Cucurbitane Glycosides Derived from Mogroside IIE: Structure-Taste Relationships, Antioxidant Activity, and Acute Toxicity
Abstract
:1. Introduction
2. Results and Discussion
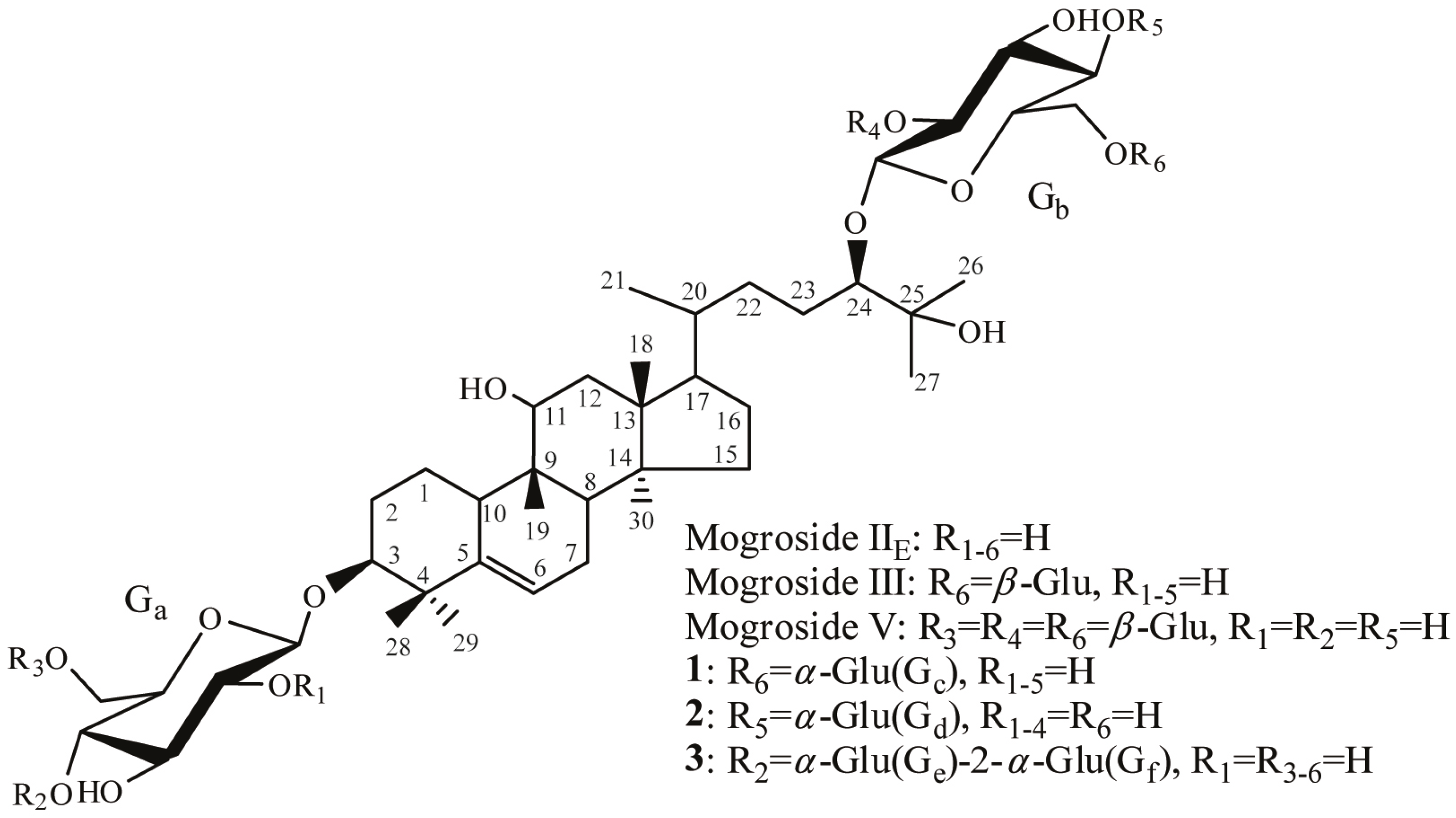
2.1. Transglycosylation of Mogroside IIE with CGTases
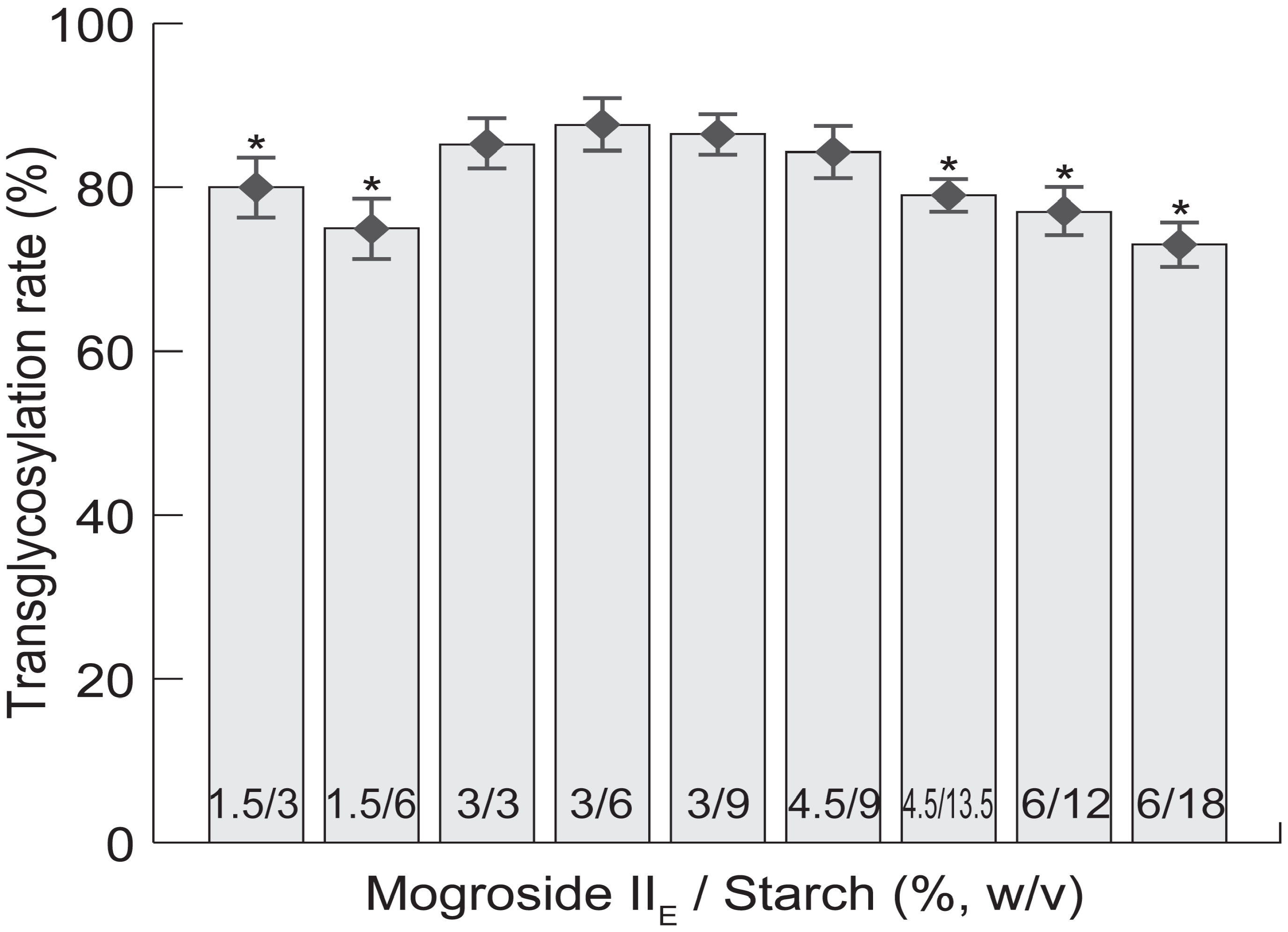
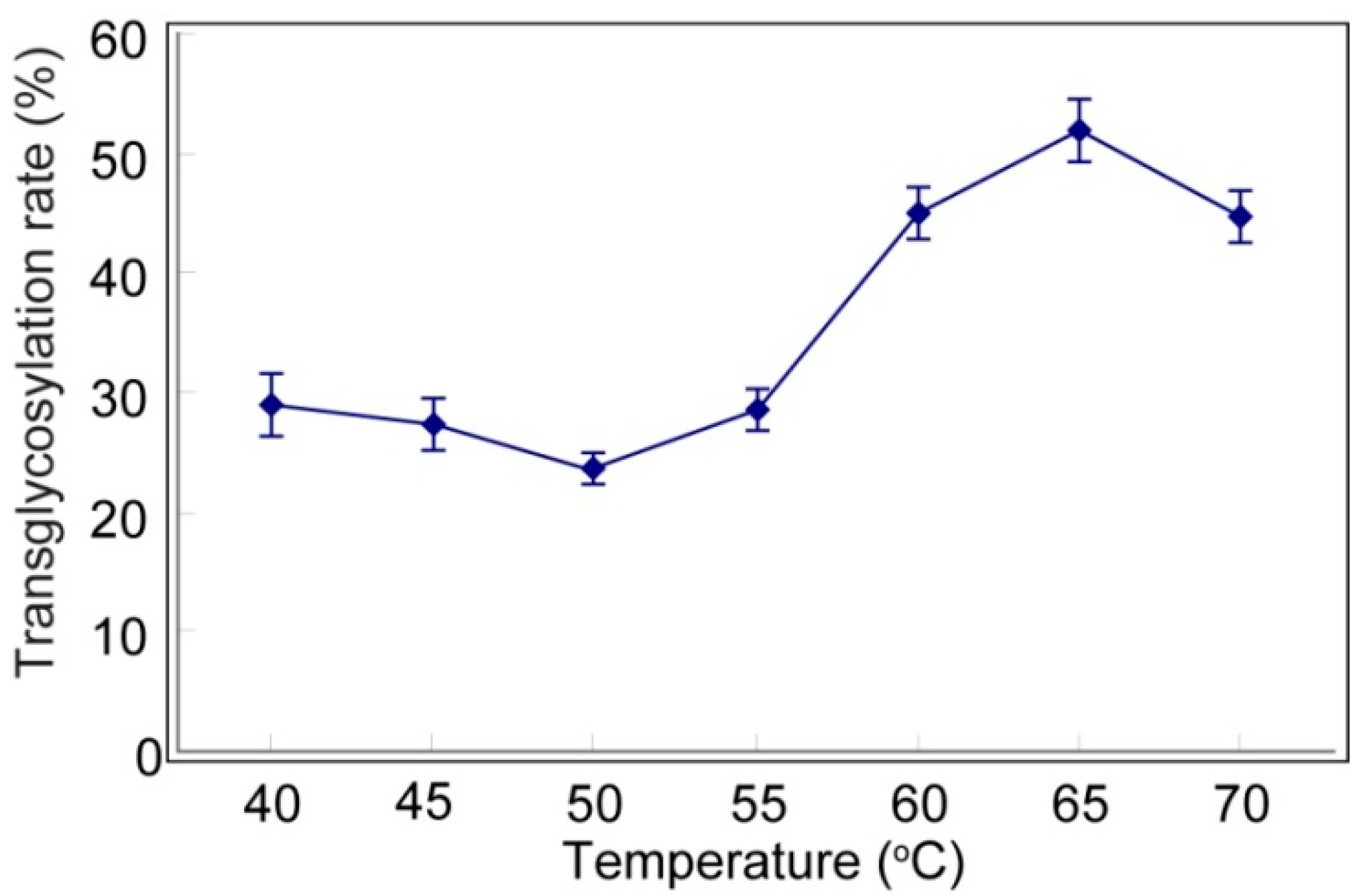
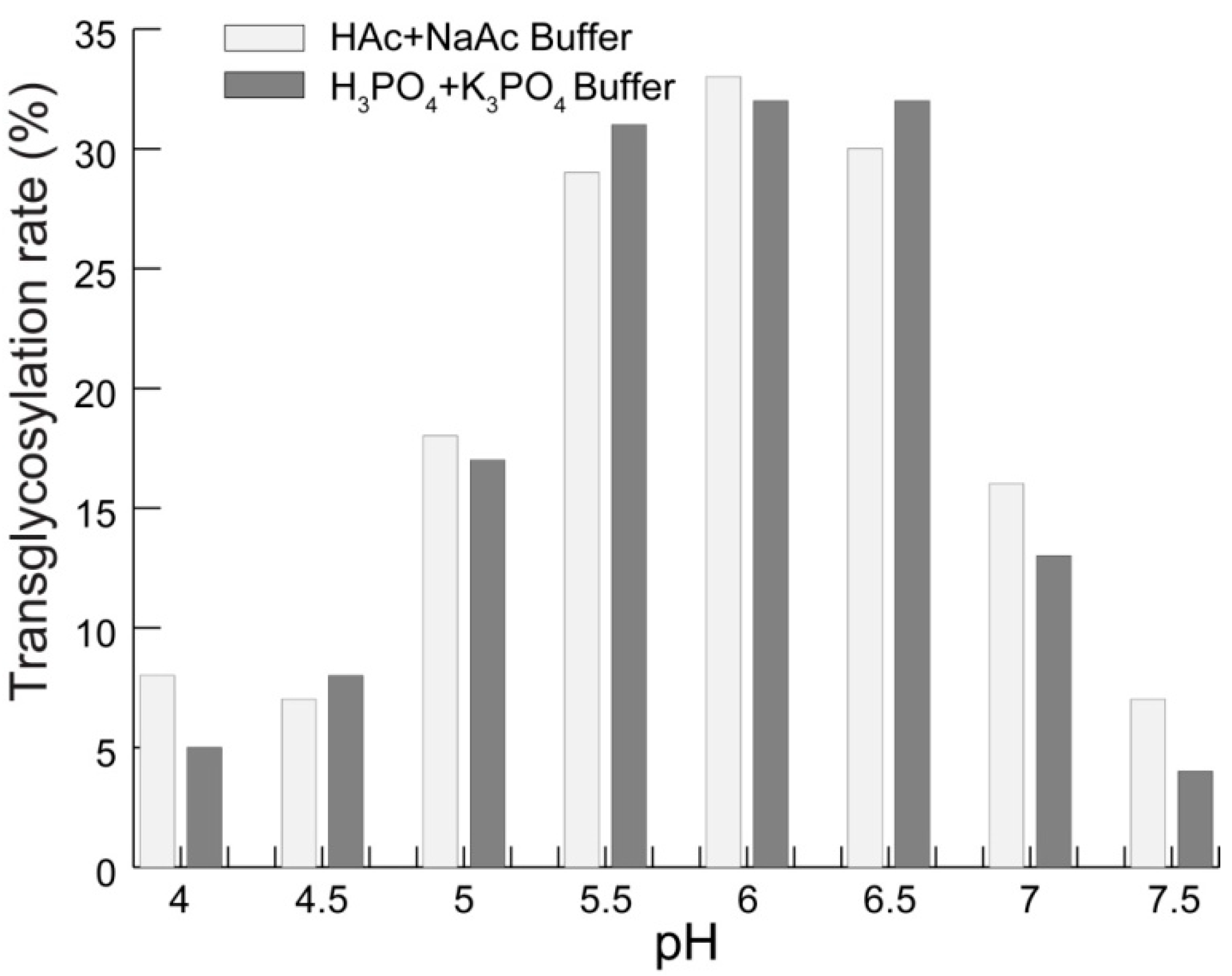
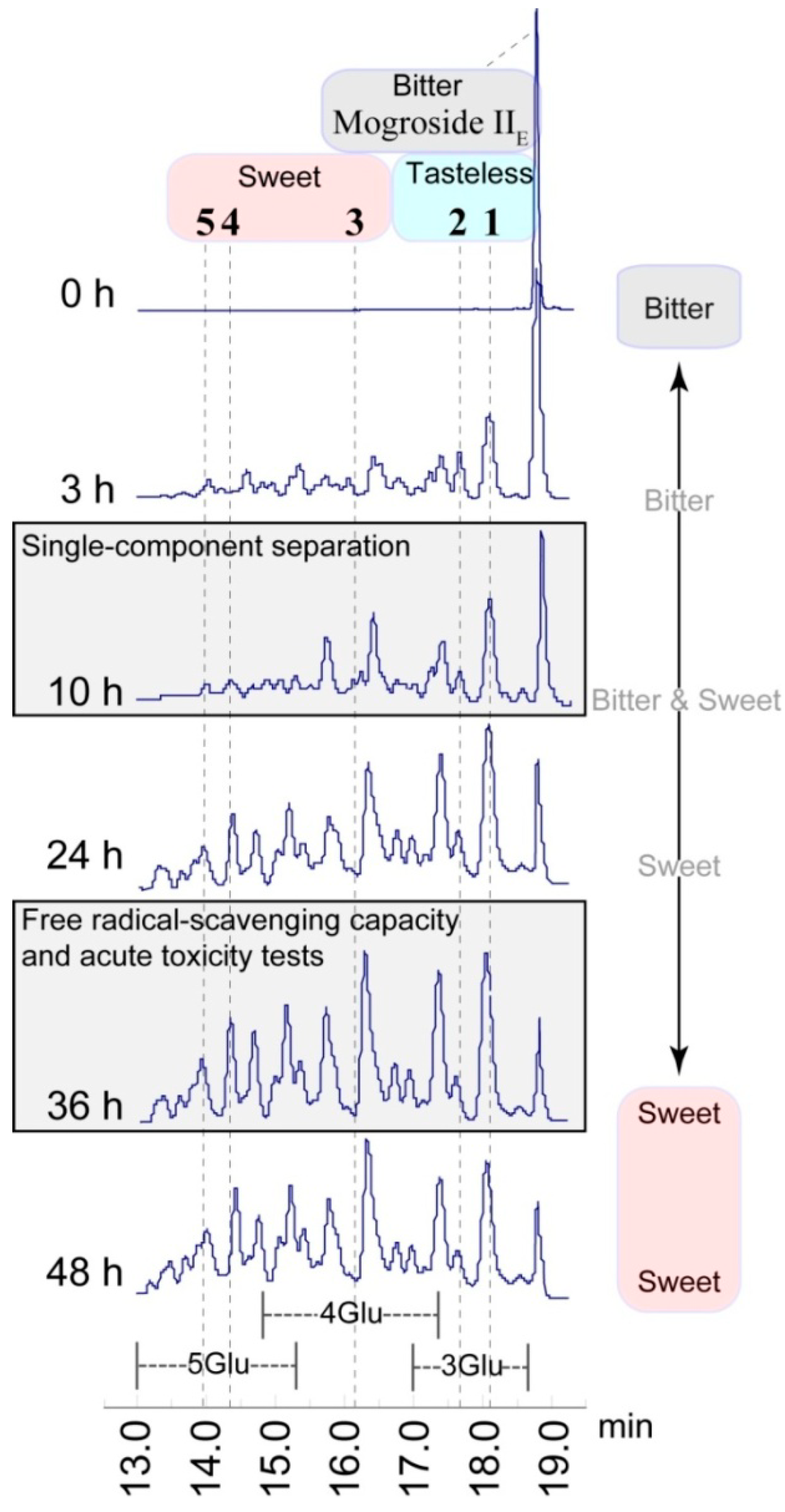
2.2. Structure-Taste Relationships
2.3. Free Radical Scavenging Capacity
| Tests | MSP | Mogroside V |
|---|---|---|
| Hydroxyl Radicals | 0.202 | 0.139 |
| Superoxide Radicals | 1.260 | 0.551 |
| DPPH | 0.954 | 0.433 |
2.4. Acute Toxicity Tests
| Parameters | Treated Group | Control Group | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| ALT (U/L) | 30.1 ± 4.9 | 25.6 ± 3.1 | 27.3 ± 3.6 | 26.0 ± 2.7 |
| AST (U/L) | 55.8 ± 7.2 | 59.4 ± 5.5 | 52.3 ± 6.1 | 56.1 ± 6.5 |
| CRE (mmol/L) | 42.7 ± 10.4 | 37.9 ± 8.3 | 43.8 ± 9.9 | 39.6 ± 10.0 |
| BUN (mmol/L) | 6.5 ± 1.2 | 6.8 ± 1.5 | 6.3 ± 1.8 | 6.6 ± 1.7 |
| Liver Weight (g) | 2.92 ± 0.087 | 1.55 ± 0.051 | 2.93 ± 0.107 | 1.57 ± 0.079 |
| Kidney Weight (g) | 0.60 ± 0.022 | 0.34 ± 0.032 | 0.58 ± 0.048 | 0.34 ± 0.035 |
| Spleen Weight (g) | 0.09 ± 0.004 | 0.10 ± 0.003 | 0.10 ± 0.006 | 0.10± 0.004 |
| Body Weight at the Beginning (g) | 29.5 ± 2.7 | 24.1 ± 2.6 | 29.5 ± 2.6 | 24.0 ± 2.8 |
| Body Weight at the End (g) | 32.7 ± 5.1 | 27.6 ± 6.3 | 32.5 ± 6.0 | 28.0 ± 5.9 |
3. Experimental Section
3.1. Chemicals and Materials
3.2. General Procedures
3.3. Transglycosylation Ratio
3.4. Hydroxyl Radical-Scavenging Assay
3.5. Superoxide Radical Scavenging Assay
3.6. DPPH Radicals Scavenging Assay
3.7. Acute Toxicity Tests
3.8. Preparation of Compounds
3.9. Taste Analysis
3.10. Identification and Characterization
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Lee, C. Intense sweetener from Lo Han Kuo (Momordica grosvenori). Experientia 1975, 31, 533–534. [Google Scholar] [CrossRef]
- Takemoto, T.; Arihara, S.; Nakajima, T.; Okuhira, M. Studies on the constituents of Fructus momordicae. I. On the sweet principle. J. Pharm. Soc. Jpn. 1983, 103, 1151–1154. [Google Scholar]
- Kasai, R.; Nie, R.L.; Nashi, K.; Ohtani, K.; Zhou, J.; Tao, G.D.; Tanaka, O. Sweet cucurbitane glycosides from fruits of Siraitia siamensis (chi-zi luo-han-guo), a Chinese folk medicine. Agric. Biol. Chem. 1989, 53, 3347–3349. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of Siraitia grosvenori (Cucurbitaceae). Chem. Pharm. Bull. 1990, 38, 2030–2032. [Google Scholar] [CrossRef]
- Qi, X.Y.; Chen, W.J.; Zhang, L.Q.; Xie, B.J. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr. Res. 2008, 28, 278–284. [Google Scholar] [CrossRef]
- Song, F.; Qi, X.; Chen, W.; Jia, W.; Yao, P.; Nussler, A.K.; Sun, X.; Liu, L. Effect of Momordica grosvenori on oxidative stress pathways in renal mitochondria of normal and alloxan-induced diabetic mice. Eur. J. Nutr. 2007, 46, 61–69. [Google Scholar] [CrossRef]
- Yasushi, A.S.; Mayuko, T.; Yuji, M.; Hiroshi, I.; Masaki, S.; Yoshihisa, N. Antidiabetic effect of long-term supplementation with Siraitia grosvenori on the spontaneously diabetic Goto-Kakizaki rat. Brit. J. Nutr. 2007, 97, 770–775. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Toriumi, M.; Mukainaka, T.; Banno, N.; Kimura, Y.; Hasegawa, J.; Nishino, H. Inhibitory effects of cucurbitane glycosides and other triterpenoids from the fruit of Momordica grosvenori on Epstein-Barr virus early antigen induced by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 2002, 50, 6710–6715. [Google Scholar] [CrossRef]
- Takao, K.; Midori, T. Cancer-chemopreventive effects of natural sweeteners and related compounds. Pure Appl. Chem. 2002, 74, 1309–1316. [Google Scholar]
- Toshihiro, A.; Yosuke, H.; Harukuni, T.; Norihiro, B.; Naoto, S.; Takashi, S.; Yumiko, K. Cucurbitane glycosides from the fruits of Siraitia grosvenori and their inhibitory effects on Epstein-Barr virus activation. J. Nat. Prod. 2007, 70, 783–788. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Murata, Y.; Sugiura, M.; Nishino, H.; Tokuda, H.; Matsumoto, K.; Kasai, R.; Yamasaki, K. Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori. Cancer Lett. 2003, 198, 37–42. [Google Scholar] [CrossRef]
- Di, R.; Huang, M.T.; Ho, C.T. Anti-inflammatory activities of mogrosides from Momordica grosvenori in murine macrophages and a murine ear edema model. J. Agric. Food Chem. 2011, 59, 7474–7481. [Google Scholar] [CrossRef]
- Li, D.; Ikeda, T.; Huang, Y.; Liu, J.; Nohara, T.; Sakamoto, T.; Nonaka, G. Seasonal variation of mogrosides in Lo Han Kuo (Siraitia grosvenori) fruits. J. Nat. Med. 2007, 61, 307–312. [Google Scholar] [CrossRef]
- Li, F.; Jiang, S.Y.; Li, D. Study on Cultivation and Chemistry of Siraitia grosvenori, 1st ed.; Guangxi Science and Technology Press: Nanning, China, 2010; pp. 20–31. [Google Scholar]
- Xia, Y.; Rivero-Huguet, M.E.; Hughes, B.H.; Marshall, W.D. Isolation of the sweet components from Siraitia grosvenori. Food Chem. 2008, 107, 1022–1028. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, X. A minor, sweet cucurbitane glycoside from Siraitia grosvenori. Nat. Prod. Commun. 2009, 4, 769–772. [Google Scholar]
- Murata, Y.; Yoshikawa, S.; Suzuki, Y.; Sugiura, M.; Inui, H.; Nakano, Y. Sweetness characteristics of the triterpene glycosides in Siraitia grosvenori. J. Jpn. Soc. Food Sci. Technol. 2006, 53, 527–533. [Google Scholar] [CrossRef]
- Uitdehaag, J.C.; Veen, B.A.; Dijkhuizen, L.; Bauke, D. Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-mylase family. Enzym. Microb. Technol. 2002, 30, 295–304. [Google Scholar] [CrossRef]
- Kometani, T.; Terada, Y.; Nishimura, T.; Nakae, T.; Takii, H.; Okada, S. Transglycosylation to hesperidin by cyclodextrin glucanotransferase from an alkalophilic Bacillus species in alkaline pH and properties of hesperidin glycosides. Biosci. Biotechnol. Biochem. 1994, 58, 1990–1994. [Google Scholar] [CrossRef]
- Kelly, R.M.; Dijkhuizen, L.; Leemhuis, H. The evolution of cyclodextrin glucanotransferase product specificity. Appl. Microbiol. Biotechnol. 2009, 84, 119–133. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Miyata, T.; Nakayasu, N.; Mizutani, K.; Kasai, R.; Tanaka, O. Enzymatic transglycosylation products of stevioside: Separation and sweetness-evaluation. Agric. Biol. Chem. 1989, 53, 603–1607. [Google Scholar]
- Ohtani, K.; Aikawa, Y.; Ishikawa, H.; Kasai, R.; Kitahata, S.; Mizutani, K.; Doi, S.; Nakaura, M.; Tanaka, O. Further study on the 1,4-α-transglucosylation of rubusoside, a sweet steviolbisglucoside from Rubus suavissimus. Agric. Biol. Chem. 1991, 55, 449–453. [Google Scholar] [CrossRef]
- Murata, Y.; Ogawa, T.; Suzuki, Y.A.; Yoshikawa, S.; Inui, H.; Sugiura, M.; Nakano, Y. Digestion and absorption of Siraitia grosvenori triterpenoids in the rat. Biosci. Biotechnol. Biochem. 2010, 74, 673–676. [Google Scholar] [CrossRef]
- Halliwell, B.; John, M.; Okezie, I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Methods and doses of acute toxicity tests are following the Chinese national standard, GB15193.3-2003 and GB21804-2008-T
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.; Yang, Z.; Lu, F.; Liu, J.; Song, Y.; Li, D. Cucurbitane Glycosides Derived from Mogroside IIE: Structure-Taste Relationships, Antioxidant Activity, and Acute Toxicity. Molecules 2014, 19, 12676-12689. https://doi.org/10.3390/molecules190812676
Wang L, Yang Z, Lu F, Liu J, Song Y, Li D. Cucurbitane Glycosides Derived from Mogroside IIE: Structure-Taste Relationships, Antioxidant Activity, and Acute Toxicity. Molecules. 2014; 19(8):12676-12689. https://doi.org/10.3390/molecules190812676
Chicago/Turabian StyleWang, Lei, Ziming Yang, Fenglai Lu, Jinglei Liu, Yunfei Song, and Dianpeng Li. 2014. "Cucurbitane Glycosides Derived from Mogroside IIE: Structure-Taste Relationships, Antioxidant Activity, and Acute Toxicity" Molecules 19, no. 8: 12676-12689. https://doi.org/10.3390/molecules190812676





