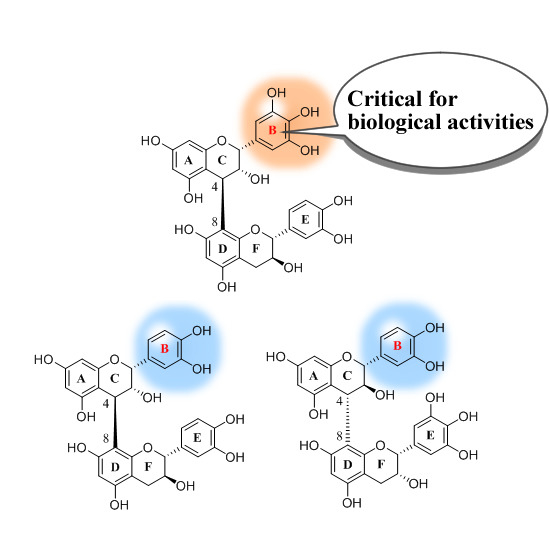
Structure–Activity Relationship of Oligomeric Flavan-3-ols: Importance of the Upper-Unit B-ring Hydroxyl Groups in the Dimeric Structure for Strong Activities
Abstract
:1. Introduction
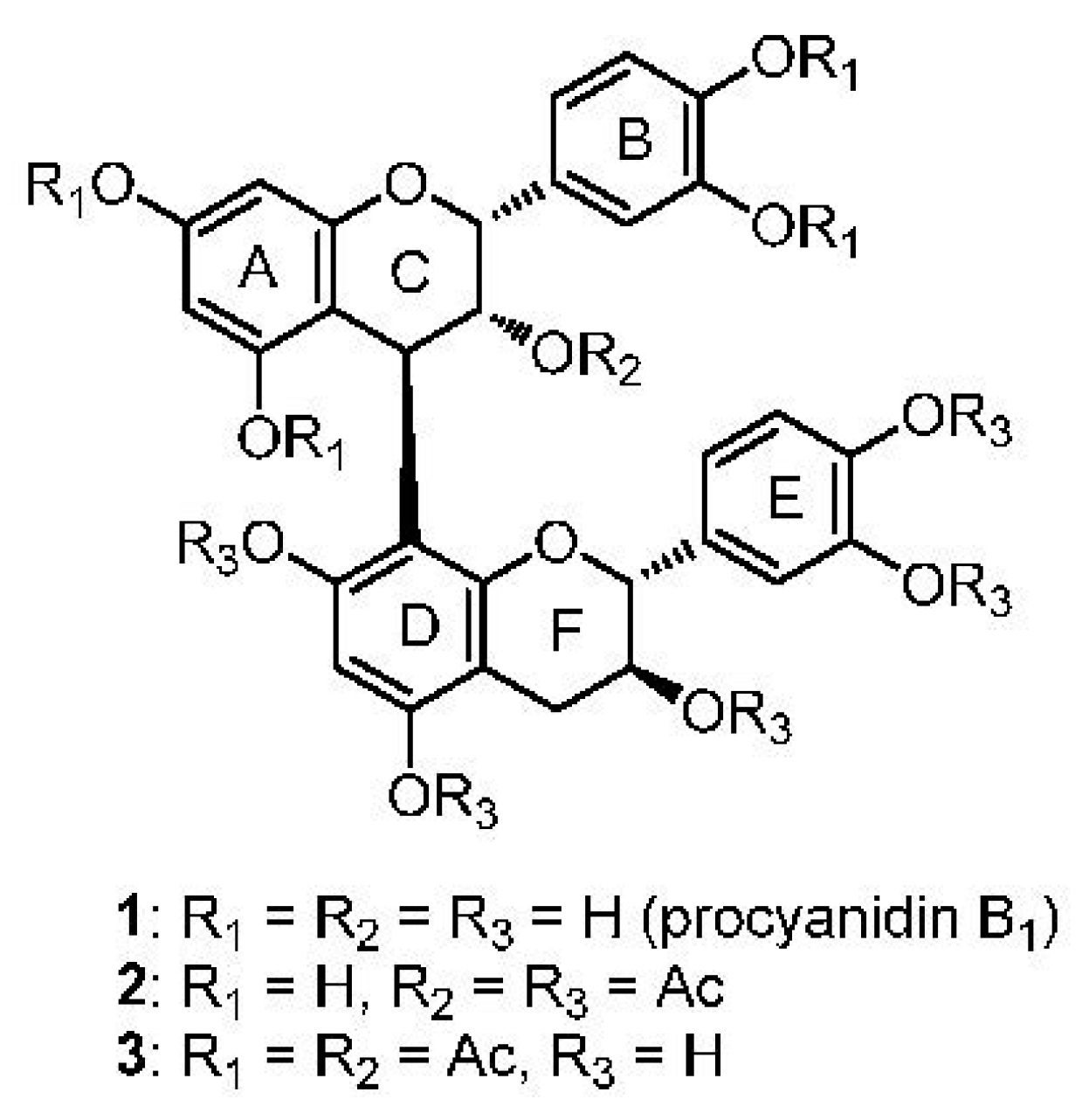

2. Results and Discussion
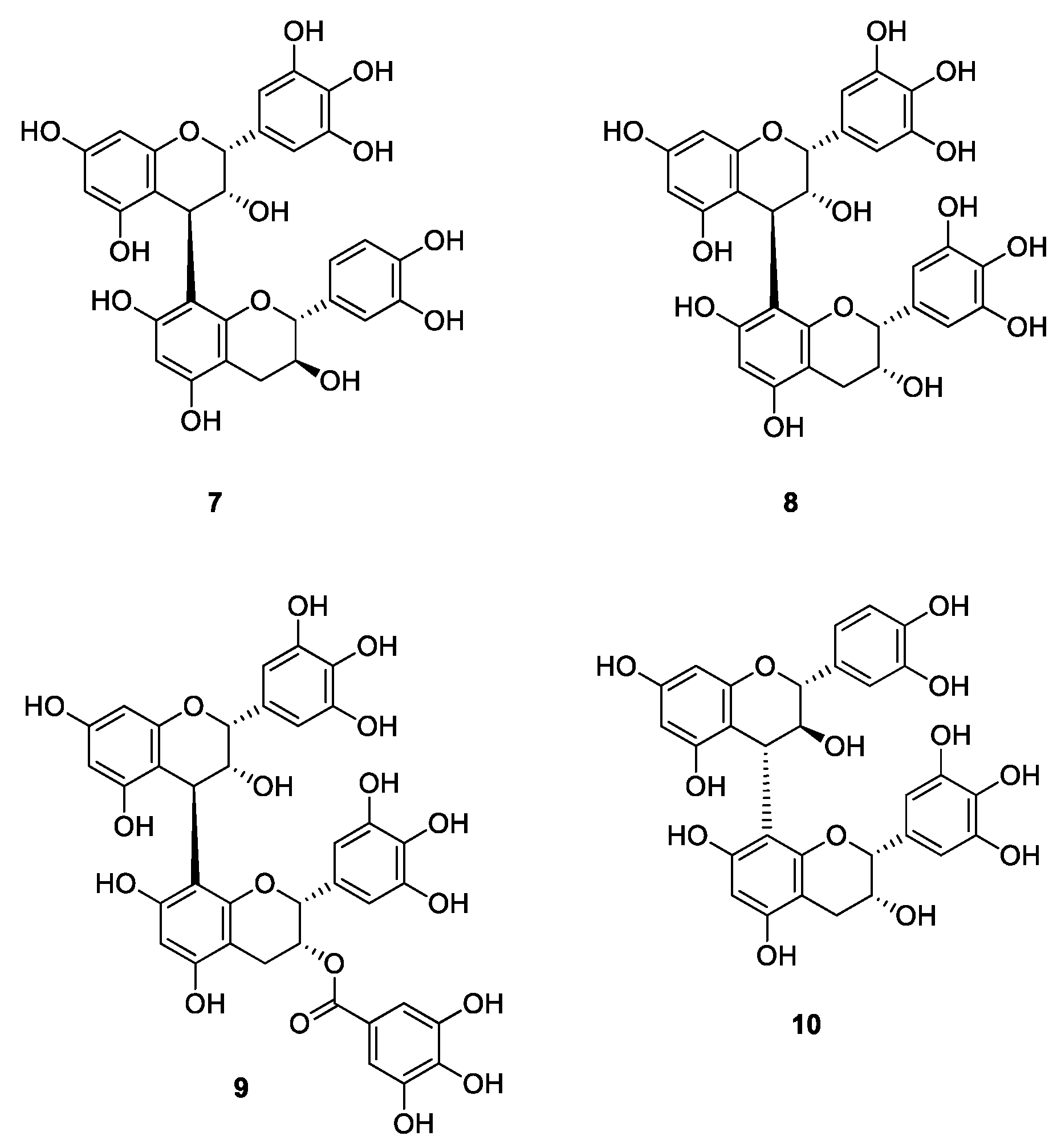

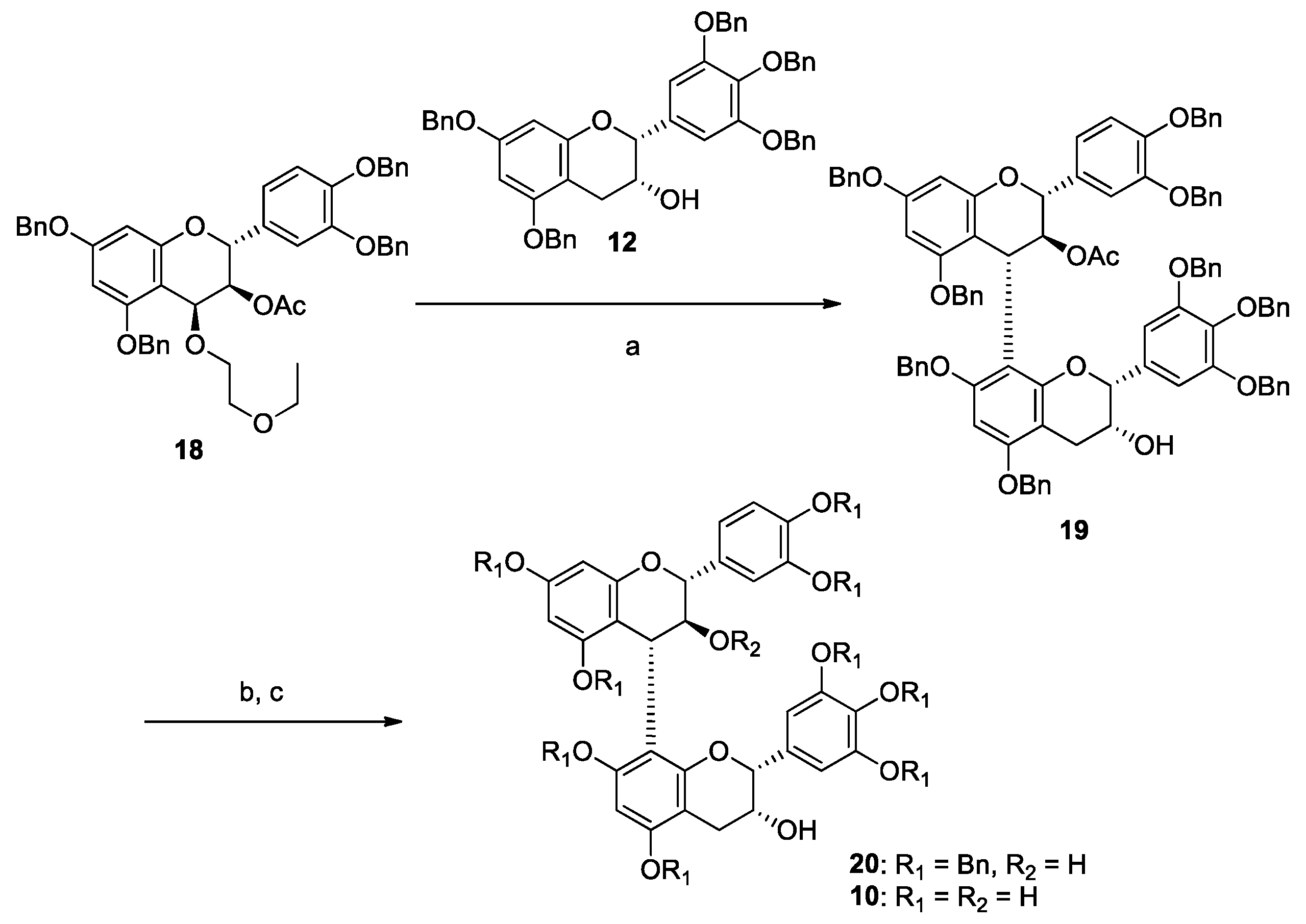
2.1. DPPH Radical Scavenging Activity
2.2. Antimicrobial Activity against S. cerevisiae
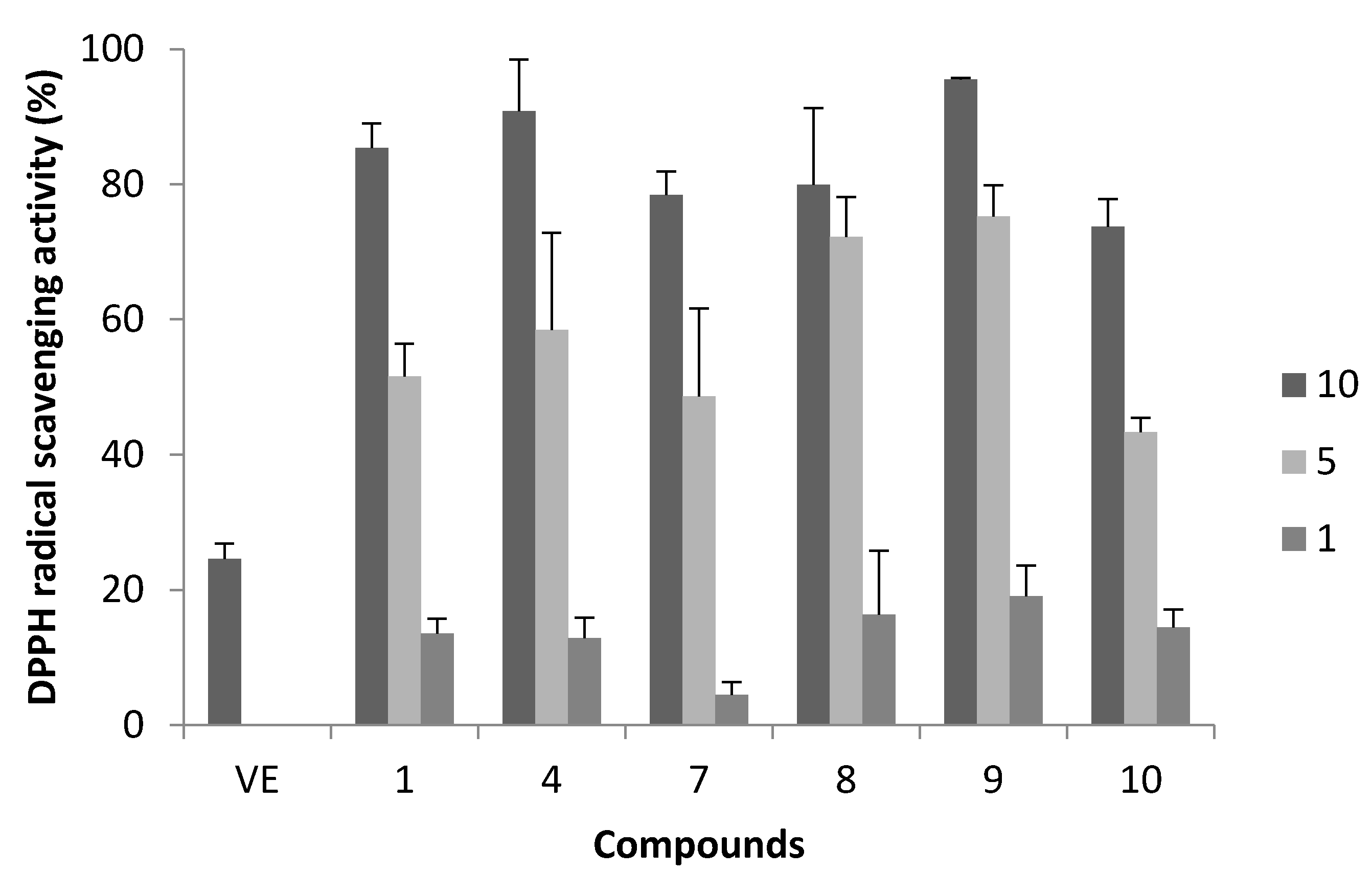

2.3. Cervical Epithelioid Carcinoma Cell Line, HeLa S3 Proliferation Inhibitory Activity

3. Experimental Section
3.1. General Information
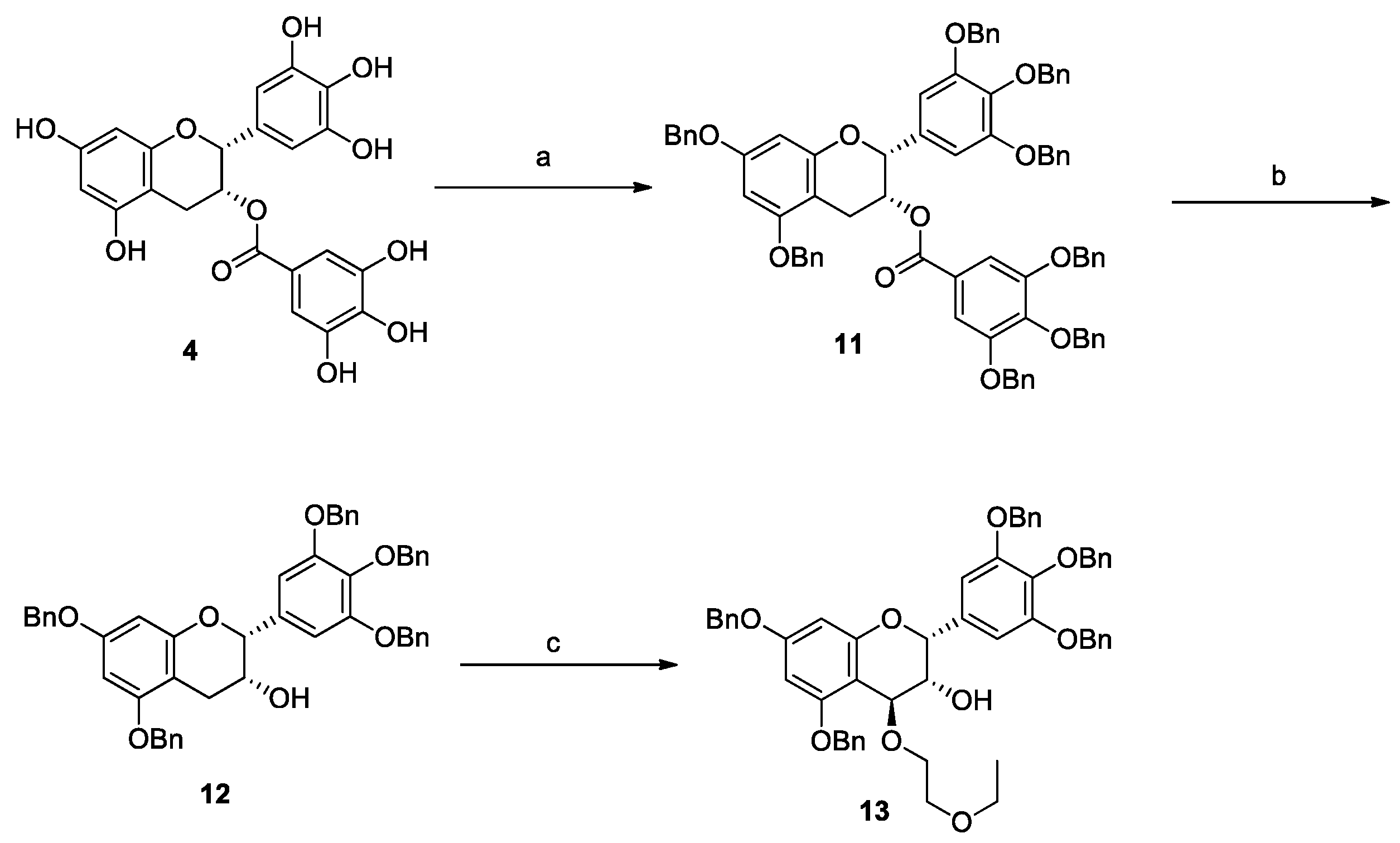
3.2. Synthesis
3.3. DPPH Radical Scavenging Activity
3.4. Antimicrobial Activity against S. cerevisiae
3.5. Inhibitory Activity of HeLa S3 Cell Proliferation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harborne, J.B. The Flavonoids: Advances in Research from 1986; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Tuckmantel, W.; Kozikowski, A.P.; Romanczyk, L.J., Jr. Studies in polyphenol chemistry and bioactivity. 1. 1reparation of building blocks from (+)-catechin. Procyanidin formation. Synthesis of the cancer cell growth inhibitor, 3-O-galloyl-(2R,3R)-epicatechin-4β,8-[3-O-galloyl-(2R,3R)-epicatechin]. J. Am. Chem. Soc. 1999, 121, 12073–12081. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Tuckmantel, W.; George, C. Studies in polyphenol chemistry and bioactivity. 2. Establishment of interflavan linkage regio- and stereochemistry by oxidative degradation of an O-alkylated derivative of procyanidin B2 to (R)-(−)-2,4-diphenylbutyric acid. J. Org. Chem. 2000, 65, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Tuckmantel, W.; Boettcher, G.; Romanczyk, L.J., Jr. Studies in polyphenol chemistry and bioactivity. 4. Synthesis of trimeric, tetrameric, pentameric, and higher oligomeric epicatechin-derived procyanidins having all-4β,8-interflavan connectivity and their inhibition of cancer cell growth through cell cycle arrest. J. Org. Chem. 2003, 68, 1641–1658. [Google Scholar] [PubMed]
- Ohmori, K.; Ushimaru, N.; Suzuki, K. Oligomeric catechins: An enabling synthetic strategy by orthogonal activation and C(8) protection. Proc. Natl. Acad. Sci. USA 2004, 101, 12002–12007. [Google Scholar] [CrossRef] [PubMed]
- Tarascou, I.; Barathieu, K.; Andre, Y.; Pianet, I.; Dufourc, E.; Fouquet, E. An improved synthesis of procyanidin dimers: Regio- and stereocontrol of the interflavan bond. Eur. J. Org. Chem. 2006, 23, 5367–5377. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kolchinski, A.; Shea, H.A.; Nair, J.J.; Gou, Y.; Romanczyk, L.J., Jr.; Schmitz, H.H. Scale-up syntheses of two naturally occurring procyanidins: (−)-epicatechin-(4β,8)-(+)-catechin and (−)-epicatechin-3-O-galloyl-(4β,8)-(−)-epicatechin-3-O-gallate. Org. Process. Res. Dev. 2007, 11, 422–430. [Google Scholar] [CrossRef]
- Mohri, Y.; Sagehashi, M.; Yamada, T.; Hattori, Y.; Morimura, K.; Kamo, T.; Hirota, M.; Makabe, H. An efficient synthesis of procyanidins. Rare earth metal Lewis acid catalyzed equimolar condensation of catechin and epicatechin. Tetrahedron Lett. 2007, 48, 5891–5894. [Google Scholar] [CrossRef]
- Oyama, K.; Kuwano, M.; Ito, M.; Yoshida, K.; Kondo, T. Synthesis of procyanidins by stepwise- and self-condensation using (+)-catechin and (−)-epicatechin as a key building monomer. Tetrahedron Lett. 2008, 49, 3176–3180. [Google Scholar] [CrossRef]
- Matthew, A.C.; Bonnet, S.L.; van der Westhuizen, J.H. Stereochemistry of sagittamide A: Prediction and confirmation. Org. Lett. 2008, 10, 3865–3868. [Google Scholar]
- Mohri, Y.; Sagehashi, M.; Yamada, T.; Hattori, Y.; Morimura, Y.; Hamauzu, K.; Kamo, T.; Hirota, M.; Makabe, H. An efficient synthesis of procyanidins using equimolar condensation of catechin and/or epicatechin catalyzed by ytterbium triflate. Heterocycles 2009, 79, 549–563. [Google Scholar] [CrossRef]
- Watanabe, G.; Ohmori, K.; Suzuki, K. First regiocontrolled synthesis of procyanidin B6, a catechin dimer with rare connectivity: A halo-capping strategy for formation of 4,6-interflavan bonds. Chem. Commun. 2013, 49, 5210–5212. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Ohmori, K.; Suzuki, K. A seco-catechin cyclization approach to 4–6-linked catechin dimers. Chem. Commun. 2014, 49, 14371–14373. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Emoto, M.; Tanaka, A.; Doi, Y.; Shoji, K.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Matsuura, N.; Nakajima, N. Stereoselective synthesis of procyanidin B3–3-O-gallate and 3,3″-di-O-gallate, and their abilities as antioxidant and DNA polymerase inhibitor. Tetrahedron 2004, 60, 12043–12049. [Google Scholar] [CrossRef]
- Saito, A.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Doi, Y.; Tanaka, A.; Nakajima, N. Systematic synthesis of galloyl-substituted procyanidin B1 and B2, and their ability of DPPH radical scavenging activity and inhibitory activity of DNA polymerases. Bioorg. Med. Chem. 2005, 13, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, H.; Saito, A.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Tanaka, A.; Nakajima, N. Synthesis of galloyl-substituted procyanidin B4 series, and their DPPH radical scavenging activity and DNA polymerase inhibitory activity. Heterocycles 2006, 67, 175–188. [Google Scholar]
- Saito, A.; Mizushina, Y.; Tanaka, A.; Nakajima, N. Versatile synthesis of epicatechin series procyanidin oligomers, and their antioxidant and DNA polymerase inhibitory activity. Tetrahedron 2009, 65, 7422–7428. [Google Scholar] [CrossRef]
- Okamoto, S.; Ishihara, S.; Okamoto, T.; Doi, S.; Harui, K.; Higashino, Y.; Kawasaki, T.; Nakajima, N.; Saito, A. Inhibitory activity of synthesized acetylated procyanidin B1 analogs against HeLa S3 cells proliferation. Molecules 2014, 19, 1775–1785. [Google Scholar] [PubMed]
- Mori, K.; Ayano, Y.; Hamada, Y.; Hojima, T.; Tanaka, R.; Higashino, Y.; Izuno, M.; Okamoto, T.; Kawasaki, T.; Hamada, M.; et al. Role of 2,3-cis structure of (−)-epicatechin-3,5-O-digallate in inhibition of HeLa S3 cell proliferation. Nat. Prod. Chem. Res. 2015, 3. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; Boyer, R.R.; O’Keefe, S.F. Application of proanthocyanidins from peanut skins as a natural yeast inhibitory agent. J. Food Sci. 2012, 77, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; Huang, W. Mechanism of proanthocyanidins-induced alcoholic fermentation enhancement in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2014, 41, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Maeta, K.; Nomura, W.; Takatsume, Y.; Izawa, S.; Inoue, Y. Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl. Environ. Microbiol. 2007, 73, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Matsukawa, M.; Yamakawa, S.; Asai, T.; Yahara, S.; Hashimoto, F.; Akizawa, T. Inhibitory effect of green tea polyphenols on membrane-type 1 matrix metalloproteinase, MT1-MMP. Biol. Pharm. Bull. 2003, 26, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Tuytens, G.M. Structure elucidation of three dimeric proanthocyanidins isolated from commercial Belgian pilsner beer. J. Inst. Brew. 1984, 90, 153–161. [Google Scholar] [CrossRef]
- Fujii, W.; Toda, K.; Matsumoto, K.; Kawaguchi, K.; Kawahara, S.; Hattori, Y.; Fujii, H.; Makabe, H. Syntheses of prodelphinidin B1, B2, and B4 and their antitumor activities against human PC-3 prostate cancer cell lines. Tetrahedron Lett. 2013, 54, 7188–7192. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 2: Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron 2002, 58, 7829–7837. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LXXVII. Novel chalcan-flavan dimers, assamicains A, B and C, and a new flavan-3-ol and proanthocyanidins from the fresh leaves of Camellia sinensis L. var. assamica KITAMURA. Chem. Pharm. Bull. 1989, 37, 77–85. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 and 7–10 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, Y.; Takano, S.; Ayano, Y.; Tokunaga, M.; Koashi, T.; Okamoto, S.; Doi, S.; Ishida, M.; Kawasaki, T.; Hamada, M.; et al. Structure–Activity Relationship of Oligomeric Flavan-3-ols: Importance of the Upper-Unit B-ring Hydroxyl Groups in the Dimeric Structure for Strong Activities. Molecules 2015, 20, 18870-18885. https://doi.org/10.3390/molecules201018870
Hamada Y, Takano S, Ayano Y, Tokunaga M, Koashi T, Okamoto S, Doi S, Ishida M, Kawasaki T, Hamada M, et al. Structure–Activity Relationship of Oligomeric Flavan-3-ols: Importance of the Upper-Unit B-ring Hydroxyl Groups in the Dimeric Structure for Strong Activities. Molecules. 2015; 20(10):18870-18885. https://doi.org/10.3390/molecules201018870
Chicago/Turabian StyleHamada, Yoshitomo, Syota Takano, Yoshihiro Ayano, Masahiro Tokunaga, Takahiro Koashi, Syuhei Okamoto, Syoma Doi, Masahiko Ishida, Takashi Kawasaki, Masahiro Hamada, and et al. 2015. "Structure–Activity Relationship of Oligomeric Flavan-3-ols: Importance of the Upper-Unit B-ring Hydroxyl Groups in the Dimeric Structure for Strong Activities" Molecules 20, no. 10: 18870-18885. https://doi.org/10.3390/molecules201018870