Quantification of the Resveratrol Analogs trans-2,3-Dimethoxy-stilbene and trans-3,4-Dimethoxystilbene in Rat Plasma: Application to Pre-Clinical Pharmacokinetic Studies
Abstract
:1. Introduction
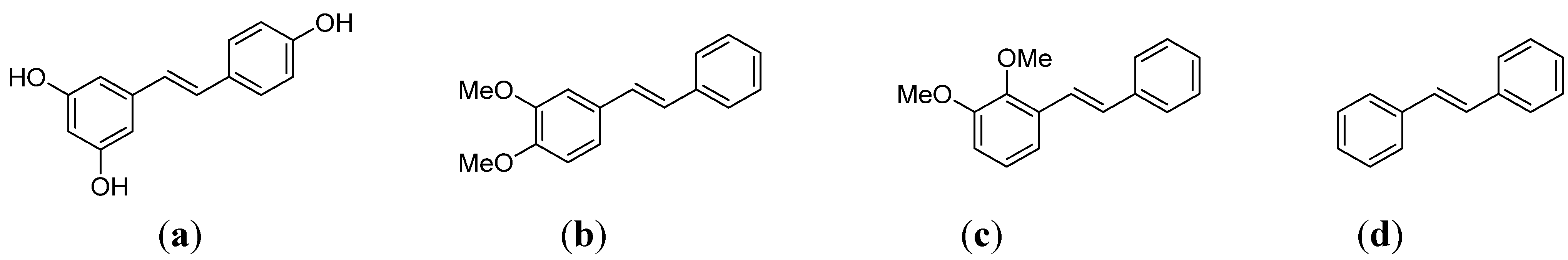
2. Results and Discussion
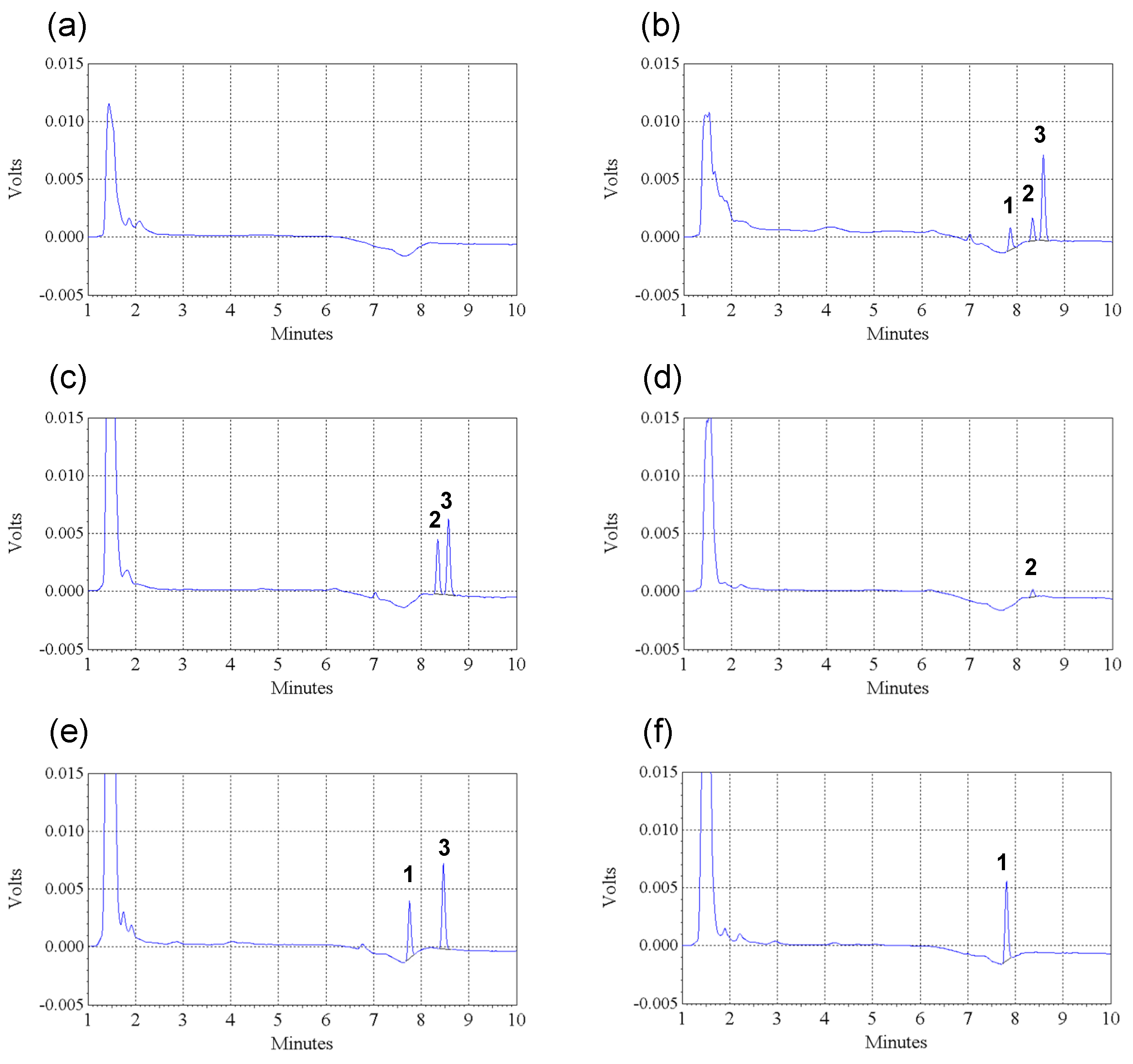
2.1. HPLC Method Validation
| Amount Spiked (ng/mL) | 2,3-DMS | 3,4-DMS | ||||
|---|---|---|---|---|---|---|
| Amount Measured (ng/mL) | Precision (RSD, %) | Bias Range (%) | Amount Measured (ng/mL) | Precision (RSD, %) | Bias Range (%) | |
| 25.0 | 24.2 ± 1.6 | 6.3 | −3.4 ~ +11.8 | 24.6 ± 1.0 | 4.2 | −3.9 ~ +7.2 |
| 400.0 | 398.2 ± 3.7 | 0.9 | −0.4 ~ +1.9 | 409.3 ± 5.1 | 1.3 | −4.0 ~ +0.6 |
| 1400.0 | 1378.0 ± 15.3 | 1.1 | 0.8 ~ +3.4 | 1398.0 ± 21.5 | 1.5 | −0.8 ~ +2.8 |
| Amount Spiked (ng/mL) | 2,3-DMS | 3,4-DMS | ||||
|---|---|---|---|---|---|---|
| Amount Measured (ng/mL) | Precision (RSD, %) | Bias Range (%) | Amount Measured (ng/mL) | Precision (RSD, %) | Bias Range (%) | |
| 25.0 | 25.2 ± 2.1 | 8.3 | −9.3 ~ +13.6 | 25.6 ± 2.0 | 7.7 | −13.1 ~ +7.2 |
| 400.0 | 382.4 ± 20.2 | 5.3 | −0.8 ~ +13.1 | 392.2 ± 19.2 | 4.8 | −3.4 ~ +9.8 |
| 1400.0 | 1340.0 ± 54.6 | 4.1 | 0.1 ~ +11.2 | 1392.0 ± 81.0 | 5.8 | −11.1 ~ +7.5 |
| 2,3-DMS | 3,4-DMS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stability (% Remained) | Stability (% Remained) | |||||||||
| Stock solution stored at 24 °C for 8 days | 98.1 ± 2.1 | 96.7 ± 2.7 | ||||||||
| Spiked Concentration (ng/mL) | Spiked Concentration (ng/mL) | |||||||||
| 25 | 400 | 1400 | 25 | 400 | 1400 | |||||
| Plasma samples stored at 4 °C for 6 h | 94.9 ± 4.4 | 95.5 ± 1.1 | 95.4 ± 0.6 | 104.3 ± 6.6 | 94.7 ± 0.7 | 96.2 ± 0.4 | ||||
| Post-preparative samples stored at 24 °C for 24 h | 97.2 ± 2.5 | 100.1 ± 0.5 | 100.6 ± 0.7 | 98.1 ± 5.7 | 98.1 ± 1.8 | 100.1 ± 0.6 | ||||
| Plasma samples after three freeze-thaw cycles | 102.3 ± 4.5 | 93.2 ± 1.2 | 91.8 ± 2.6 | 106.1 ± 5.2 | 93.4 ± 0.7 | 92.4 ± 2.4 | ||||
| Plasma samples stored at −80 °C for 10 days | 106.9 ± 6.6 | 96.6 ± 4.7 | 93.5 ± 4.1 | 91.0 ±13.8 | 93.6 ± 4.6 | 93.0 ± 3.7 | ||||
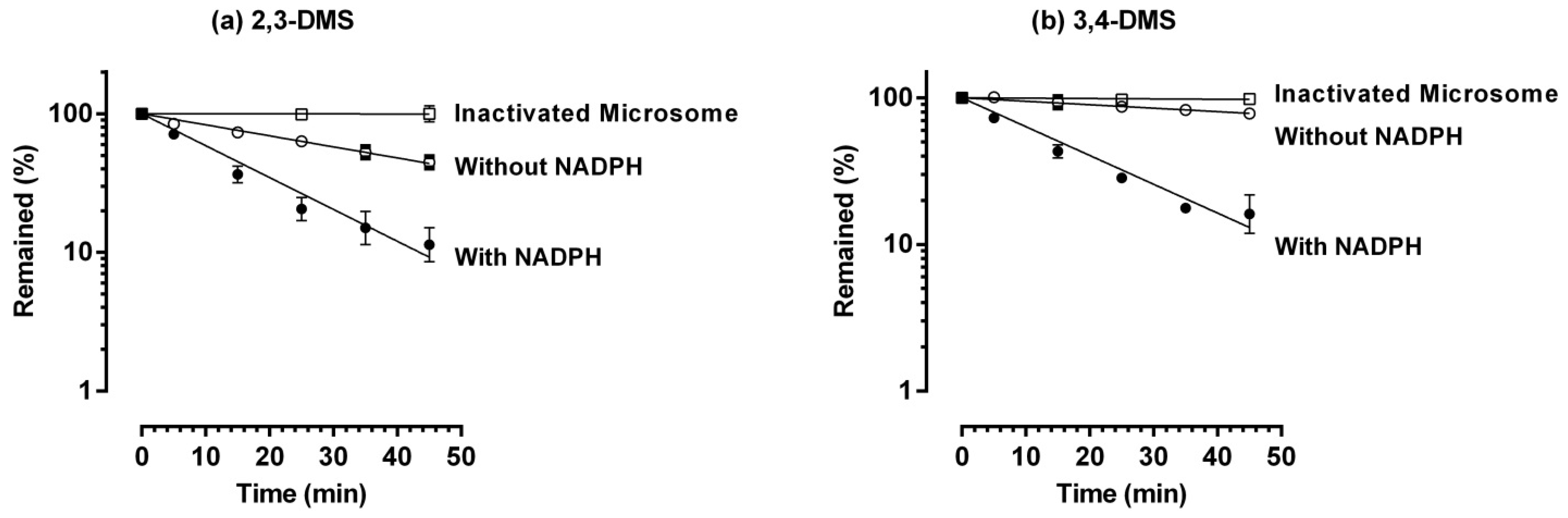
2.2. Metabolic Stability in Rat Liver Microsome
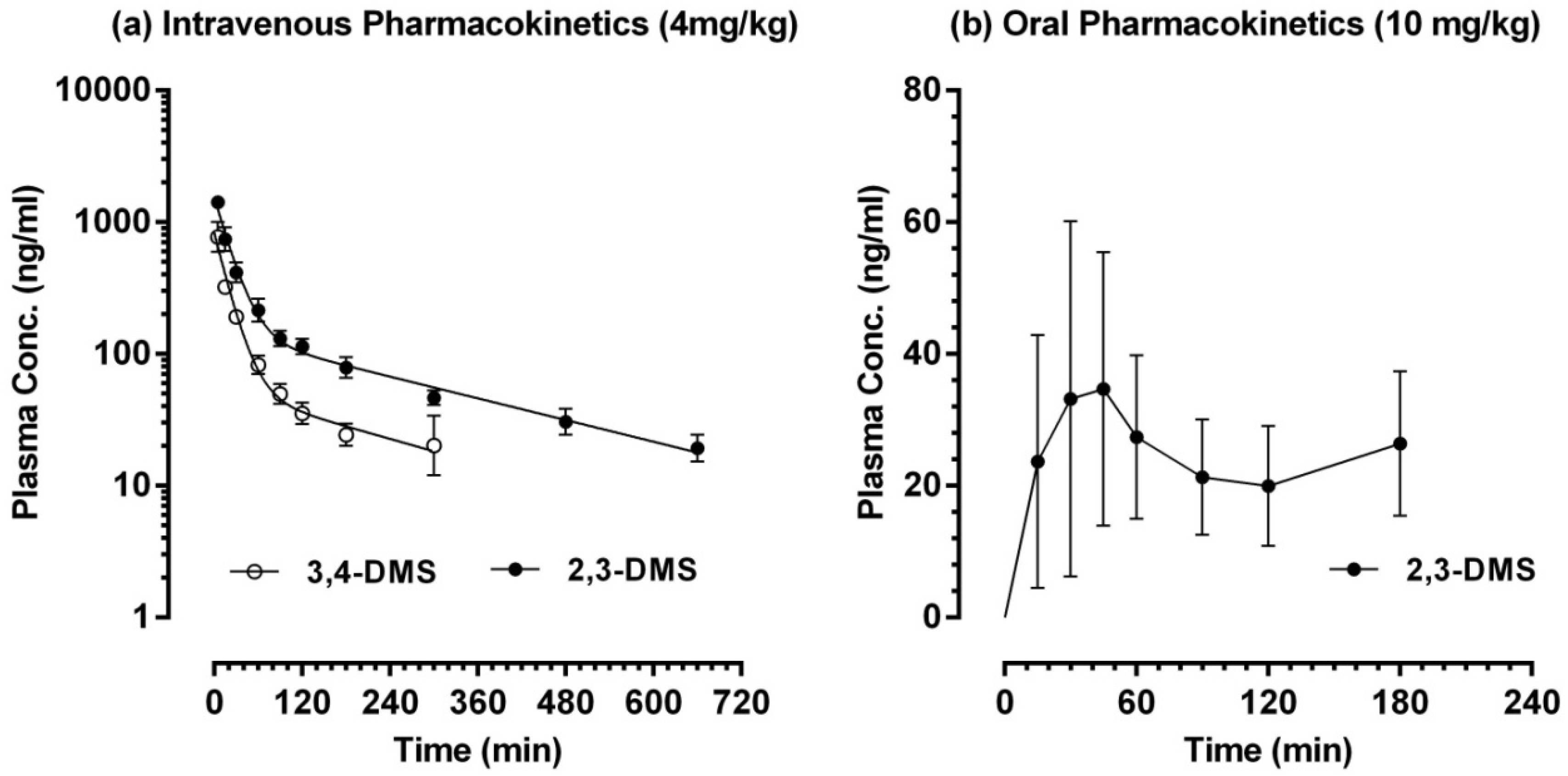
2.3. Pharmacokinetic Study in Sprague-Dawley Rats
| Parameters | 2,3-DMS | 2,3-DMS | 3,4-DMS |
|---|---|---|---|
| Dosing Route | Oral (n = 6) | Intravenous (n = 5) | Intravenous (n = 5) |
| Dose (mg·kg−1) | 10 | 4 | 4 |
| A(ng·mL−1) | - | 1384.1 ± 251.4 | 702.7 ± 148.5 ** |
| B(ng·mL−1) | - | 135.6 ± 40.6 | 55.9 ± 26.1 ** |
| α (10−2 × min−1) | - | 5.02 ± 1.76 | 5.25 ± 1.45 |
| β (10−3 × min−1) | - | 3.08 ± 0.73 | 3.85 ± 2.45 |
| Vc (L·kg−1) | - | 2.71 ± 0.51 | 5.58 ± 1.73 ** |
| AUC0→last (103 × min·ng·mL−1) | 3.86 ± 3.70 | 69.5 ± 8.5 | 25.6 ± 7.0 *** |
| Cl (mL·min−1·kg−1) | - | 52.0 ± 7.0 | 143.4 ± 40.5 *** |
| t1/2 λZ (min) | - | 288.9 ± 92.9 | 193.1 ± 123.3 |
| MTT0→last (min) | 83.2 ± 67.4 | 131.0 ± 4.5 | 61.4 ± 27.1 *** |
| Cmax (ng·mL−1) | 37.5 ± 23.7 | - | - |
| tmax (min) | 15, 30 or 300 | - | - |
| F (%) | 2.22 ± 2.13 | - | - |
3. Experimental Section
3.1. General Information
3.2. Special Precautions
3.3. Synthesis
3.4. Chromatographic Conditions
3.5. Sample Preparation
3.6. Method Validation
3.7. Rat Liver Microsome Metabolic Stability Investigation
3.8. Pharmacokinetic Profiling in Male Sprague-Dawley Rats
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Roupe, K.A.; Remsberg, C.M.; Yanez, J.A.; Davies, N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, H.; Cui, L.; Li, H.; Zhou, B.; Zhou, G.; Dai, F. 3,4-Dimethoxystilbene, a resveratrol derivative with anti-angiogenic effect, induces both macroautophagy and apoptosis in endothelial cells. J. Cell. Biochem. 2013, 114, 697–707. [Google Scholar]
- Heynekamp, J.J.; Weber, W.M.; Hunsaker, L.A.; Gonzales, A.M.; Orlando, R.A.; Deck, L.M.; Jagt, D.L. Substituted trans-stilbenes, including analogues of the natural product resveratrol, inhibit the human tumor necrosis factor alpha-induced activation of transcription factor nuclear factor kappa B. J. Med. Chem. 2006, 49, 7182–7189. [Google Scholar] [CrossRef]
- Valdameri, G.; Pereira Rangel, L.; Spatafora, C.; Guitton, J.; Gauthier, C.; Arnaud, O.; Ferreira-Pereira, A.; Falson, P.; Winnischofer, S.M.; Rocha, M.E.; et al. Methoxy stilbenes as potent, specific, untransported, and noncytotoxic inhibitors of breast cancer resistance protein. ACS Chem. Biol. 2012, 7, 322–330. [Google Scholar] [CrossRef]
- Lin, H.S.; Tringali, C.; Spatafora, C.; Choo, Q.Y.; Ho, P.C. LC determination of trans-3,5,3',4',5'-pentamethoxystilbene in rat plasma. Chromatographia 2011, 72, 827–831. [Google Scholar]
- Lin, H.S.; Spatafora, C.; Tringali, C.; Ho, P.C. Determination of trans-2,4,3',4',5'-pentamethoxystilbene in rat plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 57, 94–98. [Google Scholar] [CrossRef]
- Lin, H.S.; Choo, Q.Y.; Ho, P.C. Quantification of oxyresveratrol analog trans-2,4,3',5'-tetramethoxystilbene in rat plasma by a rapid HPLC method: Application in a pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2010, 24, 1373–1378. [Google Scholar] [CrossRef]
- Lin, H.S.; Zhang, W.; Go, M.L.; Tringali, C.; Spatafora, C.; Ho, P.C. Quantification of trans-3,4,5,4'-Tetramethoxystilbene in rat plasma by HPLC: Application to pharmacokinetic study. J. Agric. Food Chem. 2011, 59, 1072–1077. [Google Scholar]
- Lin, H.S.; Tringali, C.; Spatafora, C.; Wu, C.; Ho, P.C. A simple and sensitive HPLC-UV method for the quantification of piceatannol analog trans-3,5,3',4'-tetramethoxystilbene in rat plasma and its application for a pre-clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 679–684. [Google Scholar] [CrossRef]
- Lin, H.S.; Ho, P.C. Preclinical pharmacokinetic evaluation of resveratrol trimethyl ether in sprague-dawley rats: The impacts of aqueous solubility, dose escalation, food and repeated dosing on oral bioavailability. J. Pharm. Sci. 2011, 100, 4491–4500. [Google Scholar] [CrossRef]
- Lin, H.S.; Zhang, W.; Go, M.L.; Choo, Q.Y.; Ho, P.C. Determination of Z-3,5,4'-trimethoxystilbene in rat plasma by a simple HPLC method: Application in a pre-clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 53, 693–697. [Google Scholar] [CrossRef]
- Lou, B.S.; Wu, P.S.; Hou, C.W.; Cheng, F.Y.; Chen, J.K. Simultaneous quantification of trans-resveratrol and its sulfate and glucuronide metabolites in rat tissues by stable isotope-dilution UPLC-MS/MS analysis. J. Pharm. Biomed. Anal. 2014, 94, 99–105. [Google Scholar] [CrossRef]
- Das, S.; Lin, H.S.; Ho, P.C.; Ng, K.Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef]
- Yeo, S.C.; Luo, W.; Wu, J.; Ho, P.C.; Lin, H.S. Quantification of pinosylvin in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pre-clinical pharmacokinetic study. J. Chromatogr. B 2013, 931, 68–74. [Google Scholar] [CrossRef]
- Sample Availability: Samples of 2,3-DMS and 3,4-DMS are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.Y.; Cardullo, N.; Yeo, S.C.M.; Spatafora, C.; Tringali, C.; Ong, P.-S.; Lin, H.-S. Quantification of the Resveratrol Analogs trans-2,3-Dimethoxy-stilbene and trans-3,4-Dimethoxystilbene in Rat Plasma: Application to Pre-Clinical Pharmacokinetic Studies. Molecules 2014, 19, 9577-9590. https://doi.org/10.3390/molecules19079577
Ng SY, Cardullo N, Yeo SCM, Spatafora C, Tringali C, Ong P-S, Lin H-S. Quantification of the Resveratrol Analogs trans-2,3-Dimethoxy-stilbene and trans-3,4-Dimethoxystilbene in Rat Plasma: Application to Pre-Clinical Pharmacokinetic Studies. Molecules. 2014; 19(7):9577-9590. https://doi.org/10.3390/molecules19079577
Chicago/Turabian StyleNg, Shermain Yali, Nunzio Cardullo, Samuel Chao Ming Yeo, Carmela Spatafora, Corrado Tringali, Pei-Shi Ong, and Hai-Shu Lin. 2014. "Quantification of the Resveratrol Analogs trans-2,3-Dimethoxy-stilbene and trans-3,4-Dimethoxystilbene in Rat Plasma: Application to Pre-Clinical Pharmacokinetic Studies" Molecules 19, no. 7: 9577-9590. https://doi.org/10.3390/molecules19079577






