Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism
Abstract
:1. Introduction
2. Melatonin Synthesis
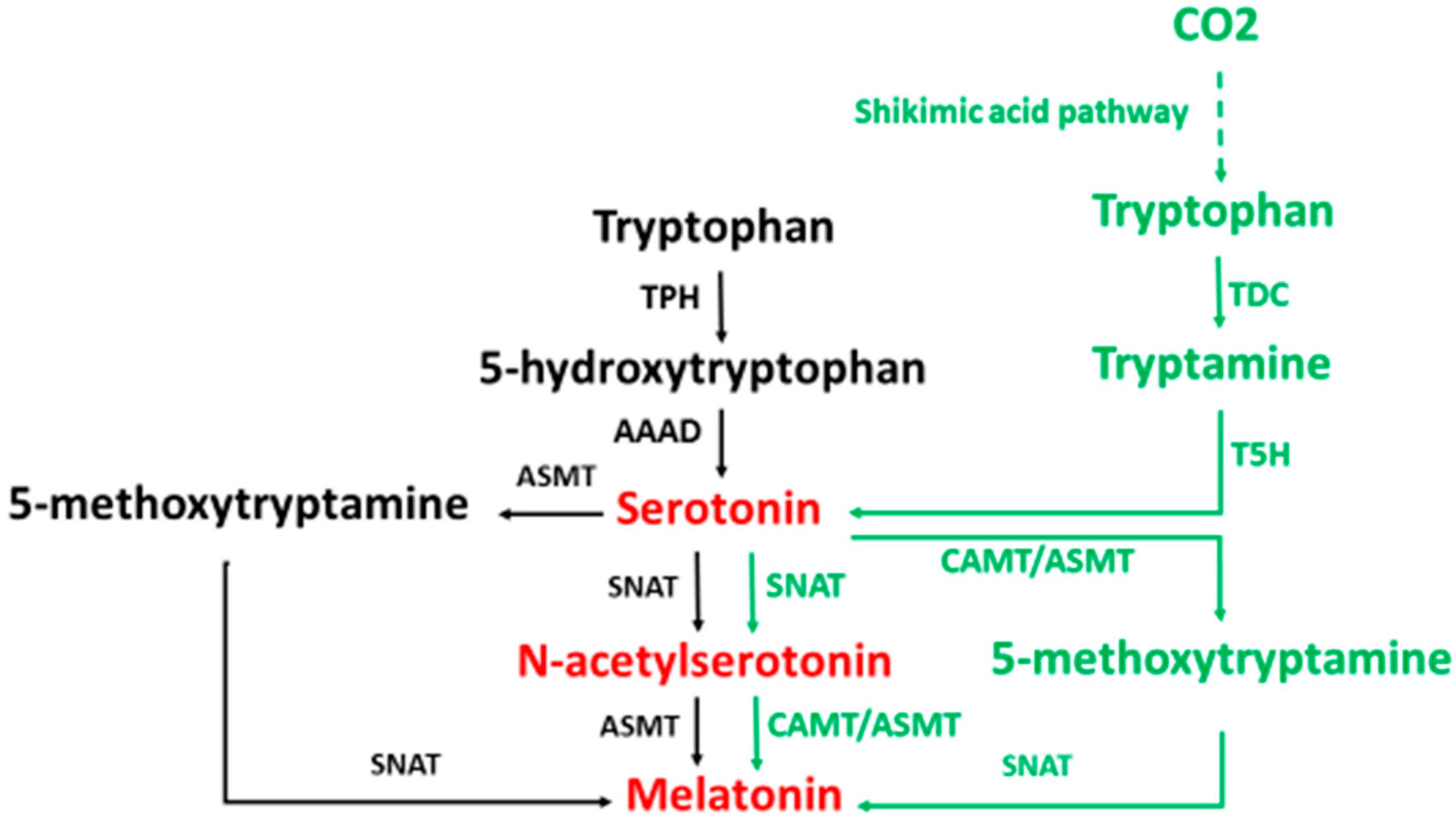
3. Melatonin Metabolism

4. Cascade Reactions of Melatonin: Interactions with ROS and NOS
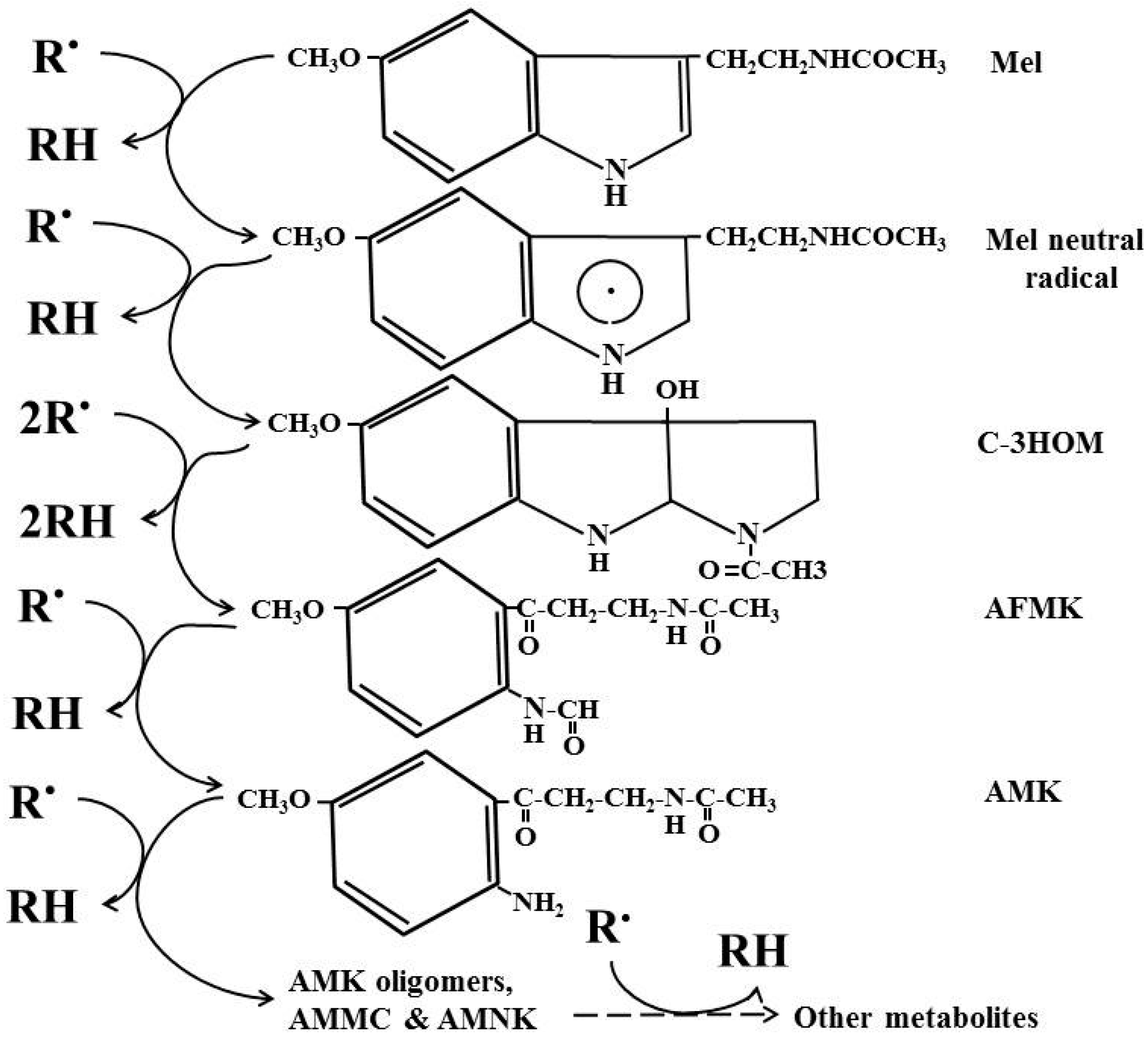
5. Melatonin, an Inducible Antioxidant
6. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D. Pigment cell regulatory factors. J. Investig. Dermatol. 1959, 32, 211–221. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Lerner, A.B. The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 1977, 45, 768–774. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Pelham, R.W. A serum melatonin rhythm in chickens and its abolition by pinealectomy. Endocrinology 1975, 96, 543–546. [Google Scholar] [CrossRef]
- Yu, H.S.; Pang, S.F.; Tang, P.L.; Brown, G.M. Persistence of circadian rhythms of melatonin and N-acetylserotonin in the serum of rats after pinealectomy. Neuroendocrinology 1981, 32, 262–265. [Google Scholar] [CrossRef]
- Pevet, P.; Challet, E. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. J. Physiol. 2011, 105, 170–182. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Legros, C.; Chesneau, D.; Boutin, J.A.; Barc, C.; Malpaux, B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 2014, 26, 151–163. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef]
- Von Gall, C.; Stehle, J.H.; Weaver, D.R. Mammalian melatonin receptors: Molecular biology and signal transduction. Cell Tiss. Res. 2002, 309, 151–162. [Google Scholar] [CrossRef]
- Weaver, D.R.; Reppert, S.M. The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport 1996, 8, 109–112. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Coto-Montes, A.; Boga, J.A.; Tan, D.X.; Davis, J.M.; Konturek, P.C.; Konturek, S.J.; Brzozowski, T. The photoperiod, circadian regulation and chronodisruption: The requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin. J. Physiol. Pharmacol. 2011, 62, 269–274. [Google Scholar]
- Pevet, P.; Agez, L.; Bothorel, B.; Saboureau, M.; Gauer, F.; Laurent, V.; Masson-Pevet, M. Melatonin in the multi-oscillatory mammalian circadian world. Chronobiol. Int. 2006, 23, 39–51. [Google Scholar] [CrossRef]
- Stehle, J.H.; von Gall, C.; Korf, H.W. Melatonin: A clock-output, a clock-input. J. Neuroendocrinol. 2003, 15, 383–389. [Google Scholar] [CrossRef]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin production in an aerobic photosynthetic bacterium: An evolutionarily early association with darkness. J. Pineal Res. 1997, 22, 102–106. [Google Scholar] [CrossRef]
- Roopin, M.; Yacobi, Y.Z.; Levy, O. Occurrence, diel patterns, and the influence of melatonin on the photosynthetic performance of cultured Symbiodinium. J. Pineal Res. 2013, 55, 89–100. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 2010, 85, 607–623. [Google Scholar] [CrossRef]
- Tosches, M.A.; Bucher, D.; Vopalensky, P.; Arendt, D. Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 2014, 159, 46–57. [Google Scholar] [CrossRef]
- Wang, F.; Yeung, K.L.; Chan, W.C.; Kwok, C.C.; Leung, S.L.; Wu, C.; Chan, E.Y.; Yu, I.T.; Yang, X.R.; Tse, L.A. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013, 24, 2724–2732. [Google Scholar] [CrossRef]
- Akerstedt, T.; Knutsson, A.; Narusyte, J.; Svedberg, P.; Kecklund, G.; Alexanderson, K. Night work and breast cancer in women: A Swedish cohort study. BMJ Open 2015, 5, e008127. [Google Scholar] [CrossRef]
- Papantoniou, K.; Castano-Vinyals, G.; Espinosa, A.; Aragones, N.; Perez-Gomez, B.; Burgos, J.; Gomez-Acebo, I.; Llorca, J.; Peiro, R.; Jimenez-Moleon, J.J.; et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int. J. Cancer 2014. [Google Scholar] [CrossRef]
- Mirick, D.K.; Bhatti, P.; Chen, C.; Nordt, F.; Stanczyk, F.Z.; Davis, S. Night shift work and levels of 6-sulfatoxymelatonin and cortisol in men. Cancer Epidemiol Biomark. Prev. 2013, 22, 1079–1087. [Google Scholar] [CrossRef]
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Slakey, L.M.; Xiang, S.; Yuan, L.; Dauchy, E.M.; Shan, B.; Brainard, G.C.; Hanifin, J.P.; et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3β. Mol. Endocrinol. 2012, 26, 1808–1820. [Google Scholar] [CrossRef]
- Stevens, R.G.; Blask, D.E.; Brainard, G.C.; Hansen, J.; Lockley, S.W.; Provencio, I.; Rea, M.S.; Reinlib, L. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect. 2007, 115, 1357–1362. [Google Scholar] [CrossRef]
- Brainard, G.C.; Petterborg, L.J.; Richardson, B.A.; Reiter, R.J. Pineal melatonin in syrian hamsters: Circadian and seasonal rhythms in animals maintained under laboratory and natural conditions. Neuroendocrinology 1982, 35, 342–348. [Google Scholar] [CrossRef]
- Reiter, R.J. The melatonin rhythm: both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef]
- Ralph, C.L.; Harlow, H.J.; Phillips, J.A. Delayed effect of pinealectomy on hibernation of the golden-mantled ground squirrel. Int. J. Biometeorol. 1982, 26, 311–328. [Google Scholar] [CrossRef]
- Reiter, R.J. Influence of pinealectomy on the breeding capability of hamsters maintained under natural photoperiodic and temperature conditions. Neuroendocrinology 1974, 13, 366–370. [Google Scholar] [CrossRef]
- Nelson, R.J.; Mason, R.T.; Krohmer, R.W.; Crews, D. Pinealectomy blocks vernal courtship behavior in red-sided garter snakes. Physiol. Behav. 1987, 39, 231–233. [Google Scholar] [CrossRef]
- Kolář, J.; Macháčková, I.; Eder, J.; Prinsen, E.; van Dongen, W.; van Onckelen, H.; Illnerová, H. Melatonin: Occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 1997, 44, 1407–1413. [Google Scholar] [CrossRef]
- Murch, S.J.; Krishna, R.S.; Saxena, P.K. Tryptophan is a precursor for melatonin and serotonin biosynththesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000, 19, 698–704. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.; Kozai, T. Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Tan, D.X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef]
- Foulkes, N.S.; Borjigin, J.; Snyder, S.H.; Sassone-Corsi, P. Rhythmic transcription: The molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997, 20, 487–492. [Google Scholar] [CrossRef]
- Ganguly, S.; Gastel, J.A.; Weller, J.L.; Schwartz, C.; Jaffe, H.; Namboodiri, M.A.; Coon, S.L.; Hickman, A.B.; Rollag, M.; Obsil, T.; et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc. Nat. Acad. Sci. USA 2001, 98, 8083–8088. [Google Scholar] [CrossRef]
- Szewczuk, L.M.; Tarrant, M.K.; Sample, V.; Drury, W.J., III; Zhang, J.; Cole, P.A. Analysis of serotonin N-acetyltransferase regulation in vitro and in live cells using protein semisynthesis. Biochemistry 2008, 47, 10407–10419. [Google Scholar] [CrossRef]
- Borjigin, J.; Liu, T. Application of long-term microdialysis in circadian rhythm research. Pharmacol. Biochem. Behav. 2008, 90, 148–155. [Google Scholar] [CrossRef]
- Johnston, J.D.; Bashforth, R.; Diack, A.; Andersson, H.; Lincoln, G.A.; Hazlerigg, D.G. Rhythmic melatonin secretion does not correlate with the expression of arylalkylamine N-acetyltransferase, inducible cyclic amp early repressor, period1 or cryptochrome1 mRNA in the sheep pineal. Neuroscience 2004, 124, 789–795. [Google Scholar] [CrossRef]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef]
- Ceinos, R.M.; Chansard, M.; Revel, F.; Calgari, C.; Miguez, J.M.; Simonneaux, V. Analysis of adrenergic regulation of melatonin synthesis in Siberian hamster pineal emphasizes the role of HIOMT. Neuro-Signals 2004, 13, 308–317. [Google Scholar] [CrossRef]
- Pang, S.F.; Yu, H.S.; Suen, H.C.; Brown, G.M. Melatonin in the retina of rats: A diurnal rhythm. J. Endocrinol. 1980, 87, 89–93. [Google Scholar] [CrossRef]
- Isorna, E.; El M’rabet, A.; Confente, F.; Falcon, J.; Munoz-Cueto, J.A. Cloning and expression of arylalkylamine N-acetyltranferase-2 during early development and metamorphosis in the sole Solea senegalensis. Gen. Comp. Endocrinol. 2009, 161, 97–102. [Google Scholar] [CrossRef]
- Isorna, E.; Aliaga-Guerrero, M.; M’Rabet, A.E.; Servili, A.; Falcon, J.; Munoz-Cueto, J.A. Identification of two arylalkylamine N-acetyltranferase 1 genes with different developmental expression profiles in the flatfish Solea senegalensis. J. Pineal Res. 2011, 51, 434–444. [Google Scholar] [CrossRef]
- Falcon, J.; Coon, S.L.; Besseau, L.; Cazamea-Catalan, D.; Fuentes, M.; Magnanou, E.; Paulin, C.H.; Boeuf, G.; Sauzet, S.; Jorgensen, E.H.; et al. Drastic neofunctionalization associated with evolution of the timezyme AANAT 500 Mya. Proc. Nat. Acad. Sci. USA 2014, 111, 314–319. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Huether, G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Park, S.; Back, K. Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 2014, 56, 107–114. [Google Scholar] [CrossRef]
- Roseboom, P.H.; Namboodiri, M.A.; Zimonjic, D.B.; Popescu, N.C.; Rodriguez, I.R.; Gastel, J.A.; Klein, D.C. Natural melatonin “knockdown” in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Mol. Brain Res. 1998, 63, 189–197. [Google Scholar] [CrossRef]
- Park, S.; Byeon, Y.; Back, K. Transcriptional suppression of tryptamine 5-hydroxylase, a terminal serotonin biosynthetic gene, induces melatonin biosynthesis in rice (Oryza sativa L.). J. Pineal Res. 2013, 55, 131–137. [Google Scholar] [CrossRef]
- Lee, H.Y.; Byeon, Y.; Lee, K.; Lee, H.J.; Back, K. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J. Pineal Res. 2014, 57, 418–426. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla Mdel, C. Production of melatonin by Saccharomyces strains under growth and fermentation conditions. J. Pineal Res. 2012, 53, 219–224. [Google Scholar] [CrossRef]
- Fernandez-Pachon, M.S.; Medina, S.; Herrero-Martin, G.; Cerrillo, I.; Berna, G.; Escudero-Lopez, B.; Ferreres, F.; Martin, F.; Garcia-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Ordonez, J.L.; Callejon, R.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is formed during winemaking at safe levels of biogenic amines. Food Chem.Toxicol. 2013, 57, 140–146. [Google Scholar] [CrossRef]
- Kocadagli, T.; Yilmaz, C.; Gokmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Yilmaz, C.; Kocadagli, T.; Gokmen, V. Formation of melatonin and its isomer during bread dough fermentation and effect of baking. J. Agric. Food Chem. 2014, 62, 2900–2905. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Rosales-Corral, S.; Coto-Montes, A.; Boga, J.A.; Reiter, R.J. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J. Pineal Res. 2012, 53, 113–121. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef]
- Gardana, C.; Iriti, M.; Stuknyte, M.; de Noni, I.; Simonetti, P. “Melatonin isomer” in wine is not an isomer of the melatonin but tryptophan-ethylester. J. Pineal Res. 2014, 57, 435–441. [Google Scholar] [CrossRef]
- Knight, E.M.; Zhu, J.; Forster, J.; Luo, H. Microorganisms for the Production of Melatonin. WO Patent 20150024440 A1, 28 February 2013. [Google Scholar]
- Byeon, Y.; Lee, K.; Park, Y.I.; Park, S.; Back, K. Molecular cloning and functional analysis of serotonin N-acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 2013, 55, 371–376. [Google Scholar]
- Semak, I.; Korik, E.; Antonova, M.; Wortsman, J.; Slominski, A. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J. Pineal Res. 2008, 45, 515–523. [Google Scholar] [CrossRef]
- Dibrova, D.V.; Cherepanov, D.A.; Galperin, M.Y.; Skulachev, V.P.; Mulkidjanian, A.Y. Evolution of cytochrome bc complexes: From membrane-anchored dehydrogenases of ancient bacteria to triggers of apoptosis in vertebrates. Biochim. Biophys. Acta 2013, 1827, 1407–1427. [Google Scholar] [CrossRef]
- Tesoriere, L.; Avellone, G.; Ceraulo, L.; D’Arpa, D.; Allegra, M.; Livrea, M.A. Oxidation of melatonin by oxoferryl hemoglobin: A mechanistic study. Free Radic. Res. 2001, 35, 633–642. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Galano, A.; Reiter, R.J. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr. Med. Chem. 2014, 21, 1557–1565. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Bałabusta, M.; Szewczyk, R.; Posmyk, M.M. The levels of melatonin and its metabolites in conditioned corn (Zea mays L.) and cucumber (Cucumis sativus L.) seeds during storage. Acta Physiol. Plant 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Predominance of 2-hydroxymelatonin over melatonin in plants. J. Pineal Res. 2015. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Poeggeler, B.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 2003, 34, 249–259. [Google Scholar] [CrossRef]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef]
- Sharma, S.; Haldar, C. Melatonin prevents X-ray irradiation induced oxidative damagein peripheral blood and spleen of the seasonally breeding rodent, Funambulus pennanti during reproductively active phase. Int. J. Radiat. Biol. 2006, 82, 411–419. [Google Scholar] [CrossRef]
- Tunez, I.; Munoz, M.C.; Medina, F.J.; Salcedo, M.; Feijoo, M.; Montilla, P. Comparison of melatonin, vitamin E and l-carnitine in the treatment of neuro- and hepatotoxicity induced by thioacetamide. Cell Biochem. Funct. 2007, 25, 119–127. [Google Scholar] [CrossRef]
- Elbe, H.; Vardi, N.; Esrefoglu, M.; Ates, B.; Yologlu, S.; Taskapan, C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum. Exp. Toxicol. 2015, 34, 100–113. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moises, F.P.; Gomez-Rodriguez, V.M.; Gonzalez-Renovato, E.D.; Torres-Sanchez, E.D.; Ramirez-Anguiano, A.C. Fish oil, melatonin and vitamin E attenuates midbrain cyclooxygenase-2 activity and oxidative stress after the administration of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine. Metab. Brain Dis. 2013, 28, 705–709. [Google Scholar] [CrossRef]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin reduces lipid peroxidation and membrane viscosity. Front Physiol. 2014, 5, 377. [Google Scholar] [CrossRef]
- Zavala-Oseguera, C.; Galano, A.; Merino, G. Computational study on the kinetics and mechanism of the carbaryl + OH reaction. J. Phys. Chem. A 2014, 118, 7776–7781. [Google Scholar] [CrossRef]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sainz, R.M.; Tan, D.X.; Poeggeler, B.; Antolin, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxy-kynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.S.; Hardeland, R.; Reiter, R.J. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef]
- Johns, J.R.; Platts, J.A. Theoretical insight into the antioxidant properties of melatonin and derivatives. Org. Biomol. Chem. 2014, 12, 7820–7827. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.K.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102. [Google Scholar] [CrossRef]
- Kelly, R.W.; Amato, F.; Seamark, R.F. N-acetyl-5-methoxy kynurenamine, a brain metabolite of melatonin, is a potent inhibitor of prostaglandin biosynthesis. Biochem. Biophys. Res. Commun. 1984, 121, 372–379. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Manda, K.; Ueno, M.; Anzai, K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J. Pineal Res. 2007, 42, 386–393. [Google Scholar] [CrossRef]
- Manda, K.; Ueno, M.; Anzai, K. Space radiation-induced inhibition of neurogenesis in the hippocampal dentate gyrus and memory impairment in mice: Ameliorative potential of the melatonin metabolite, AFMK. J. Pineal Res. 2008, 45, 430–438. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Escribano, B.M.; Colin-Gonzalez, A.L.; Santamaria, A.; Tunez, I. The role of melatonin in multiple sclerosis, Huntington’s disease and cerebral ischemia. CNS Neurol. Disord. Drug Targets 2014, 13, 1096–1119. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Clinical uses of melatonin: Evaluation of human trials. Curr. Med. Chem. 2010, 17, 2070–2095. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Esfandiar, A.; Akhavan, O.; Irajizadab, A. Melatonin as a powerful bio-antioxidant for reduction of graphene oxide. J. Mater. Chem. 2011, 21, 10907–10914. [Google Scholar] [CrossRef]
- Reiter, R.J. Normal patterns of melatonin levels in the pineal gland and body fluids of humans and experimental animals. J. Neural Transm. 1986, 21, 35–54. [Google Scholar]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human melatonin production decreases with age. J. Pineal Res. 1986, 3, 379–388. [Google Scholar] [CrossRef]
- Reiter, R.J.; Richardson, B.A.; Johnson, L.Y.; Ferguson, B.N.; Dinh, D.T. Pineal melatonin rhythm: Reduction in aging Syrian hamsters. Science 1980, 210, 1372–1373. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef]
- Ebihara, S.; Marks, T.; Hudson, D.J.; Menaker, M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 1986, 231, 491–493. [Google Scholar] [CrossRef]
- Troiani, M.E.; Reiter, R.J.; Tannenbaum, M.G.; Puig-Domingo, M.; Guerrero, J.M.; Menendez-Pelaez, A. Neither the pituitary gland nor the sympathetic nervous system is responsible for eliciting the large drop in elevated rat pineal melatonin levels due to swimming. J. Neural Transm. 1988, 74, 149–160. [Google Scholar] [CrossRef]
- Ueck, M.; Troiani, M.E.; Reiter, R.J. Transient reduction in pineal melatonin levels but not N-acetyltransferase activity in rats forced to swim for 15 minutes at night. Neuroendocrinol. Lett. 1988, 10, 81–90. [Google Scholar]
- Liu, R.Y.; Zhou, J.N.; van Heerikhuize, J.; Hofman, M.A.; Swaab, D.F. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J. Clin. Endocrinol. Metab. 1999, 84, 323–327. [Google Scholar]
- Maurizi, C.P. Loss of intraventricular fluid melatonin can explain the neuropathology of Alzheimer’s disease. Med. Hypotheses 1997, 49, 153–158. [Google Scholar] [CrossRef]
- Zhou, J.N.; Liu, R.Y.; Kamphorst, W.; Hofman, M.A.; Swaab, D.F. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 2003, 35, 125–130. [Google Scholar] [CrossRef]
- Dzida, G.; Prystupa, A.; Lachowska-Kotowska, P.; Kadas, T.; Kamienski, P.; Kimak, E.; Halabis, M.; Kicinski, P. Alteration in diurnal and nocturnal melatonin serum level in patients with chronic heart failure. Ann. Agric. Environ. Med. 2013, 20, 745–748. [Google Scholar]
- Sakotnik, A.; Liebmann, P.M.; Stoschitzky, K.; Lercher, P.; Schauenstein, K.; Klein, W.; Eber, B. Decreased melatonin synthesis in patients with coronary artery disease. Eur. Heart J. 1999, 20, 1314–7. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Arroyo-Ucar, E.; Reiter, R.J. Decreased level of melatonin in serum predicts left ventricular remodelling after acute myocardial infarction. J. Pineal Res. 2012, 53, 319–323. [Google Scholar] [CrossRef]
- Pohjanvirta, R.; Tuomisto, J.; Linden, J.; Laitinen, J. TCDD reduces serum melatonin levels in Long-Evans rats. Pharmacol. Toxicol. 1989, 65, 239–240. [Google Scholar] [CrossRef]
- Linden, J.; Pohjanvirta, R.; Rahko, T.; Tuomisto, J. TCDD decreases rapidly and persistently serum melatonin concentration without morphologically affecting the pineal gland in TCDD-resistant Han/Wistar rats. Pharmacol. Toxicol. 1991, 69, 427–432. [Google Scholar] [CrossRef]
- Pohjanvirta, R.; Laitinen, J.; Vakkuri, O.; Linden, J.; Kokkola, T.; Unkila, M.; Tuomisto, J. Mechanism by which 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) reduces circulating melatonin levels in the rat. Toxicology 1996, 107, 85–97. [Google Scholar] [CrossRef]
- Uzun, A.; Baltaci, A.K.; Kilic, M.; Mogulkoc, R. The effect of acute swimming exercise on plasma melatonin levels in rats. Bratislav. Lekar. Listy 2012, 113, 64–66. [Google Scholar] [CrossRef]
- Troiani, M.E.; Reiter, R.J.; Vaughan, M.K.; Oaknin, S.; Vaughan, G.M. Swimming depresses nighttime melatonin content without changing N-acetyltransferase activity in the rat pineal gland. Neuroendocrinology 1988, 47, 55–60. [Google Scholar] [CrossRef]
- Oxenkrug, G.F.; McIntyre, I.M. Stress-induced synthesis of melatonin: possible involvement of the endogenous monoamine oxidase inhibitor (tribulin). Life Sci. 1985, 37, 1743–1746. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Nonaka, K.O.; Lerchl, A.; Vaughan, M.K.; Reiter, R.J. Low temperature stimulates pineal activity in Syrian hamsters. J. Pineal Res. 1991, 10, 43–48. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Reiter, R.J.; Vaughan, M.K.; Nonaka, K.O.; Lerchl, A. Endocrine and metabolic effects of life-long food restriction in rats. Acta Endocrinol. 1991, 125, 93–100. [Google Scholar] [CrossRef]
- Mattison, J.A.; Lane, M.A.; Roth, G.S.; Ingram, D.K. Calorie restriction in rhesus monkeys. Exp. Gerontol. 2003, 38, 35–46. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S.; Holloszy, J.O. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age 2010, 32, 97–108. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Sainz, R.M.; Mayo, J.C.; Leon, J.; Reiter, R.J. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production: A hypothesis. Endocrine 2005, 27, 149–158. [Google Scholar] [CrossRef]
- Jaworek, J.; Leja-Szpak, A.; Bonior, J.; Nawrot, K.; Tomaszewska, R.; Stachura, J.; Sendur, R.; Pawlik, W.; Brzozowski, T.; Konturek, S.J. Protective effect of melatonin and its precursor l-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J. Pineal Res. 2003, 34, 40–52. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef]
- Tal, O.; Haim, A.; Harel, O.; Gerchman, Y. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J. Exp. Bot. 2011, 62, 1903–1910. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Growth conditions determine different melatonin levels in Lupinus albus L. J. Pineal Res. 2013, 55, 149–155. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, L.; Tan, D.X.; Zhao, Y.; Zheng, X.D.; Chen, H.; Li, Q.T.; Zuo, B.X.; Kong, J. Identification of genes for melatonin synthetic enzymes in “Red Fuji” apple (Malus domestica Borkh.cv.Red) and their expression and melatonin production during fruit development. J. Pineal Res. 2013, 55, 443–451. [Google Scholar]
- Park, S.; Byeon, Y.; Back, K. Functional analyses of three ASMT gene family members in rice plants. J. Pineal Res. 2013, 55, 409–415. [Google Scholar] [CrossRef]
- Kang, K.; Lee, K.; Park, S.; Byeon, Y.; Back, K. Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J. Pineal Res. 2013, 55, 7–13. [Google Scholar] [CrossRef]
- Fuhrberg, B.; Hardeland, R.; Poeggeler, B.; Behrmann, G. Dramatic rises of melatonin and 5-methoxytryptamine in Gonyaulax exposed to decreased temperature. Biol. Rhythm. Res. 1997, 28, 144–150. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886-18906. https://doi.org/10.3390/molecules201018886
Tan D-X, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules. 2015; 20(10):18886-18906. https://doi.org/10.3390/molecules201018886
Chicago/Turabian StyleTan, Dun-Xian, Lucien C. Manchester, Eduardo Esteban-Zubero, Zhou Zhou, and Russel J. Reiter. 2015. "Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism" Molecules 20, no. 10: 18886-18906. https://doi.org/10.3390/molecules201018886
APA StyleTan, D.-X., Manchester, L. C., Esteban-Zubero, E., Zhou, Z., & Reiter, R. J. (2015). Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules, 20(10), 18886-18906. https://doi.org/10.3390/molecules201018886





