Optimization of Astilbin Extraction from the Rhizome of Smilax glabra, and Evaluation of Its Anti-Inflammatory Effect and Probable Underlying Mechanism in Lipopolysaccharide-Induced RAW264.7 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Box-Behnken Design Analysis
| Run | Extract Time (A, min) | Ethanol Concentration (B, %) | Temperature (C, °C) | Liquid-Solid Ratio (D, mL/g) | EYA (mg/g) | |
|---|---|---|---|---|---|---|
| Observed | Predicted | |||||
| 1 | 40(1) | 55(0) | 65(0) | 10(−1) | 12.25 | 12.13 |
| 2 | 40(1) | 55(0) | 65(0) | 30(1) | 14.35 | 14.26 |
| 3 | 40(1) | 60(1) | 65(0) | 20(0) | 13.91 | 14.00 |
| 4 | 40(1) | 55(0) | 75(1) | 20(0) | 14.40 | 14.22 |
| 5 | 10(−1) | 55(0) | 55(−1) | 20(0) | 13.60 | 13.75 |
| 6 | 25(0) | 55(0) | 55(−1) | 10(−1) | 12.04 | 12.06 |
| 7 | 10(−1) | 55(0) | 65(0) | 30(1) | 14.06 | 13.99 |
| 8 | 25(0) | 55(0) | 65(0) | 20(0) | 14.17 | 14.39 |
| 9 | 25(0) | 55(0) | 65(0) | 20(0) | 14.50 | 14.39 |
| 10 | 25(0) | 55(0) | 75(1) | 10(−1) | 12.80 | 12.81 |
| 11 | 25(0) | 50(−1) | 65(0) | 10(−1) | 12.61 | 12.73 |
| 12 | 25(0) | 55(0) | 75(1) | 30(1) | 14.20 | 14.40 |
| 13 | 25(0) | 55(0) | 55(−1) | 30(1) | 14.05 | 14.27 |
| 14 | 40(1) | 50(−1) | 65(0) | 20(0) | 12.52 | 12.66 |
| 15 | 10(−1) | 50(−1) | 65(0) | 20(0) | 14.04 | 14.17 |
| 16 | 40(1) | 55(0) | 55(−1) | 20(0) | 12.87 | 13.03 |
| 17 | 25(0) | 50(−1) | 55(−1) | 20(0) | 13.78 | 13.46 |
| 18 | 25(0) | 60(1) | 65(0) | 10(−1) | 11.47 | 11.54 |
| 19 | 25(0) | 50(−1) | 65(0) | 30(1) | 13.76 | 13.66 |
| 20 | 10(−1) | 55(1) | 65(0) | 20(0) | 12.32 | 12.41 |
| 21 | 25(0) | 50(−1) | 75(1) | 20(0) | 13.76 | 13.79 |
| 22 | 25(0) | 60(1) | 55(−1) | 20(0) | 13.37 | 13.14 |
| 23 | 25(0) | 55(0) | 65(0) | 20(0) | 14.64 | 14.39 |
| 24 | 25(0) | 60(1) | 65(0) | 30(1) | 14.57 | 14.42 |
| 25 | 25(0) | 55(0) | 65(0) | 20(0) | 14.31 | 14.39 |
| 26 | 25(0) | 55(0) | 65(0) | 20(0) | 14.33 | 14.39 |
| 27 | 10(−1) | 55(0) | 75(1) | 20(0) | 13.62 | 13.43 |
| 28 | 25(0) | 60(1) | 75(1) | 20(0) | 13.55 | 13.68 |
| 29 | 10(−1) | 55(0) | 65(0) | 10(−1) | 12.43 | 12.32 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value (prob>) | Remarks |
|---|---|---|---|---|---|---|
| Sequntial model sum of squares | ||||||
| Mean | 5306.33 | 1 | 5306.33 | |||
| Linear | 11.52 | 4 | 2.88 | 6.89 | 0.0008 | |
| 2FI | 4.10 | 6 | 0.68 | 2.07 | 0.1086 | |
| Quadratic | 5.29 | 4 | 1.32 | 28.19 | <0.0001 | Suggested |
| Cubic | 0.35 | 8 | 0.043 | 0.84 | 0.6032 | Aliased |
| Residual | 0.31 | 6 | 0.052 | |||
| Total | 5327.90 | 29 | 183.72 | |||
| Lack of fit tests | ||||||
| Linear | 9.91 | 20 | 0.50 | 14.90 | 0.0089 | |
| 2FI | 5.81 | 14 | 0.42 | 12.48 | 0.0128 | |
| Quadratic | 0.52 | 10 | 0.052 | 1.57 | 0.3510 | Suggested |
| Cubic | 0.18 | 2 | 0.089 | 2.66 | 0.1839 | Aliased |
| Pure error | 0.13 | 4 | 0.033 | |||
| Model summary statistics | ||||||
| Source | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | Press | Remarks |
| Linear | 0.65 | 0.5344 | 0.4568 | 0.3588 | 13.83 | |
| 2FI | 0.57 | 0.7244 | 0.5712 | 0.4870 | 11.06 | |
| Quadratic | 0.22 | 0.9696 | 0.9391 | 0.8505 | 3.22 | Suggested |
| Cubic | 0.23 | 0.9856 | 0.9329 | -0.1926 | 25.72 | Aliased |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value (prob>) |
|---|---|---|---|---|---|
| Model | 20.91 | 14 | 1.49 | 31.85 | <0.0001 |
| A | 4.408 × 10−3 | 1 | 4.408 × 10−3 | 0.094 | 0.7637 |
| B | 0.14 | 1 | 0.14 | 2.91 | 0.1100 |
| C | 0.57 | 1 | 0.57 | 12.20 | 0.0036 |
| D | 10.81 | 1 | 10.81 | 230.54 | <0.0001 |
| AB | 2.42 | 1 | 2.42 | 51.56 | <0.0001 |
| AC | 0.57 | 1 | 0.57 | 12.16 | 0.0036 |
| AD | 0.055 | 1 | 0.055 | 1.18 | 0.2962 |
| BC | 0.010 | 1 | 0.010 | 0.21 | 0.6513 |
| BD | 0.95 | 1 | 0.95 | 20.27 | 0.0005 |
| CD | 0.093 | 1 | 0.093 | 1.98 | 0.1808 |
| A2 | 1.59 | 1 | 1.59 | 34.01 | <0.0001 |
| B2 | 2.22 | 1 | 2.22 | 47.27 | <0.0001 |
| C2 | 0.53 | 1 | 0.53 | 11.40 | 0.0045 |
| D2 | 3.35 | 1 | 3.35 | 71.37 | <0.0001 |
| Residual | 0.66 | 14 | 0.047 | ||
| Lack of fit | 0.52 | 10 | 0.052 | 1.57 | 0.3510 |
| Pure error | 0.13 | 4 | 0.033 | ||
| Cor toal | 21.56 | 28 |
2.2. Optimization of Extraction Conditions of Astilbin
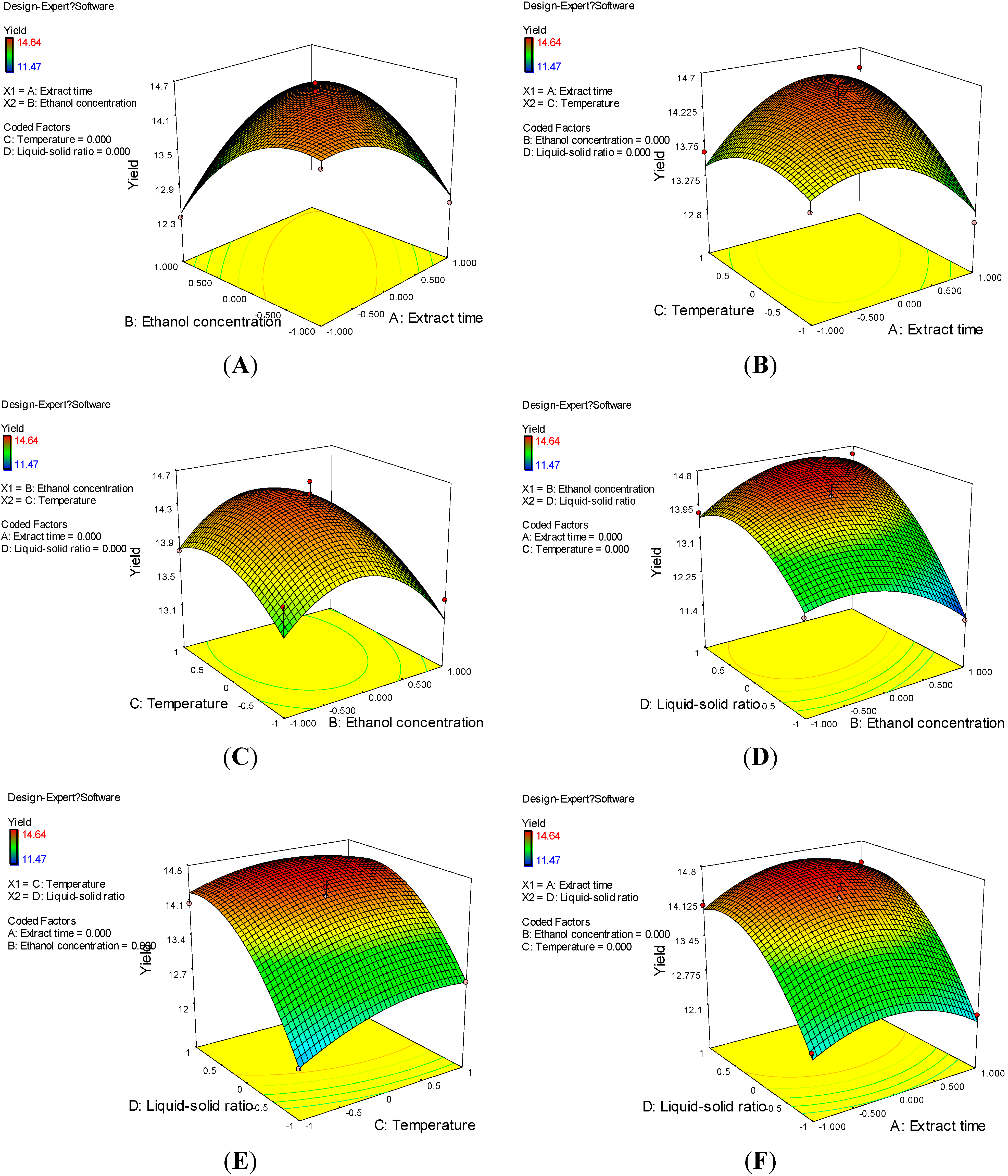
2.3. Verification of Predictive Model
2.4. Effects of Astilbin on RAW264.7 Cell Viability and Production of Pro-Inflammatory Mediators
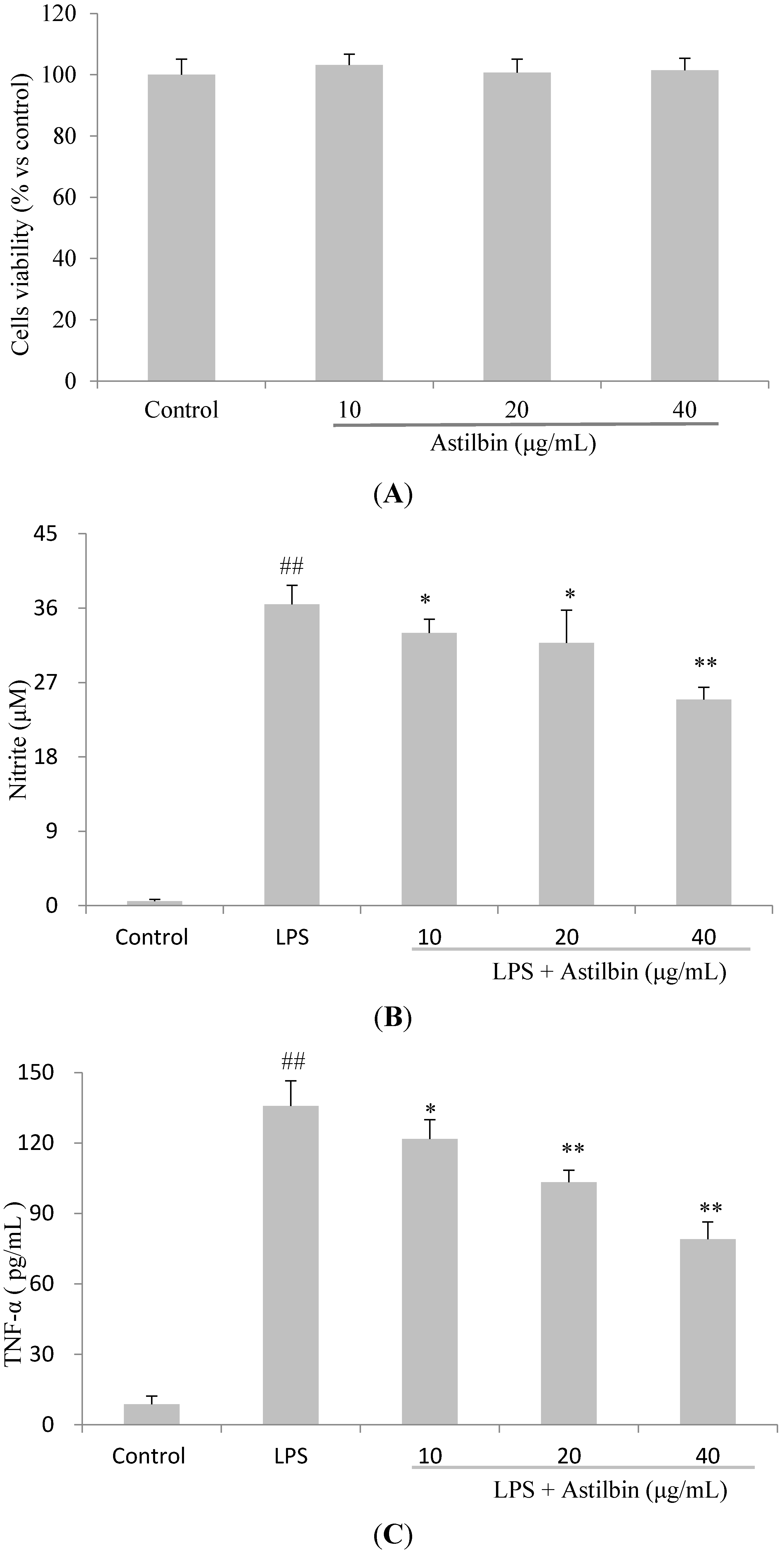
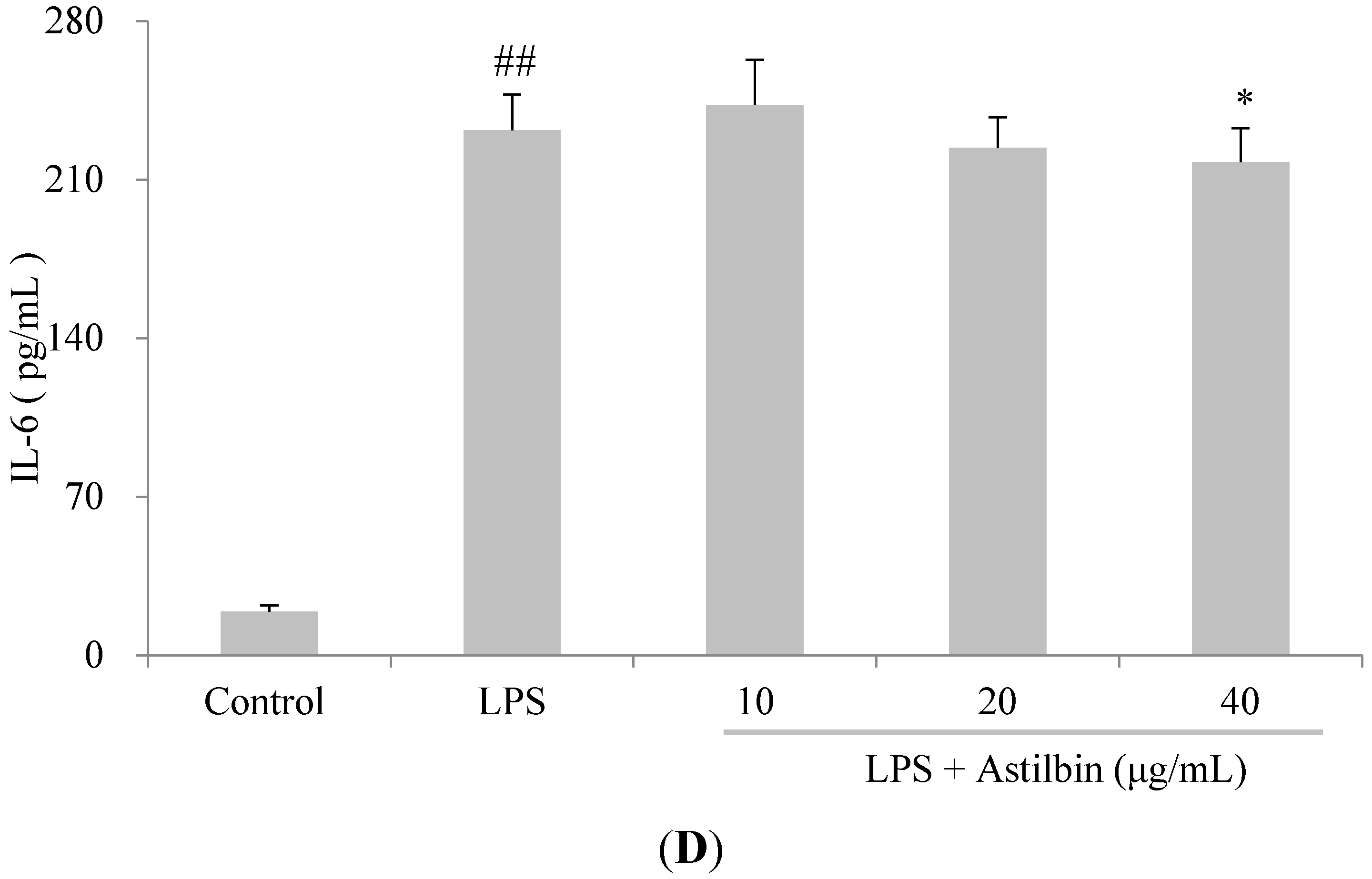


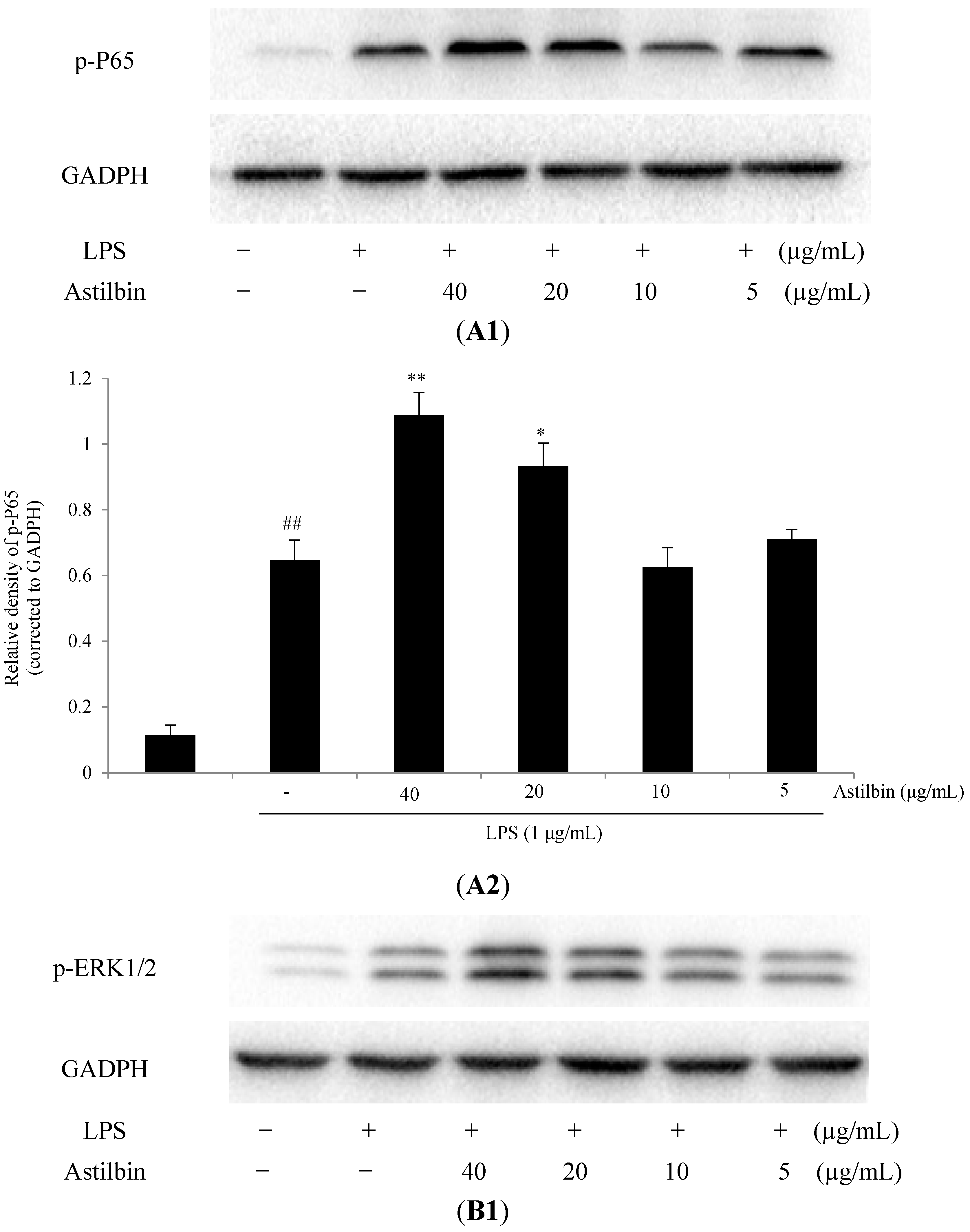
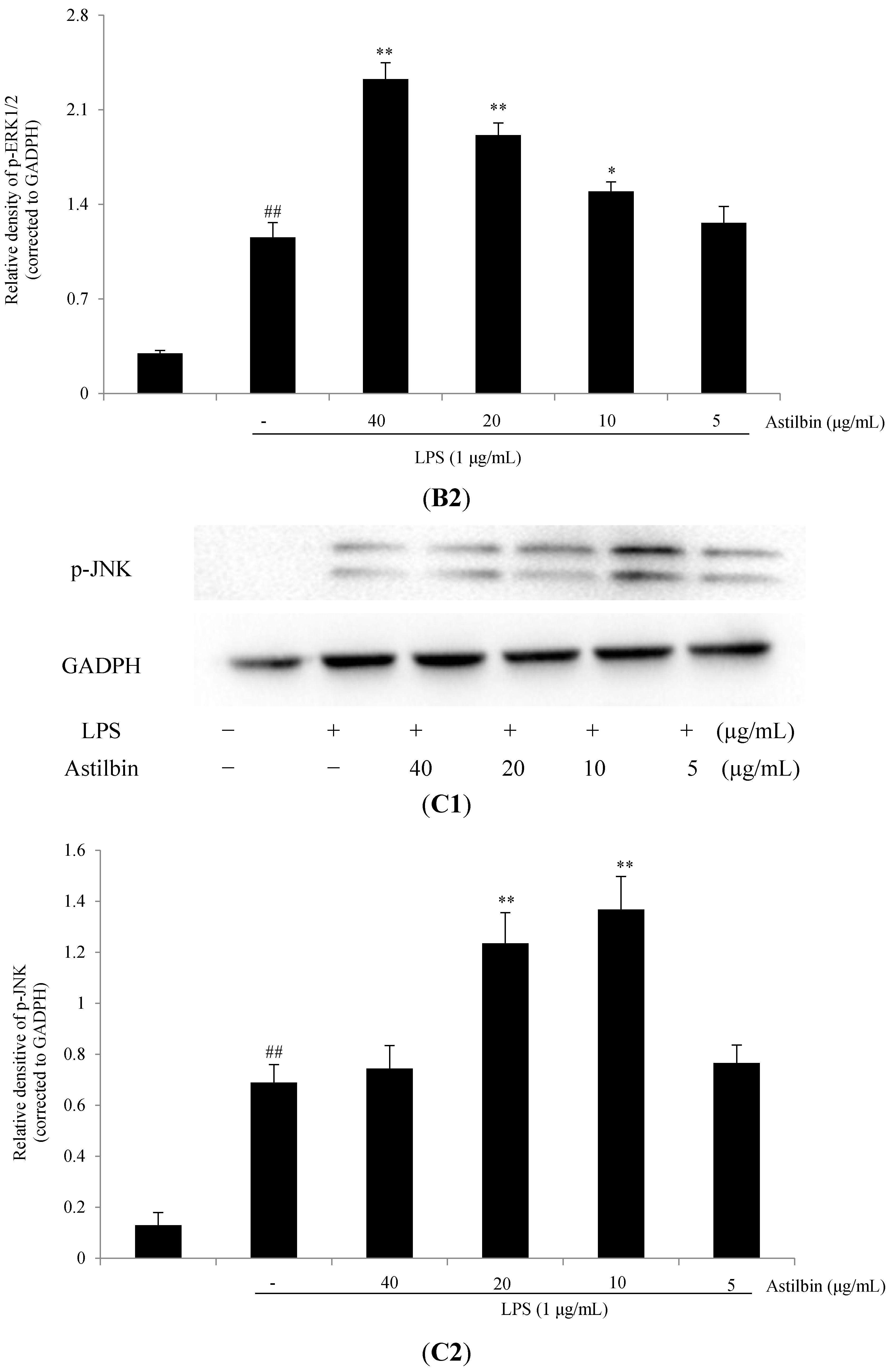
2.5. Effects of Astilbin on NF-κB and MAPKs Pathways

3. Materials and Methods
3.1. Plant & Materials
3.2. Extraction Procedure
3.3. Determination of Astilbin
3.4. Experimental Design
3.5. Determination of the Anti-Inflammatory Efficiency of Astilbin
3.5.1. Cell Culture and Treatment
3.5.2. Cell Viability Assay
3.5.3. Measurement of Nitric Oxide Production
3.5.4. Determination of Production of Pro-Inflammation Cytokines
3.5.5. RNA Extractions and Quantitative Real-Time PCR
3.5.6. Western Blot Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.; Zong, C.; Gao, Y.; Cai, R.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264.7 cell. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Kim, D.H.; Hwang, J.Y.; Yun, N.Y.; Lee, Y.H.; Han, S.B.; Hwang, B.Y.; Lee, M.S.; Jeong, H.S.; Hong, J.T. Anti-inflammatory effect of 4′-O-(threo-β-guaiacylglyceryl) ether, a novel flavonolignan compound isolated from Njavara on in RAW264.7 cells and in ear mice edema. Toxicol. Appl. Pharmacol. 2014, 277, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Tae, N.; Lee, J.J.; Kim, T.; Lee, J.H. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264.7 cells by inducing proteasomal degradation of TRAF6. Eur. J. Pharmacol. 2010, 636, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Moon, J.Y.; Song, G.; Lee, Y.K.; Han, M.S.; Lee, J.S.; Ihm, B.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW264.7 macrophages. Food Chem. Toxicol. 2010, 48, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ouchi, K. Glycosidic structure of astilbin. Misc. Rep. Res. Inst. Nat. Resources 1952, 26, 22–26. [Google Scholar]
- Fouad, H.A.; Faroni, L.R.D.; Tavaes, W.D.; Ribeiro, R.C.; Freitas, S.D.; Zanuncio, J.C. Botanical extracts of plants from the Brazilian Cerrado for the integrated management of sitotroga cerealella (Lepidoptera: Gelechiidas) in stored grain. J. Stored Prod. Res. 2014, 57, 6–11. [Google Scholar] [CrossRef]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A new cinnamoylglycoflavonoid, antimycodacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Q.; Cheng, Z.H.; Shi, H.M.; Xin, W.B.; Wang, T.T.Y.; Yu, L.L. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J. Agric. Food Chem. 2011, 59, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.B.; Huang, H.Q.; Yu, L.; Shi, H.M.; Sheng, Y.; Wang, T.T.Y.; Yu, L.L. Three new flavanonol glycosides from leaves of Engelhardtia roxburghiana, and their anti-inflammation, antiproliferative and antioxidant properties. Food Chem. 2012, 132, 788–798. [Google Scholar] [CrossRef]
- Xu, W.A.; Yin, L.; Pan, H.Y.; Shi, L.; Xu, L.; Zhang, X.; Duan, J.A. Study on the correlation between constituents detected in serum from Rhizoma Smilacis Glabrae and the reduction of uric acid levels in hyperuricemia. J. Ethnopharmacol. 2013, 150, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Guo, Y.X.; Shangguan, X.C.; Zheng, G.D.; Wang, W.J. Antioxidant and anti-proliferative activity of Rhizoma Smilacis Chinae extracts and main constituents. Food Chem. 2012, 133, 140–145. [Google Scholar] [CrossRef]
- Mecchi, M.C.; Lago, J.H.G. Chemical constituents derived from Drimys Brasiliensis Miers (Winteraceae). Nat. Prod. Res. 2013, 27, 1927–1929. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, G.P.; Gois, R.W.D.; de Brito, T.S.; de Lima, F.J.; Bandeira, M.A.M.; Romero, N.P.; Magalhaes, P.J.; Santiago, G.M. Phytochemical study guided by the myorelaxant activity of the crude extract, fractions and constituent from stem bark of Hymenaea courbaril L. J. Ethnopharmacol. 2013, 149, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Dimech, G.S.; Soares, L.A.L.; Ferreira, M.A.; de Oliveira, A.G.V.; Carvalho, M.D.; Ximenes, E.A. Phytochemical and antibacterial investigations of the extracts and fractions from the stem bark of Hymenaea stigonocarpa Mart. Ex Hayne and effect on ultrastructure of staphylococcus aureus induced by hydroalcoholic extract. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Li, Y.P.; Li, Y.H.; Zhong, J.D.; Li, R.T. Antioxidant phenolic glycoside and flavonoids from Pieris japonica. J. Asian Nat. Prod. Res. 2013, 15, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Xu, Q. Astilbin prevents concanavalin A-induced liver injury by reducing TNF-α production and T lymphocyte adhesion. J. Pharm. Pharmacol. 2004, 56, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lu, W.Y.; Niu, Y.G.; Liu, J.; Zhang, X.W.; Gao, B.Y.; Akoh, C.C.; Shi, H.M.; Yu, L.L. Identification and quantification of phytochemical composition and anti-inflammatory, cellular antioxidant, and radical scavenging activities of 12 plantago species. J. Agric. Food Chem. 2013, 61, 6693–6702. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, H.Q.; Yu, L.L.; Wang, T.T.Y. Utility of hesperidinase for food function research: Enzymatic digestion of botanical extracts alters cellular antioxidant capacities and anti-inflammatory properties. J. Agric. Food Chem. 2014, 62, 8640–8647. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, T.; Xu, Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm. Res. 2003, 52, 334–340. [Google Scholar] [CrossRef]
- Li, G.S.; Jiang, W.L.; Yue, X.D.; Qu, G.W.; Tian, J.W.; Wu, J.; Fu, F.H. Effect of astilbin on experimental diabetic nephropathy in vivo and in vitro. Planta Med. 2009, 75, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Eikani, M.H.; Golmohammad, F.; Amoli, H.S.; Sadr, Z.B. An experimental design approach for pressurized liquid extraction from cardamom seeds. Sep. Sci. Technol. 2013, 48, 1194–1200. [Google Scholar] [CrossRef]
- Rai, C.; Majumdar, G.C.; De, S. Optimization of process parameters for water extraction of stevioside using response surface methodology. Sep. Sci. Technol. 2012, 47, 1014–1022. [Google Scholar] [CrossRef]
- Kwon, O.K.; Lee, M.Y.; Yuk, J.E.; Oh, S.R.; Chin, Y.W.; Lee, H.K.; Ahn, K.S. Anti-inflammatory effects of methanol extracts of the root of lilium lancifolium on LPS-stimulated RAW264.7 cells. J. Ethnopharmacol. 2010, 130, 28–34. [Google Scholar] [CrossRef]
- Lee, K.C.; Chang, H.H.; Chung, Y.H.; Lee, T.Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT2-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Shimomura-Shimizu, M.; Sugiyama, K.I.; Muroi, M.; Tanamoto, K.I. Alachlor and carbaryl suppress lipopolysaccharide-induced iNOS expression by differentially inhibiting NF-κB activation. Biochem. Biophys. Res. Commun. 2005, 332, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Liang, Y.; Deng, B.; Qiao, A.H.; Wu, K.Y.; Xiao, W.M.; Gong, W. Induction of TGF-beta and IL-10 production in dendritic cells using astilbin to inhibit dextran sulfate sodium-induced colitis. Biochem. Biophys. Res. Commun. 2014, 446, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ruangnoo, S.; Jaiaree, N.; Makchuchit, S.; Panthong, S.; Thongdeeying, P.; Itharat, A. An in vitro in vitro inhibitory effect on RAW264.7 cells by anti-inflammatory compounds from Smilax corbularia Kunth. Asian Pac. J. Allergy Immunol. 2012, 30, 268–274. [Google Scholar] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Karin, M. The two NF-kappa B activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Rimoldi, M.; Raes, G.; Brys, L.; Ghezzi, P.; Di Liberto, D.; Dieli, F.; Ghisletti, S.; Natoli, G.; de Baetselier, P.; et al. Tolerance and M2 (alternative) macrophages polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl. Acad. Sci. USA 2009, 106, 14978–14983. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Li, Y.M.; Fu, G.Q.; Yang, L.; Jiang, J.G.; Zhu, L.; Lin, F.L.; Chen, J.; Lin, Q.S. Extraction optimization of daphnoretin from root bark of Wikstroemia indica (L.) C.A. and its anti-tumor activity test. Food Chem. 2011, 124, 1500–1506. [Google Scholar] [CrossRef]
- Kim, Y.; Han, J.; Sung, J.; Sung, M.; Choi, Y.; Jeong, H.S.; Lee, J. Anti-inflammatory activity of Chrysanthemum zawadskii var. latilobum leaf extract through haem oxygenase-1 induction. J. Funct. Foods 2012, 4, 474–479. [Google Scholar] [CrossRef]
- Yang, D.J.; Lin, J.T.; Chen, Y.C.; Liu, S.C.; Lu, F.J.; Chang, T.J.; Wang, M.; Lin, H.W.; Chang, Y.Y. Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264.7 cells via NF-kappa B and JNK inactivation. J. Funct. Foods 2013, 5, 607–615. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound astilbin is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-L.; Zhu, Y.-F.; Hu, M.-M.; Wang, D.-M.; Xu, X.-J.; Lu, C.-J.; Zhu, W. Optimization of Astilbin Extraction from the Rhizome of Smilax glabra, and Evaluation of Its Anti-Inflammatory Effect and Probable Underlying Mechanism in Lipopolysaccharide-Induced RAW264.7 Macrophages. Molecules 2015, 20, 625-644. https://doi.org/10.3390/molecules20010625
Lu C-L, Zhu Y-F, Hu M-M, Wang D-M, Xu X-J, Lu C-J, Zhu W. Optimization of Astilbin Extraction from the Rhizome of Smilax glabra, and Evaluation of Its Anti-Inflammatory Effect and Probable Underlying Mechanism in Lipopolysaccharide-Induced RAW264.7 Macrophages. Molecules. 2015; 20(1):625-644. https://doi.org/10.3390/molecules20010625
Chicago/Turabian StyleLu, Chuan-Li, Yan-Fang Zhu, Meng-Mei Hu, Dong-Mei Wang, Xiao-Jie Xu, Chuan-Jian Lu, and Wei Zhu. 2015. "Optimization of Astilbin Extraction from the Rhizome of Smilax glabra, and Evaluation of Its Anti-Inflammatory Effect and Probable Underlying Mechanism in Lipopolysaccharide-Induced RAW264.7 Macrophages" Molecules 20, no. 1: 625-644. https://doi.org/10.3390/molecules20010625
APA StyleLu, C.-L., Zhu, Y.-F., Hu, M.-M., Wang, D.-M., Xu, X.-J., Lu, C.-J., & Zhu, W. (2015). Optimization of Astilbin Extraction from the Rhizome of Smilax glabra, and Evaluation of Its Anti-Inflammatory Effect and Probable Underlying Mechanism in Lipopolysaccharide-Induced RAW264.7 Macrophages. Molecules, 20(1), 625-644. https://doi.org/10.3390/molecules20010625





