Olefin Metathesis Reaction in Water and in Air Improved by Supramolecular Additives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Screening
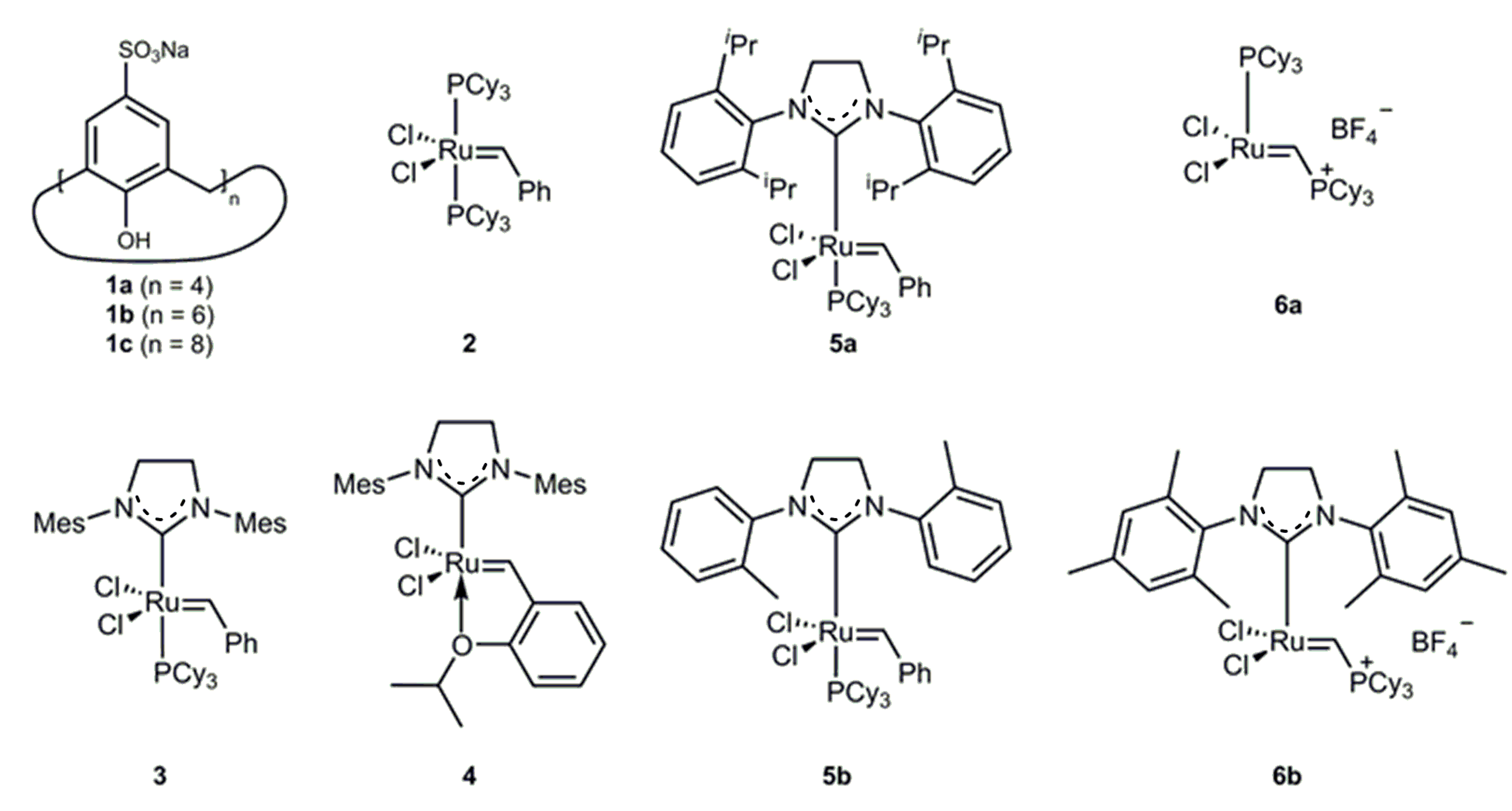

| Entry | Substrate | Cat. | t | Conversion [%] |
|---|---|---|---|---|
| 1 | 7 | 2 | 4 h | 95 |
| 2 | 7 | 3 | 4 h | 75 |
| 3 | 7 | 4 | 4 h | 89 |
| 4 | 7 | 5a | 4 h | 83 |
| 5 | 7 | 5b | 4 h | 5 |
| 6 | 7 | 6a | 5 h | 3 |
| 7 | 7 | 6b | 4 h | 97 |
| 8 | 9 | 4 | 1 h | >99 |
| 9 | 9 | 5a | 5 min | >99 a |
| 10 | 9 | 5b | 4 h | 36 |
| 11 | 9 | 6a | 21 h | 3 |
| 12 | 9 | 6b | 4 h | 84 |
| 13 | 9 | 6a | 2 h | 0 b |
| 14 | 9 | 6a | 2 h | 7 c |
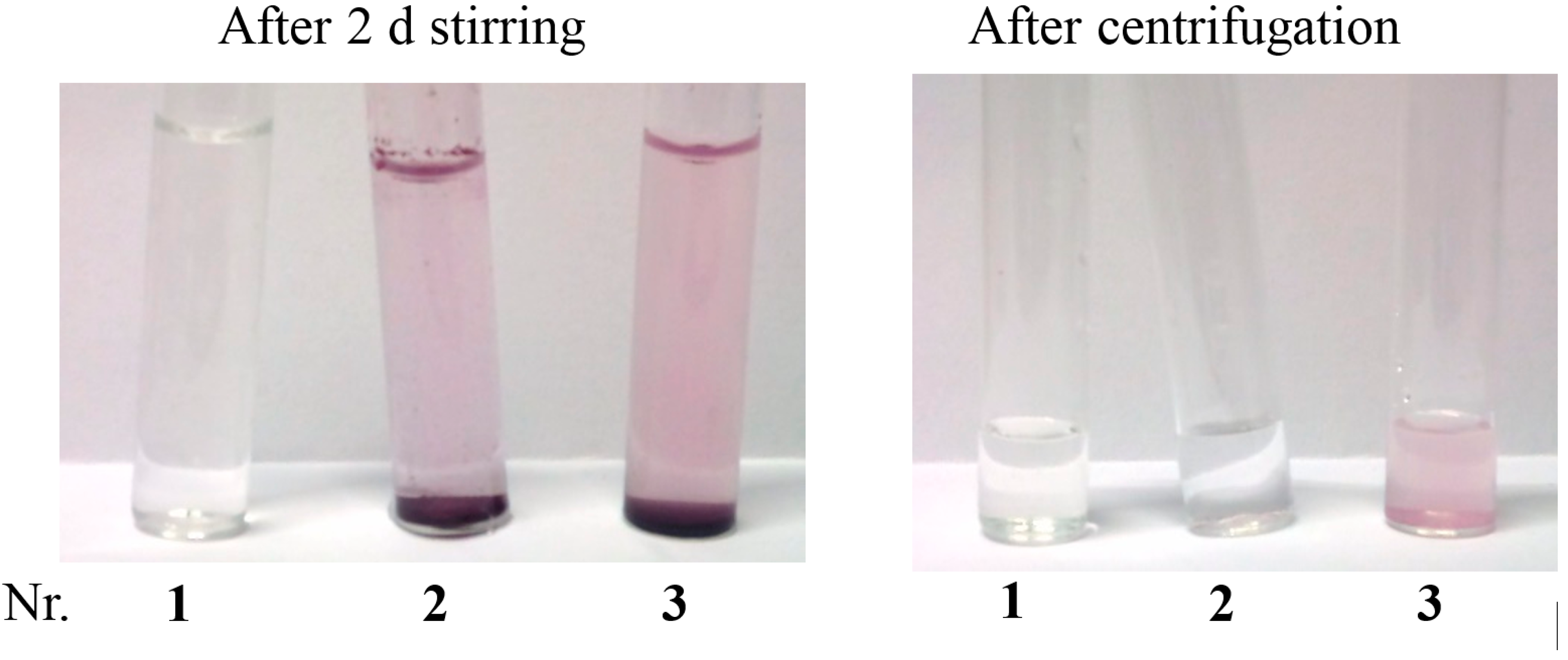
2.2. Solubilisation
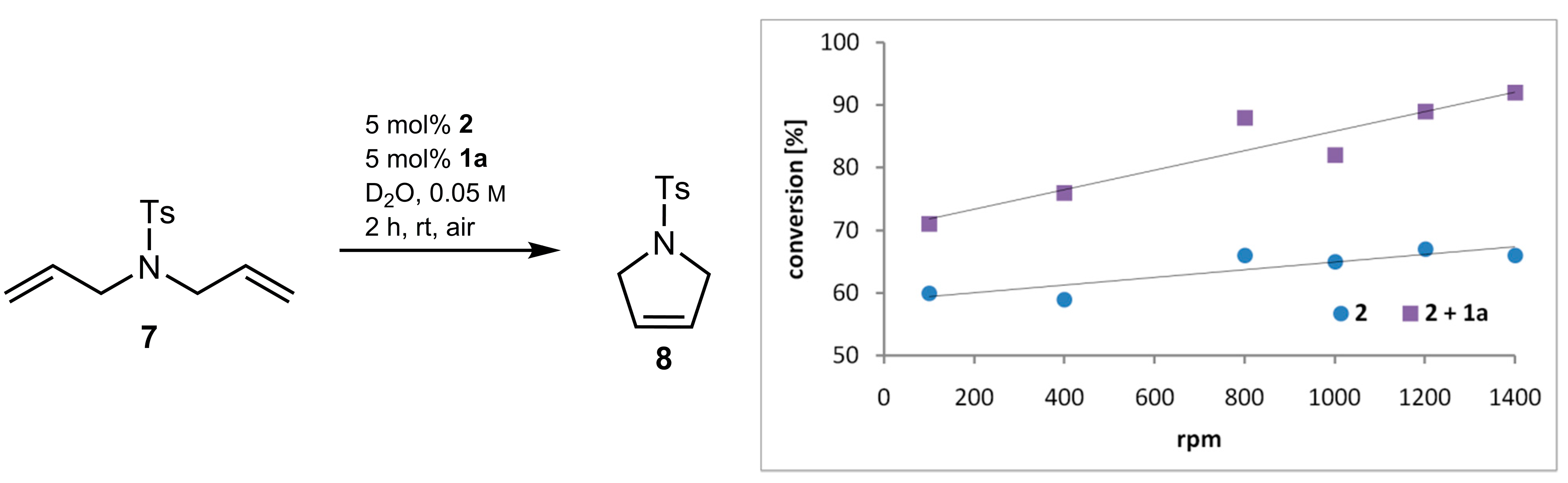
2.3. Mass Transfer Effect
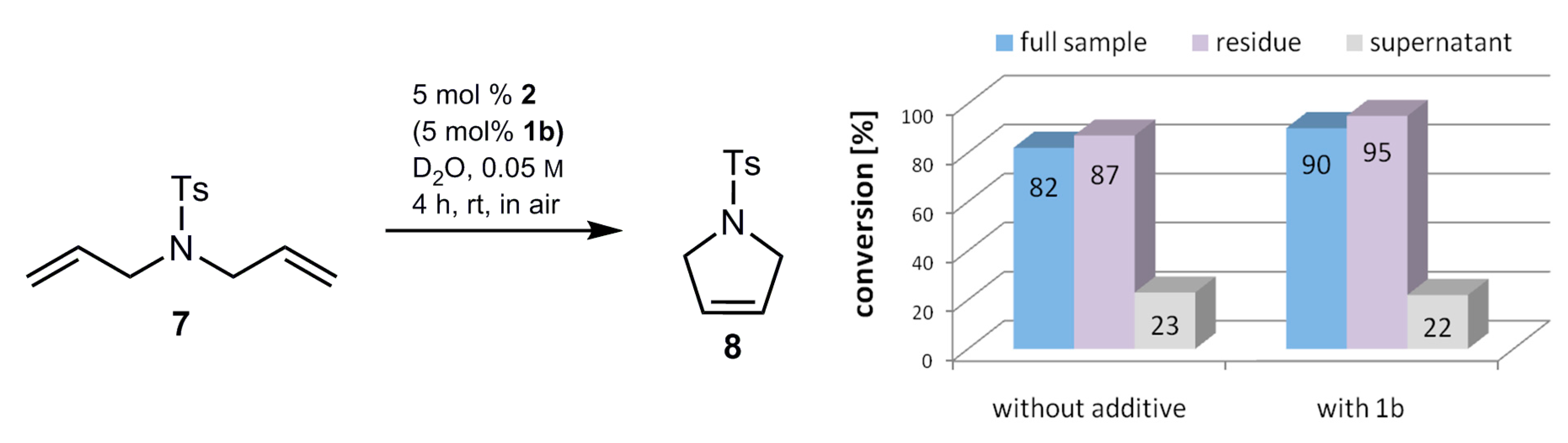
2.4. Homogeneous vs. Heterogeneous Conditions
2.5. Influence of Additive Concentration
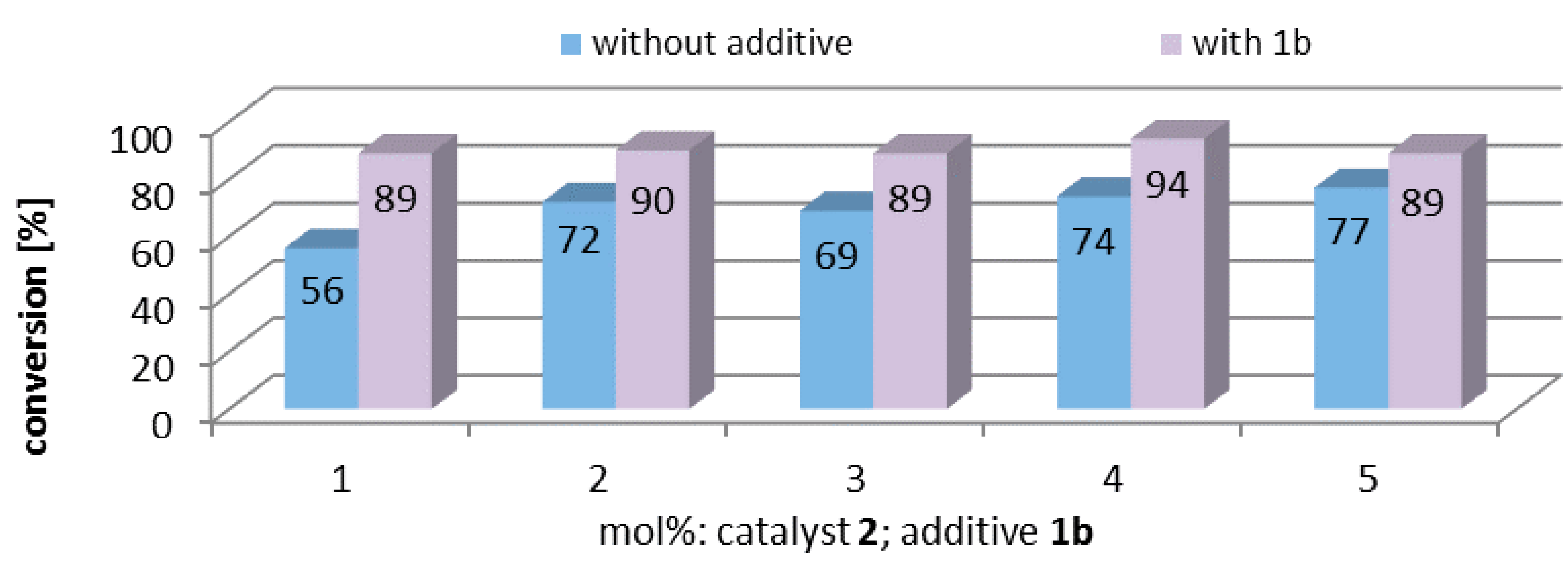
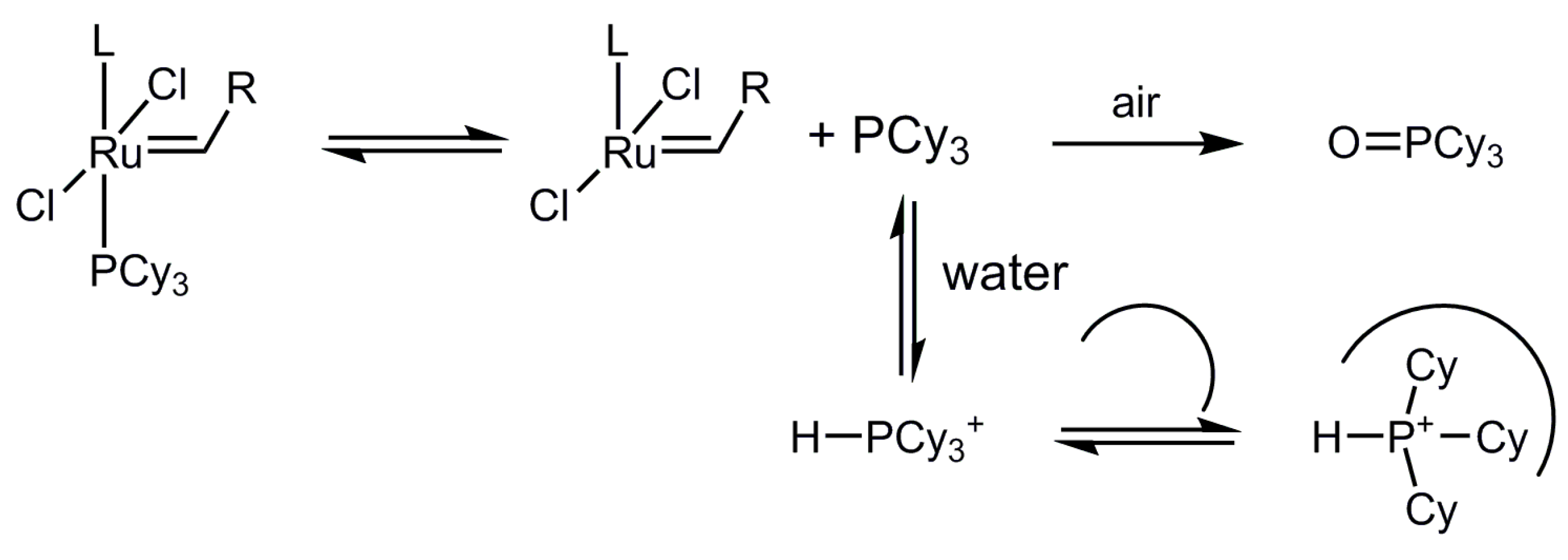
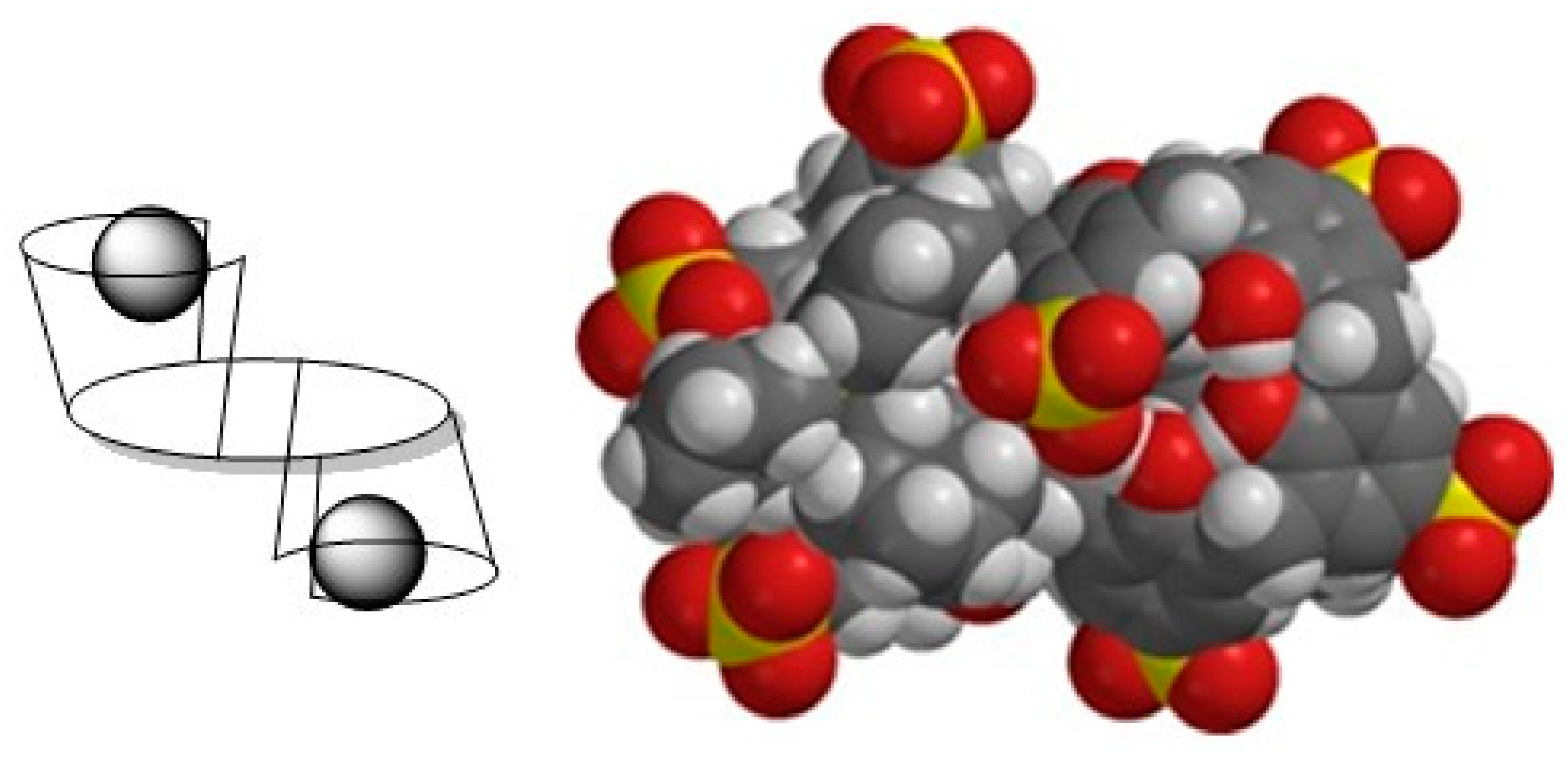
2.6. Binding Studies
| Guest | Host | log Kass | Δδ (ppm) |
|---|---|---|---|
 | 1a | 4.9 ± 0.1 | −0.42 (H1) |
| −0.50 (H2) | |||
| 1b | 4.0 ± 0.1 | −0.54 (H1) | |
| −0.58 (H2) | |||
| 1c | log K1 = 4.4 ± 0.1 | −0.61 (H1) | |
| log K2 = 7.3 ± 0.1 | −0.59 (H2) | ||
| Guest | Host | Kass (M−1) | Δδ (ppm) |
 | 1b | 29 ± 1 | −0.54 (H1) |
| −0.39 (H2) | |||
| −0.94 (H3) | |||
| −0.67 (H4) | |||
| −0.40 (H5) | |||
| −0.29 (H6) | |||
 | 1a | 22 | −0.66 (H1) |
| +0.25 (H2) | |||
| −0.11 (H3) | |||
| +0.19 (H4) | |||
| +0.26 (H5) | |||
| +0.30 (H6) | |||
| 1b | 31 | −0.40 (H1) | |
| −0.11 (H2) | |||
| −0.22 (H3) | |||
| −0.02 (H4) | |||
| −0.17 (H5) | |||
| −0.11 (H6) |
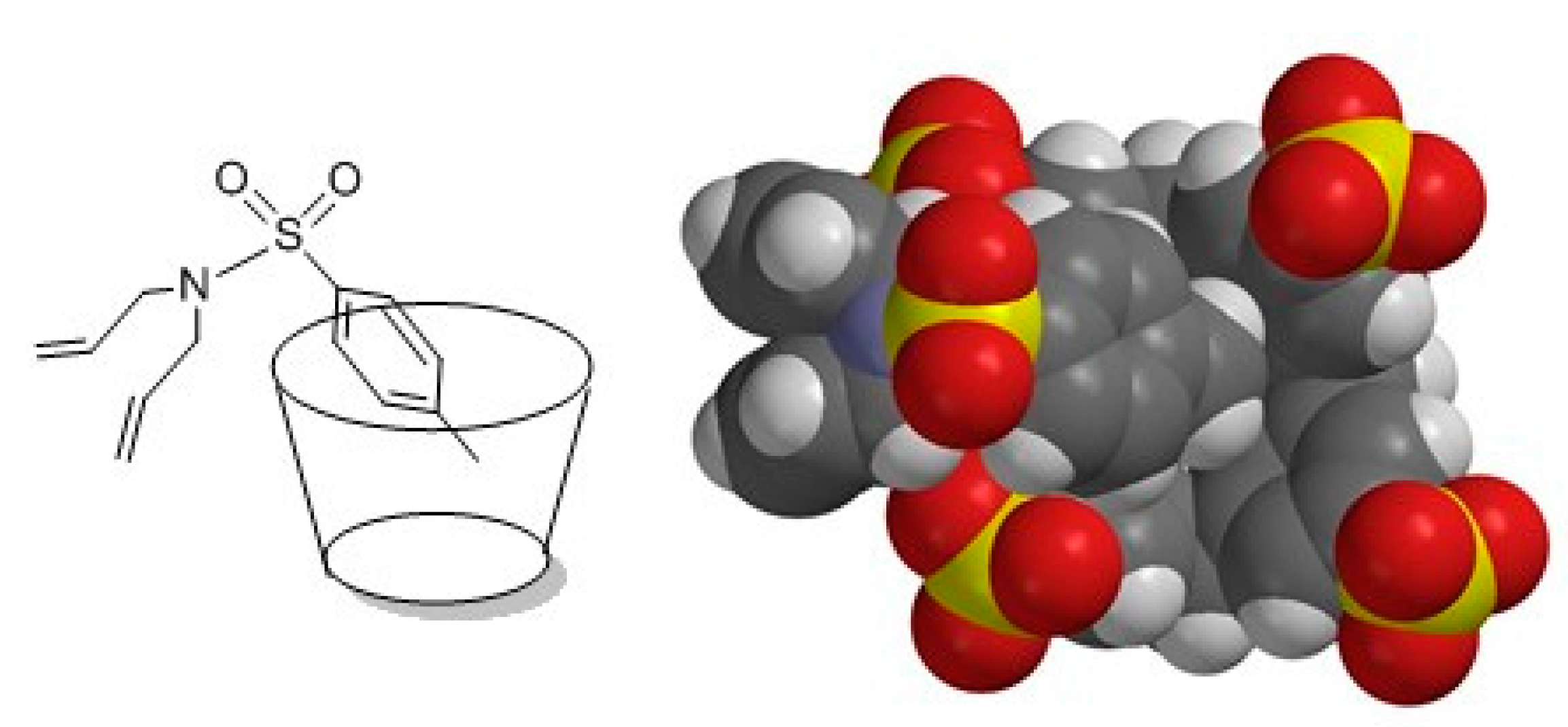
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grubbs, R.H. Handbook of Metathesis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2003; Volume 1. [Google Scholar]
- Cornils, B.; Herrmann, W.A. Aqueous-Phase Organometallic Catalysis: Concepts and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Guidone, S.; Songis, O.; Nahra, F.; Cazin, C.S.J. Conducting olefin metathesis reactions in air: Breaking the paradigm. ACS Catal. 2015, 5, 2697–2701. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Olefin metathesis for chemical biology. Curr. Opin. Chem. Biol. 2008, 12, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, D.; Grela, K. Aqueous olefin metathesis. Angew. Chem. Int. Ed. 2009, 48, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Ghorai, S. Transition metal catalyzed cross-couplings going green: In Water at room temperature. Aldrichim. Acta 2008, 41, 59–72. [Google Scholar]
- Zaman, S.; Curnow, O.J.; Abell, A.D. Development of aqueous metathesis catalysts. Aust. J. Chem. 2009, 62, 91–100. [Google Scholar] [CrossRef]
- Grela, K.; Gułajski, Ł.; Skowerski, K. Alkene metathesis in water. In Metal-Catalyzed Reactions in Water; Dixneuf, P.H., Cadierno, V., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 291–336. [Google Scholar]
- Torborg, C.; Samjlowicz, C.; Grela, K. Science of Synthesis: Water in Organic Synthesis, Section 3.6; Kobayashi, S., Ed.; Georg-Thieme Verlag KG: Stuttgart, Germany, 2012; Volume 5, pp. 225–256. [Google Scholar]
- Tomasek, J.; Schatz, J. Olefin metathesis in aqueous media. Green Chem. 2013, 15, 2317–2338. [Google Scholar] [CrossRef]
- Connon, S.J.; Blechert, S. A solid-supported phosphine-free ruthenium alkylidene for olefin metathesis in methanol and water. Bioorg. Med. Chem. Lett. 2002, 12, 1873–1876. [Google Scholar] [CrossRef]
- Connon, S.J.; Rivard, M.; Zaja, M.; Blechert, M.S. Practical olefin metathesis in protic media under an air atmosphere. Adv. Synth. Catal. 2003, 345, 572–575. [Google Scholar] [CrossRef]
- Binder, J. B.; Blank, J.J.; Raines, R.T. Olefin metathesis in homogeneous aqueous media catalyzed by conventional ruthenium catalysts. Org. Lett. 2007, 9, 4885–4888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castagnolo, D.; Botta, L.; Botta, M. Alkyne-enol ether cross-metathesis in the presence of CuSO4: Direct formation of 3-substituted crotonaldehydes in aqueous medium. J. Org. Chem. 2009, 74, 3172–3174. [Google Scholar] [CrossRef] [PubMed]
- Gułajski, Ł.; Śledz, P.; Lupa, A.; Grela, K. Olefin metathesis in water using acoustic emulsification. Green Chem. 2008, 10, 271–274. [Google Scholar]
- Brendgen, T.; Fahlbusch, T.; Frank, M.; Schühle, D.T.; Seßler, M.; Schatz, J. Metathesis in pure water mediated by supramolecular additives. Adv. Synth. Catal. 2009, 351, 303–307. [Google Scholar] [CrossRef]
- Rehm, M.; Frank, M.; Schatz, J. Water-soluble calixarenes-self-aggregation and complexation of noncharged aromatic guests in buffered aqueous solution. Tetrahedron Lett. 2009, 50, 93–96. [Google Scholar] [CrossRef]
- Monflier, E.; Bricout, H.; Hapiot, F.; Tilloy, S.; Aghmiz, A.; Masdeu-Bulto, A.M. High-pressure 31P{1H}NMR studies of RhH(CO)(TPPTS)3 in the presence of methylated cyclodextrins: new light on rhodium-catalyzed hydroformylation reaction assisted by cyclodextrins. Adv. Synth. Catal. 2004, 346, 425–431. [Google Scholar] [CrossRef]
- Binkowski-Machut, C.; Canipelle, M.; Bricout, H.; Tilloy, S.; Hapiot, F.; Monflier, E. Supramolecular trapping of phosphanes by cyclodextrins: A general approach to generate phosphane coordinatively unsaturated organometallic complexes. Eur. J. Inorg. Chem. 2006, 1611–1619. [Google Scholar] [CrossRef]
- Clavier, H.; Nolan, S.P. Percent buried volume for phosphine and N-heterocyclic carbene ligands: Steric properties in organometallic chemistry. Chem. Commun. 2010, 46, 841–861. [Google Scholar] [CrossRef] [PubMed]
- Grisi, F.; Mariconda, A.; Costabile, C.; Bertolasi, V.; Longo, P. Influence of syn and anti-configurations of NHC backbone on Ru-catalyzed olefin metathesis. Organometallics 2009, 28, 4988–4995. [Google Scholar] [CrossRef]
- Seßler, M.B. Metathese in Wasser. Ph.D. Thesis, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany, 2011. [Google Scholar]
- Bocchinfuso, G.; Mazzuca, C.; Saracini, C.; Venanzi, M.; Micheli, L.; Palleschi, G.; Palleschi, A. Receptors for organochlorine pesticides based on Calixarenes. Mikrochim. Acta 2008, 163, 195–202. [Google Scholar] [CrossRef]
- Shinkai, S.; Araki, K.; Matsuda, T.; Manabe, O. NMR determination of association constants for aqueous calixarene complexes and guest template effects on the conformational freedom. Bull. Chem. Soc. Jpn. 1989, 62, 3856–3862. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes. Acc. Chem. Res. 1983, 16, 161–170. [Google Scholar] [CrossRef]
- Gutsche, C.D. The calixarenes. In Structural Chemistry, Top. Curr. Chem.; Springer Verlag: Berlin, Germany, 1984; Volume 123, pp. 1–47. [Google Scholar]
- Hamai, S. Association of inclusion compounds of β-cyclodextrin in aqueous solution. Bull. Chem. Soc. Jpn. 1982, 55, 2721–2729. [Google Scholar] [CrossRef]
- Danyluk, O.; Perret, F.; Coleman, A.; Suwinska, W.K. The solid-state complex of para-sulphonato-calix[8]arene anion with dimethylammonium cations. Crystallogr. J. 2008, 1, 18–23. [Google Scholar] [CrossRef]
- Backes, A.C.; Schatz, J.; Siehl, H.-U. GIAO-DFT calculated and experimentally derived complexation-induced chemical shifts of calix[4]arene-solvent inclusion complexes. J. Chem. Soc. Perkin Trans. 1 2002, 2, 484–488. [Google Scholar] [CrossRef]
- Tashika, Y.; Nitta, Y.; Yomoda, J. Synthesis of p-(dipropylsulfamyl)-benzoic acid. Yakugaku Zasshi I 1954, 74, 1193–1195. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- MestRe-C 4.8.6.0; Mestrelab Research S.L.: A Coruña, Spain, 2005.
- MestReNova 5.0.4; Mestrelab Research S.L.: A Coruña, Spain, 2007.
- Gans, P. HypNMR 2008; Protonic Software: Hanau, Germany, 2008. [Google Scholar]
- Frassineti, C.; Ghelli, S.; Gans, P.; Sabatina, A.; Moruzzi, M.S.; Vacca, A. Nuclear magnetic resonance as a tool for determining protonation constants of natural polyprotic bases in solution. Anal. Biochem. 1995, 231, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasek, J.; Seßler, M.; Gröger, H.; Schatz, J. Olefin Metathesis Reaction in Water and in Air Improved by Supramolecular Additives. Molecules 2015, 20, 19130-19141. https://doi.org/10.3390/molecules201019130
Tomasek J, Seßler M, Gröger H, Schatz J. Olefin Metathesis Reaction in Water and in Air Improved by Supramolecular Additives. Molecules. 2015; 20(10):19130-19141. https://doi.org/10.3390/molecules201019130
Chicago/Turabian StyleTomasek, Jasmine, Miriam Seßler, Harald Gröger, and Jürgen Schatz. 2015. "Olefin Metathesis Reaction in Water and in Air Improved by Supramolecular Additives" Molecules 20, no. 10: 19130-19141. https://doi.org/10.3390/molecules201019130
APA StyleTomasek, J., Seßler, M., Gröger, H., & Schatz, J. (2015). Olefin Metathesis Reaction in Water and in Air Improved by Supramolecular Additives. Molecules, 20(10), 19130-19141. https://doi.org/10.3390/molecules201019130








