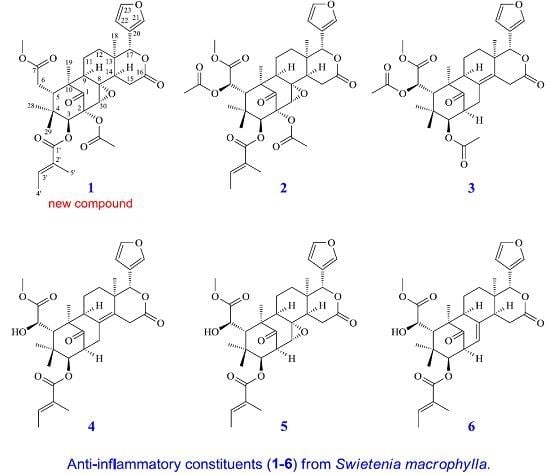
Limonoids from the Seeds of Swietenia macrophylla and Their Anti-Inflammatory Activities
Abstract
:1. Introduction
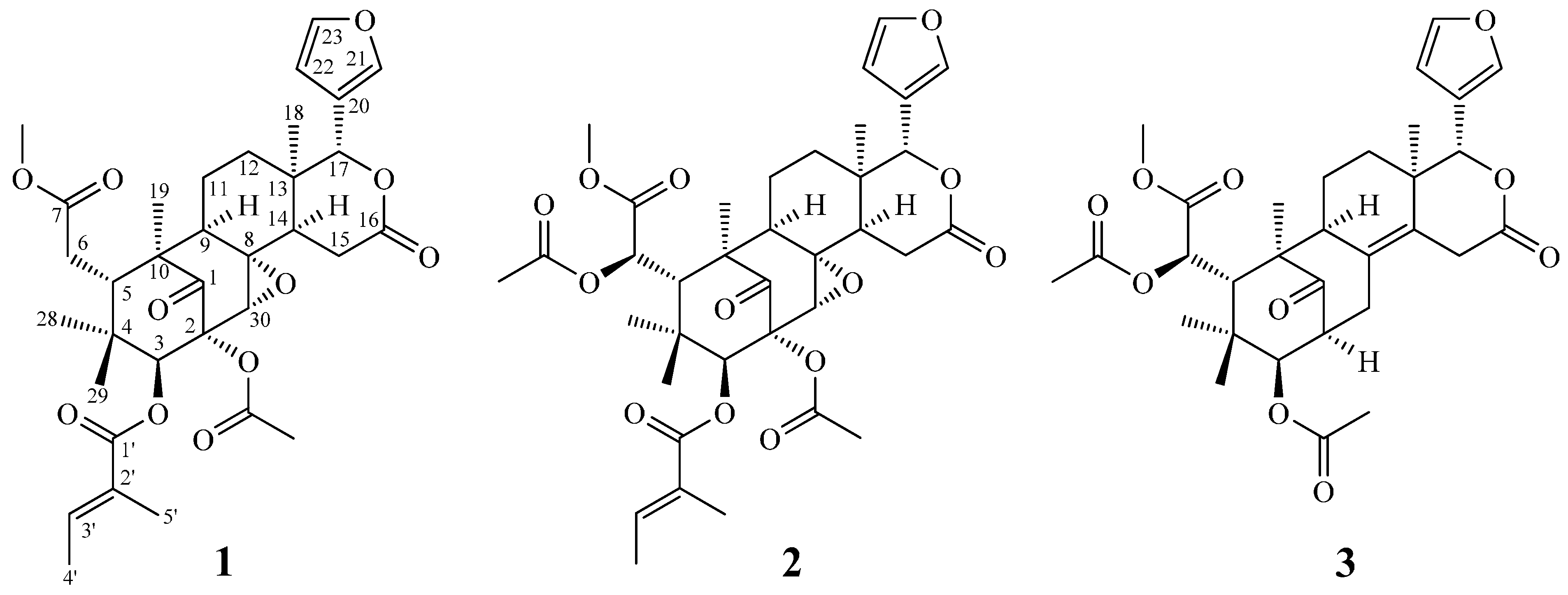
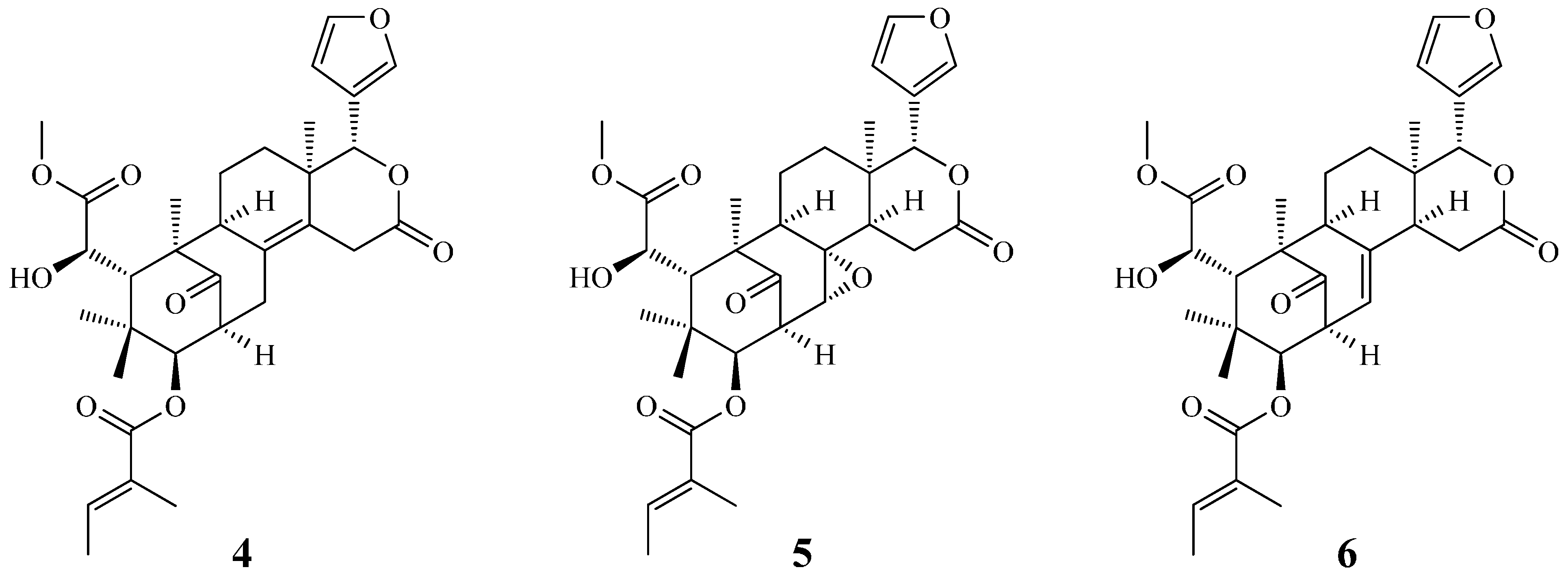
2. Results and Discussion
2.1. General
2.2. Structure Elucidation of the New Limonoid
2.3. Structure Identification of the Known Isolates
| Atom | 1 a | 2 a,b | ||
|---|---|---|---|---|
| δH | NOESY | HMBC | δH | |
| H-3 | 5.71 s | 28, 2-AcO | 1, 2, 5, 30, 1′ | 5.72 s |
| H-5 | 3.23 dd (J = 9.0, 2.5) | 6, 29 | 1, 3, 4, 7 | 3.45 s |
| H-6 | 2.33–2.37 m | 19, 28 | 4, 7, 10 | 5.49 s |
| H-9 | 1.92–1.96 m | 11, 19 | 1, 12, 14 | 1.95 m |
| H-11 | 1.79 m | 12 | 13 | |
| 1.90 m | 12 | 8, 13 | ||
| H-12 | 1.44 m | 11 | 9 | |
| 1.99 m | 11 | 9, 17 | ||
| H-14 | 1.61 m | 15 | 16 | |
| H-15 | 2.81 dd (J = 17.0, 6.5) | 14, 30 | 8, 13, 16 | 2.80 dd (J = 17.0, 6.0) |
| 3.43 dd (J = 17.0, 12.5) | 30 | 8, 16 | 3.42 dd (J = 16.0, 1.3) | |
| H-17 | 5.18 s | 12, 21, 22 | 12, 14, 16, 21 | 5.18 s |
| H-18 | 1.01 s | 12, 22 | 12, 14, 17 | 1.01 s |
| H-19 | 1.17 s | 6, 9 | 1, 5, 10 | 1.29 s |
| H-21 | 7.49 br s | 17 | 17, 20, 22 | 7.46 dd (J = 1.8, 0.9) |
| H-22 | 6.44 br s | 17, 18 | 17, 21 | 6.41 dd (J = 1.8, 0.9) |
| H-23 | 7.43 br s | 22 | 20, 21 | 7.43 dd (J = 1.8, 1.8) |
| H-28 | 0.79 s | 3, 6 | 3, 4, 5 | 1.18 s |
| H-29 | 0.81 s | 5, 3′ | 3, 5, 28 | 0.92 s |
| H-30 | 3.49 s | 15 | 1, 2, 9 | 3.48 s |
| H-3′ | 7.04 br q (J = 7.0) | 29, 4′ | 1′, 2′, 5′ | 6.98 qq (J = 7.5, 1.8) |
| H-4′ | 1.93 br d (J = 7.0) | 3′, 5′ | 2′, 3′ | 1.94 d (J = 7.5) |
| H-5′ | 1.97 s | 4′ | 1′, 2′, 3′ | 1.93 s |
| 2-OAc | 2.18 s | 3 | 2-OCOMe | 2.18 s |
| 6-OAc | 2.19 s | |||
| 7-OMe | 3.74 s | 6 | 7 | 3.82 s |
| Position | 1 a,b | 2 a,c |
|---|---|---|
| 1 | 206.1 | 206.0 |
| 2 | 80.7 | 80.8 |
| 3 | 85.6 | 85.6 |
| 4 | 40.2 | 42.1 |
| 5 | 42.4 | 45.1 |
| 6 | 33.2 | 72.0 |
| 7 | 174.2 | 171.1 |
| 8 | 62.7 | 62.6 |
| 9 | 55.3 | 55.3 |
| 10 | 50.5 | 50.6 |
| 11 | 19.7 | 19.9 |
| 12 | 33.2 | 33.4 |
| 13 | 36.2 | 36.1 |
| 14 | 45.0 | 44.9 |
| 15 | 33.3 | 33.1 |
| 16 | 169.3 | 169.2 |
| 17 | 79.3 | 79.5 |
| 18 | 26.5 | 26.7 |
| 19 | 16.1 | 16.1 |
| 20 | 120.3 | 120.2 |
| 21 | 141.0 | 140.9 |
| 22 | 110.2 | 110.1 |
| 23 | 143.3 | 143.3 |
| 28 | 22.0 | 21.0 |
| 29 | 20.5 | 21.3 |
| 30 | 65.3 | 65.2 |
| 1′ | 166.8 | 166.6 |
| 2′ | 127.6 | 127.4 |
| 3′ | 139.7 | 139.7 |
| 4′ | 14.8 | 14.9 |
| 5′ | 12.6 | 12.6 |
| 2-OCOMe | 22.5 | 22.5 |
| 2-OCOMe | 171.0 | 171.1 |
| 6-OCOMe | 23.9 | |
| 6-OCOMe | 169.7 | |
| 7-OMe | 52.4 | 53.3 |
2.4. Biological Studies
| Compounds | IC50 (μM) a |
|---|---|
| Swietemacrophin (1) | 45.44 ± 3.76 * |
| Humilinolide F (2) | 27.13 ± 1.82 ** |
| 3,6-O,O-Diacetylswietenolide (3) | 29.36 ± 1.75 * |
| 3-O-Tigloylswietenolide (4) | 35.58 ± 2.12 |
| Swietemahonin E (5) | 33.64 ± 2.05 * |
| Swietenine (6) | >100 |
| LY294002 b | 1.12 ± 0.11 * |
| Compounds | IC50 (μM) a |
|---|---|
| Swietemacrophin (1) | 33.45 ± 1.88 ** |
| Humilinolide F (2) | 49.36 ± 4.01 |
| 3,6-O,O-Diacetylswietenolide (3) | 64.21 ± 5.67 |
| 3-O-Tigloylswietenolide (4) | 32.62 ± 3.27 ** |
| Swietemahonin E (5) | 29.70 ± 2.11 * |
| Swietenine (6) | 36.32 ± 2.84 |
| Quercetin b | 32.24 ± 2.05 * |
2.5. Discussion
3. Experimental Section
3.1. Ethics Statement
3.2. General Experimental Procedures
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Biological Assay
3.5.1. Preparation of Human Neutrophils
3.5.2. Measurement of Superoxide Anion Generation
3.5.3. Determination of NO Production
3.5.4. Cell Viability Assay
3.5.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Connolly, J.D.; Labbé, C. Tetranortriterpenoids and related compounds. Part 24. The interrelation of swietenine and swietenolide, the major tetranortriterpenoids from the seeds of Swietenia macrophylla (Meliaceae). J. Chem. Soc., Perkin Trans. 1 1980, 2, 529–530. [Google Scholar] [CrossRef]
- Taylor, A.H.; Taylor, D.A.H. Limonoid extractives from Swietenia macrophylla. Phytochemistry 1983, 22, 2870–2871. [Google Scholar] [CrossRef]
- Mootoo, B.S.; Ali, A.; Motilal, R.; Pingal, R.; Ramlal, A.; Khan, A.; Reynolds, W.F.; McLean, S. Limonoids from Swietenia macrophylla and S. aubrevilleana. J. Nat. Prod. 1999, 62, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.N.; Arruda, M.S.P.; Castro, K.C.F.; da Silva, M.F.; Das, G.F. Limonoids of the phragmalin type from Swietenia macrophylla and their chemotaxonomic significance. J. Nat. Prod. 2008, 71, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Osman, H.; Wong, K.-C.; Boey, P.-L. New phragmalin-type limonoids from Swietenia macrophylla King. Food Chem. 2009, 115, 1279–1285. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, S.S.; Liao, C.H.; Wei, D.C.; Sung, P.J.; Wang, T.C.; Cheng, M.J. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010, 120, 379–384. [Google Scholar] [CrossRef]
- Jean, B.; Njikam, N.; Johnson, A.F.; Leonardo, K.B.; Pascal, R. In vitro antimalarial activity of limonoids from Khaya gradifoliola C.D.C. (Meliaceae). J. Ethnopharm. 2000, 69, 27–33. [Google Scholar]
- Govindachari, T.R.; Suresh, G.; Banumathy, B.; Masilamani, S.; Gopalakrishnan, G.; Kumari, G.N.K. Antifungal activity of some B,D-seco limonoids from two Meliaceous plants. J. Chem. Ecol. 1999, 25, 923–933. [Google Scholar] [CrossRef]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Cho, J.Y.; Hwang, T.L.; Chen, I.S. Benzoic acid derivatives, acetophenones, and anti-inflammatory constituents from Melicope semecarpifolia. J. Nat. Prod. 2008, 71, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Luo, Y.T.; Hwang, T.L.; Sung, P.J.; Wang, T.C.; Chen, I.S. A new indole alkaloid and anti-inflammatory constituents from Strychnos cathayensis. Chem. Biodivers. 2008, 5, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ting, C.W.; Hwang, T.L.; Chen, I.S. Benzophenone derivatives from the fruits of Garcinia multiflora and their anti-inflammatory activity. J. Nat. Prod. 2009, 72, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lin, Y.H.; Day, S.H.; Hwang, T.L.; Chen, I.S. New benzenoids and anti-inflammatory constituents from Zanthoxylum nitidum. Food Chem. 2011, 125, 282–287. [Google Scholar] [CrossRef]
- Chen, J.J.; Tsai, Y.C.; Hwang, T.L.; Wang, T.C. Thymol, benzofuranoid, and phenylpropanoid derivatives: Anti-inflammatory constituents from Eupatorium cannabinum. J. Nat. Prod. 2011, 74, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Villarreal, C.; Toscano, R.A.; Cook, M.; Arnason, J.T.; Byek, R.; Mata, R. Limonoids from Swietenia humilis and Guarea grandiflora (Meliaceae). Phytochemistry 1998, 49, 1981–1988. [Google Scholar] [CrossRef]
- Kadota, S.; Marpaung, L.; Kikuchi, T.; Ekimoto, H. Constituents of the seeds of Swietenia mahagoni JACQ. I. Isolation, structures, and 1H- and 13C-nuclear magnetic resonance signal assignments of new tetranortriterpenoids related to swietenine and swietenolide. Chem. Pharm. Bull. 1990, 38, 639–651. [Google Scholar] [CrossRef]
- Kadota, S.; Marpaung, L.; Kikuchi, T.; Ekimoto, H. Constituents of the seeds of Swietenia mahagoni Jacq. II. Structures of swietemahonin A, B, C, D, E, F, and G and swietemahonolide. Chem. Pharm. Bull. 1990, 38, 894–901. [Google Scholar] [CrossRef]
- Wang, L.Y.; Unehara, T.; Kitanaka, S. Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem. Pharm. Bull. 2005, 53, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Motai, T.; Kitanaka, S. Sesquiterpene chromones from Ferula fukanensis and their nitric oxide production inhibitory effects. J. Nat. Prod. 2005, 68, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar] [CrossRef]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes. Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. 1968, 97, 77–89. [Google Scholar]
- Jauregui, H.O.; Hayner, N.T.; Driscoll, J.L.; Williams-Holland, R.; Lipsky, M.H.; Galletti, P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. Vitro 1981, 17, 1100–1110. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Köpcke, B.; Anke, H.; Sterner, O. Biologically active secondary metabolites from the ascomycete A111–95. 2. Structure elucidation. J. Antibiot. 2002, 55, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Liao, H.-R.; Chen, P.-Y.; Kuo, W.-L.; Chang, T.-H.; Sung, P.-J.; Wen, Z.-H.; Chen, J.-J. Limonoids from the Seeds of Swietenia macrophylla and Their Anti-Inflammatory Activities. Molecules 2015, 20, 18551-18564. https://doi.org/10.3390/molecules201018551
Chen L-C, Liao H-R, Chen P-Y, Kuo W-L, Chang T-H, Sung P-J, Wen Z-H, Chen J-J. Limonoids from the Seeds of Swietenia macrophylla and Their Anti-Inflammatory Activities. Molecules. 2015; 20(10):18551-18564. https://doi.org/10.3390/molecules201018551
Chicago/Turabian StyleChen, Li-Chai, Hsiang-Ruei Liao, Pei-Yu Chen, Wen-Lung Kuo, Tsung-Hsien Chang, Ping-Jyun Sung, Zhi-Hong Wen, and Jih-Jung Chen. 2015. "Limonoids from the Seeds of Swietenia macrophylla and Their Anti-Inflammatory Activities" Molecules 20, no. 10: 18551-18564. https://doi.org/10.3390/molecules201018551