=N), 1103, 1052 (C=S); 1H-NMR (DMSO-d6): 12.58 (s, 1H, NH), 12.50 (s, 1H, NH), 8.32 (s, 1H, NH (10)), 8.15–8.13 (d, J = 5.9, 1H, arom.), 7.94–7.92 (d, J = 8.9, 1H, arom.), 7.69–7.67 (d, J = 6.0, 1H, arom.), 7.57–7.55 (d, J = 8.8, 1H, arom.), 6.08 (s, 1H, CH-5), 3.57 (s, 3H, NCH3), 3.52 (s, 3H, NCH3); 13C-NMR (DMSO-d6): δ = 33.46 (CH), 45.9 (CH3), 125.08, 127.9, 128.2, 128.9, 129.52, 131.20, 161.9 (C=O), 169.8 (C=S) ppm; MS: m/z (%) = M+ + 2, 422 (0.3), M+, 420 (1), 129 (19), 74 (39), 69 (100); Anal. calcd. for C17H14ClN5O2S2 (419.90): C, 48.63; H, 3.63; N, 16.68. Found: C, 49.02; H, 2.86; N, 17.01.
1,9-Dimethyl-5-(3-nitrophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrimido [5',4':5,6]pyrido[2,3-d] pyrimidine-4,6(1H,7H)-dione (9b). Yield (method A): 86%; m.p. >300 °C; reflux 5 h; IR (KBr) νmax (cm−1): 3461 (NH), 3060 (CH arom.), 2856 (CH aliph.), 1729 (C=O), 1582 (C=N), 1166, 1092 (C=S); 1H-NMR (DMSO-d6): 12.56 (s, 1H, NH), 12.05 (s, 1H, NH), 8.15 (s, 1H, NH(10)), 8.06 (s, 1H, arom.), 7.96–7.48 (m, 3H, arom.), 6.22 (s, 1H, CH-5), 3.58 (s, 3H, NCH3), 3.52 (s, 3H, NCH3); 13C-NMR (DMSO-d6): δ = 33.56 (CH), 46.33 (CH3), 125.24, 126.72, 127.45, 128.12, 128.43, 129.21, 129.91, 131.82, 162.4 (C=O), 171.3 (C=S) ppm; MS: m/z (%) = M+, 430 (5), 428 (16), 299 (18), 106 (100); Anal. calcd. for C17H14N6O4S2 (430.46): C, 47.43; H, 3.28; N, 19.52. Found: C, 47.94; H, 2.85; N, 19.97.
1,9-Dimethyl-5-(4-nitrophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrimido [5',4':5,6]pyrido[2,3-d] pyrimidine-4,6(1H,7H)-dione (9c). Yield (method A): 83%; m.p. >300 °C; reflux 4 h, IR (KBr) νmax (cm−1): 3384 (NH), 3034 (CH arom.), 2918 (CH aliph.), 1705 (C=O), 1520 (C=N), 1134, 1086 (C=S); 1H-NMR (DMSO-d6): 11.93 (s, 2H, 2NH), 8.46 (s, 1H, NH(10)), 8.06–8.04 (s, J = 7.5, 2H, arom.), 7.29–7.27 (s, J = 7.5, 2H, arom.), 6.20 (s, 1H, CH-5), 3.51 (s, 6H, 2 NCH3); 13C-NMR (DMSO-d6): δ = 33.55 (CH), 46.41 (CH3), 125.27, 128.11, 128.43, 128.93, 129.86, 131.34, 161.93 (C=O), 169.78 (C=S) ppm; MS: m/z (%) = M+, 430 (1), 332 (24), 106 (68), 91 (14), 78 (100); Anal. calcd. for C17H14N6O4S2 (430.46): C, 47.43; H, 3.28; N, 19.52. Found: C, 48.01; H, 2.86; N, 19.93.
5-(4-Bromophenyl)-1,9-dimethyl-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrimido [5',4':5,6]pyrido[2,3-d] pyrimidine-4,6(1H,7H)-dione (9d). Yield (method A): 90%; m.p. >300 °C; reflux 4 h; IR (KBr) νmax (cm−1): 3220 (NH), 3046 (CH arom.), 2818 (CH aliph.), 1721 (C=O), 1567 (C=N), 1107, 1068 (C=S); 1H-NMR (DMSO-d6): 12.61 (s, 1H, NH), 12.52 (s, 1H, NH), 8.28–8.26 (d, 1H, arom.), 8.05–8.03 (d, J = 8.6, 1H, arom.), 7.99–7.97 (d, J = 8.8, 2H, arom.), 7.84 (s, 1H, NH(10)), 6.06 (s, 1H, CH-5), 3.56 (s, 3H, NCH3), 3.51 (s, 3H, NCH3); 13C-NMR (DMSO-d6): δ = 33.48 (CH), 45.88 (CH3), 125.16, 127.89, 128.16, 128.91, 129.47, 131.16, 161.89 (C=O), 169.77 (C=S) ppm; MS: m/z (%) = M+ + 2, 466 (2), M+, 464 (6), 200 (58), 95 (100); Anal. calcd. for C17H14BrN5O2S2 (464.35): C, 43.97; H, 3.04; N, 15.08 Found: C, 44.34; H, 2.60; N, 15.31.
5-(2-Hydroxyphenyl)-1,9-dimethyl-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrimido [5',4':5,6]pyrido[2,3-d]pyrimidine-4,6(1H,7H)-dione (9e). Yield (method A): 81%; m.p. >300 °C; reflux 6 h; IR (KBr) νmax (cm−1): 3321 (NH), 3096 (CH arom.), 2901 (CH aliph.), 1684 (C=O), 1580 (C=N), 1141, 1081 (C=S); 1H-NMR (DMSO-d6): 12.44 (s, 1H, OH), 12.36 (s, 1H, NH), 10.96 (s, 1H, NH), 8.40–8.38 (d, 1H, arom.), 8.36–8.34 (d, 1H, arom.), 8.28–8.26 (d, 1H, arom.), 7.88 (s, 1H, NH(10)), 6.91–6.89 (d, 1H, arom.), 6.17 (s, 1H, CH-5), 3.56 (s, 6H, 2 NCH3); 13C-NMR (DMSO-d6): δ = 33.46 (CH), 45.64 (CH3), 125.02, 127.84, 128.12, 128.76, 129.32, 131.14, 161.85 (C=O), 169.73 (C=S) ppm; MS: m/z (%) = M+, 401 (0.4), 109 (36), 106 (100), 78 (51), 43 (52); Anal. calcd. for C17H15N5O3S2 (401.46): C, 50.86; H, 3.77; N, 17.44. Found: C, 51.27; H, 3.31; N, 17.70.