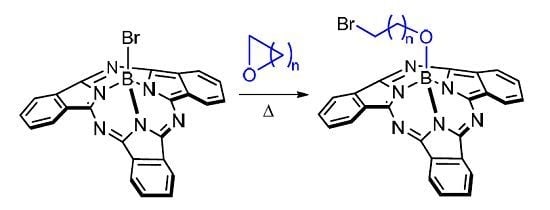
Ring Opening Reactions through C-O Bond Cleavage Uniquely Adding Chemical Functionality to Boron Subphthalocyanine
Abstract
:1. Introduction
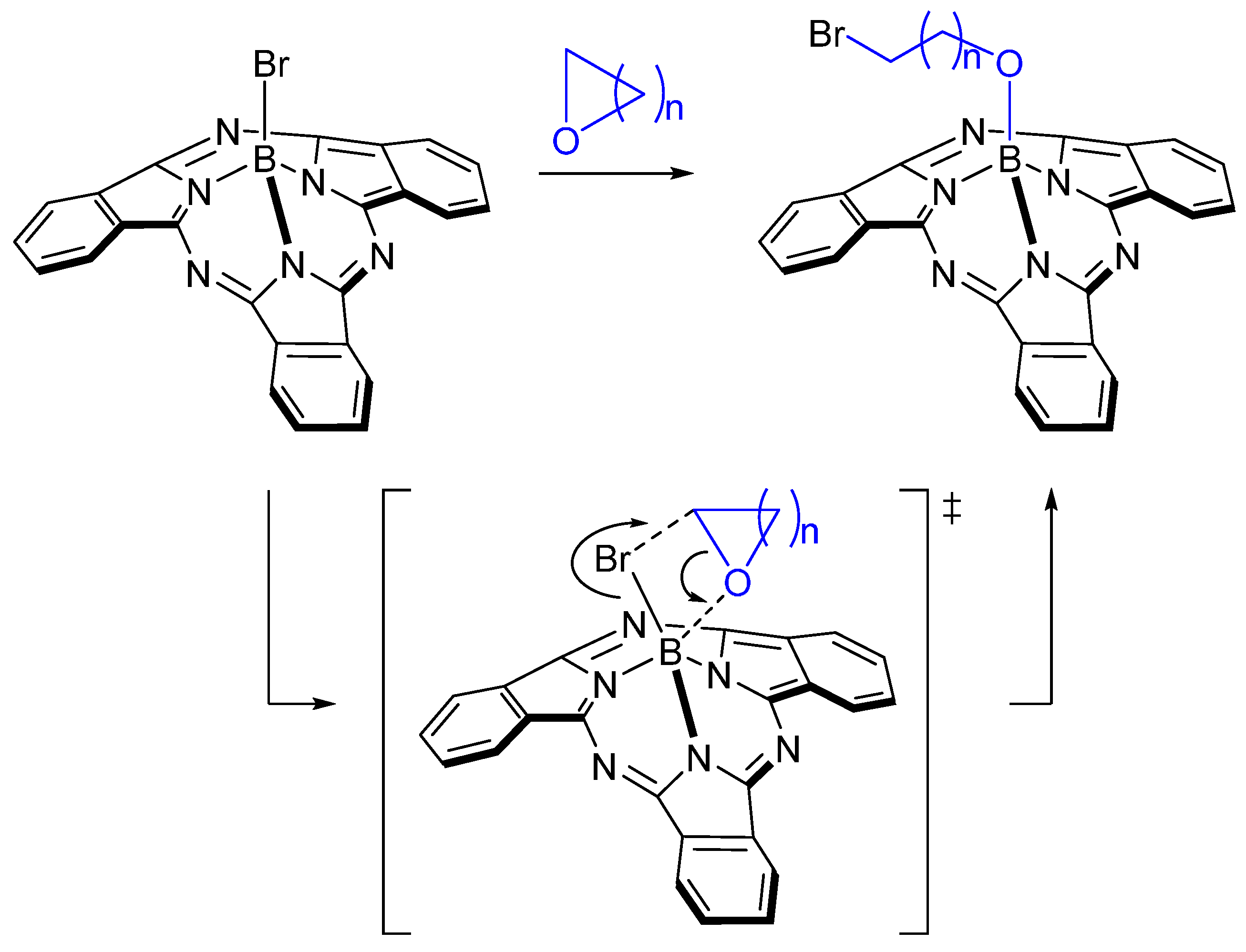
2. Results and Discussion
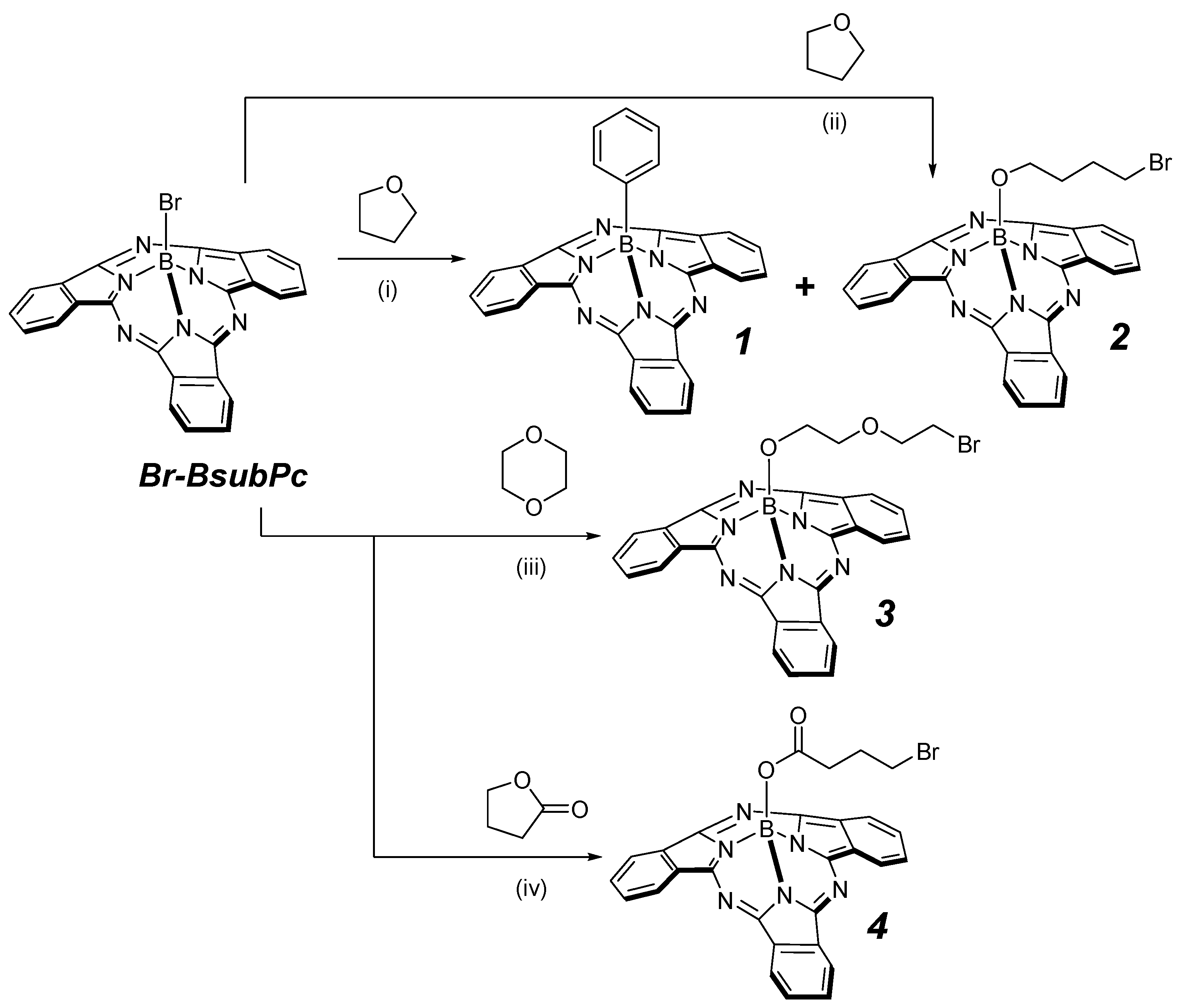
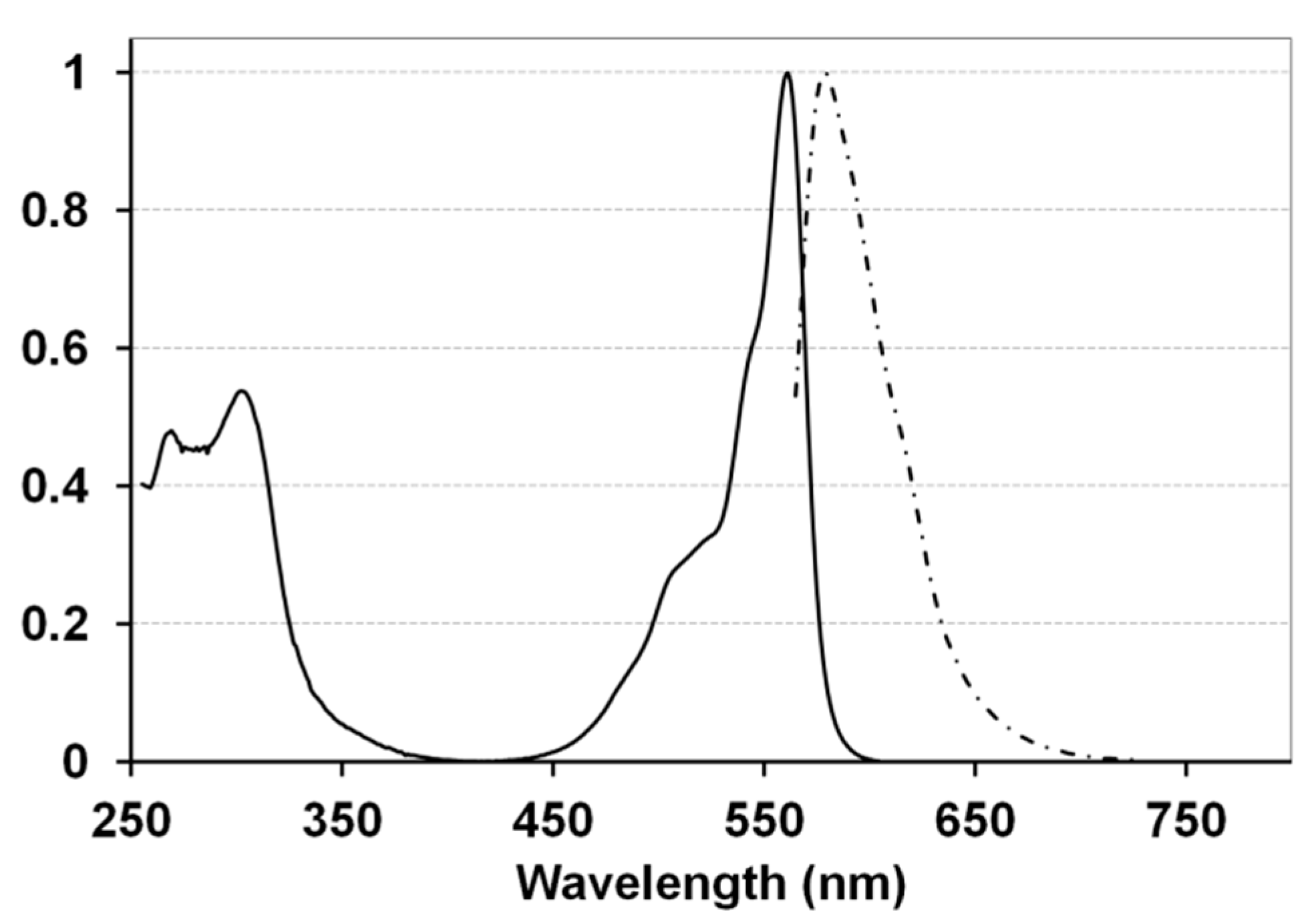
| Q band | Soret Band | Fluorescence | ||||
|---|---|---|---|---|---|---|
| λmax (nm) | Log ε | λmax (nm) | Log ε | λmax (nm) | ϕFL b | |
| 2 | 561 | 4.73 | 302 | 4.46 | 579 | 0.44 ± 0.05 |
| 3 | 561 | 4.94 | 303 | 4.61 | 581 | 0.45 ± 0.03 |
| 4 | 564 | 4.96 | 304 | 4.63 | 585 | 0.45 ± 0.04 |
3. Experimental Section
3.1. General Considerations
3.2. Syntheses
4. Conclusion
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Claessens, C.G.; González-Rodríguez, D.; Rodríguez-Morgade, M.S.; Medina, A.; Torres, T. Subphthalocyanines, subporphyrazines, and subporphyrins: Singular nonplanar aromatic systems. Chem. Rev. 2014, 114, 2192–2277. [Google Scholar] [CrossRef] [PubMed]
- Morse, G.E.; Bender, T.P. Boron subphthalocyanines as organic electronic materials. ACS Appl. Mater. Interfaces 2012, 4, 5055–5068. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, N.; Castrucci, J.S.; Sullivan, P.; Morse, G.E.; Paton, A.S.; Lu, Z.-H.; Bender, T.P.; Jones, T.S. Acceptor properties of boron subphthalocyanines in fullerene free photovoltaics. J. Phys. Chem. C 2014, 118, 14813–14823. [Google Scholar] [CrossRef]
- Castrucci, J.S.; Josey, D.S.; Thibau, E.; Lu, Z.-H.; Bender, T.P. Boron subphthalocyanines as triplet harvesting materials within organic photovoltaics. J. Phys. Chem. Lett. 2015, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- Josey, D.S.; Castrucci, J.S.; Dang, J.D.; Lessard, B.H.; Bender, T.P. Evaluating thiophene electron-donor layers for the rapid assessment of boron subphthalocyanines as electron acceptors in organic photovoltaics: Solution or vacuum deposition? ChemPhysChem 2015, 16, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Morse, G.E.; Gantz, J.L.; Steirer, K.X.; Armstrong, N.R.; Bender, T.P. Pentafluorophenoxy boron subphthalocyanine (F5BsubPc) as a multifunctional material for organic photovoltaics. ACS Appl. Mater. Interfaces 2014, 6, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Cnops, K.; Rand, B.P.; Cheyns, D.; Heremans, P. Enhanced photocurrent and open-circuit voltage in a 3-layer cascade organic solar cell. Appl. Phys. Lett. 2012, 101, 143301. [Google Scholar] [CrossRef]
- Cnops, K.; Rand, B.P.; Cheyns, D.; Verreet, B.; Empl, M.A.; Heremans, P. 8.4% Efficient fullerene-free organic solar cells exploiting long-range exciton energy transfer. Nat. Commun. 2014, 5, 3406. [Google Scholar] [CrossRef] [PubMed]
- Cnops, K.; Zango, G.; Genoe, J.; Heremans, P.; Martinez-Diaz, M.V.; Torres, T.; Cheyns, D. Energy level tuning of non-fullerene acceptors in organic solar cells. J. Am. Chem. Soc. 2015, 137, 8991–8997. [Google Scholar] [CrossRef] [PubMed]
- Verreet, B.; Cnops, K.; Cheyns, D.; Heremans, P.; Stesmans, A.; Zango, G.; Claessens, C.G.; Torres, T.; Rand, B.P. Decreased recombination through the use of a non-fullerene acceptor in a 6.4% efficient organic planar heterojunction solar cell. Adv. Energy Mater. 2014, 4, 1301413. [Google Scholar] [CrossRef]
- Mauldin, C.E.; Piliego, C.; Poulsen, D.; Unruh, D.A.; Woo, C.; Ma, B.; Mynar, J.L.; Fréchet, J.M.J. Axial thiophene-boron(subphthalocyanine) dyads and their application in organic photovoltaics. ACS Appl. Mater. Interfaces 2010, 2, 2833–2838. [Google Scholar] [CrossRef]
- Claessens, C.G.; González-Rodríguez, D.; McCallum, C.M.; Nohr, R.S.; Schuchmann, H.-P.; Torres, T. On the mechanism of boron-subphthalocyanine chloride formation. J. Porphyr. Phthalocyanines 2007, 11, 181–188. [Google Scholar] [CrossRef]
- Morse, G.E.; Helander, M.G.; Stanwick, J.; Sauks, J.M.; Paton, A.S.; Lu, Z.-H.; Bender, T.P. Experimentally validated model for the prediction of the homo and lumo energy levels of boronsubphthalocyanines. J. Phys. Chem. C 2011, 115, 11709–11718. [Google Scholar] [CrossRef]
- Paton, A.S.; Lough, A.J.; Bender, T.P. A role for π-Br interactions in the solid-state molecular packing of para-halo-phenoxy-boronsubphthalocyanines. CrystEngComm 2011, 13, 3653–3656. [Google Scholar] [CrossRef]
- Paton, A.S.; Morse, G.E.; Castelino, D.; Bender, T.P. Pseudohalides of boron subphthalocyanine. J. Org. Chem. 2012, 77, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Fulford, M.V.; Jaidka, D.; Paton, A.S.; Morse, G.E.; Brisson, E.R.L.; Lough, A.J.; Bender, T.P. Crystal structures, reaction rates, and selected physical properties of halo-boronsubphthalocyanines (halo = fluoride, chloride, and bromide). J. Chem. Eng. Data 2012, 57, 2756–2765. [Google Scholar] [CrossRef]
- Geyer, M.; Plenzig, F.; Rauschnabel, J.; Hanack, M.; Del Rey, B.; Sastre, A.; Torres, T. Subphthalocyanines: Preparation, reactivity, and physical properties. Synthesis 1996, 1139–1151. [Google Scholar] [CrossRef]
- Hanack, M.; Geyer, M. Synthesis and separation of structural isomers of tri-tert-butylsubphthalocyaninatophenylboron (III). J. Chem. Soc., Chem. Commun. 1994, 19, 2253–2254. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Mori, T. Subphthalocyanines having axial substituent with direct B-C bond: General preparation and physical properties. Chem. Lett. 2010, 39, 1108–1109. [Google Scholar] [CrossRef]
- Kipp, R.A.; Simon, J.; Beggs, M.; Ensley, H.E.; Schmehl, R.H. Photophysical and photochemical investigation of a dodecafluorosubphthalocyanine derivative. J. Phys. Chem. A 1998, 102, 5659–5664. [Google Scholar] [CrossRef]
- Bonnier, C.; Josey, D.S.; Bender, T.P. Aryl-substituted boron subphthalocyanines and their application in organic photovoltaics. Aust. J. Chem. 2015. [Google Scholar] [CrossRef]
- Tohyama, M.; Hirao, A.; Nakahama, S.; Takenaka, K. Synthesis of end-functionalized polymer by means of living anionic polymerization, 5. Syntheses of polystyrenes and polyisoprenes with hydroxy and mercapto end groups by reactions of the living polymers with haloalkanes containing silyl ether and silyl thioether functions. Macromol. Chem. Phys. 1996, 197, 3135–3148. [Google Scholar]
- Brown, H.C.; Ramachandran, P.V.; Chandrasekharan, J. Selective reductions. 56. Exploration of the B-haloisopinocampheylboranes for asymmetric reduction of ketones. Heteroat. Chem. 1995, 6, 117–131. [Google Scholar] [CrossRef]
- Heilmann, J.B.; Qin, Y.; Jäkle, F.; Lerner, H.-W.; Wagner, M. Boron-bridged poly(ferrocenylene)s as promising materials for nanoscale molecular wires. Inorg. Chimica Acta 2006, 359, 4802–4806. [Google Scholar] [CrossRef]
- Guilleme, J.; Gonzalez-Rodriguez, D.; Torres, T. Triflate-subphthalocyanines: Versatile, reactive intermediates for axial functionalization at the boron atom. Angew. Chem. Int. Ed. 2011, 50, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Pyrrolic macrocycles with stabilized triplet states: Metal-centered and ligand-centered separation of unpaired electrons. Polyhedron 2012, 42, 249–257. [Google Scholar] [CrossRef]
- Yang, Y. Electron localization in nonplanar conjugated macrocycles: Failure of electron buffers to delocalize electrons. Chem. Phys. Lett. 2011, 516, 268–271. [Google Scholar] [CrossRef]
- Guilleme, J.; Martínez-Fernández, L.; González-Rodríguez, D.; Corral, I.; Yáñez, M.; Torres, T. An insight into the mechanism of the axial ligand exchange reaction in boron subphthalocyanine macrocycles. J. Am. Chem. Soc. 2014, 136, 14289–14298. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, X.-J.; Chan, E.Y.M.; Fong, W.-P.; Ng, D.K.P. Synthesis, photophysical properties and in vitro photodynamic activity of axially substituted subphthalocyanines. Org. Biomol. Chem. 2007, 5, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, D.; Torres, T.; Guldi, D.M.; Rivera, J.; Herranz, M.Á.; Echegoyen, L. Subphthalocyanines: Tuneable molecular scaffolds for intramolecular electron and energy transfer processes. J. Am. Chem. Soc. 2004, 126, 6301–6313. [Google Scholar] [CrossRef] [PubMed]
- Fery-Forgues, S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Education 1999, 76. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compoundsare not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnier, C.; Bender, T.P. Ring Opening Reactions through C-O Bond Cleavage Uniquely Adding Chemical Functionality to Boron Subphthalocyanine. Molecules 2015, 20, 18237-18245. https://doi.org/10.3390/molecules201018237
Bonnier C, Bender TP. Ring Opening Reactions through C-O Bond Cleavage Uniquely Adding Chemical Functionality to Boron Subphthalocyanine. Molecules. 2015; 20(10):18237-18245. https://doi.org/10.3390/molecules201018237
Chicago/Turabian StyleBonnier, Catherine, and Timothy P. Bender. 2015. "Ring Opening Reactions through C-O Bond Cleavage Uniquely Adding Chemical Functionality to Boron Subphthalocyanine" Molecules 20, no. 10: 18237-18245. https://doi.org/10.3390/molecules201018237