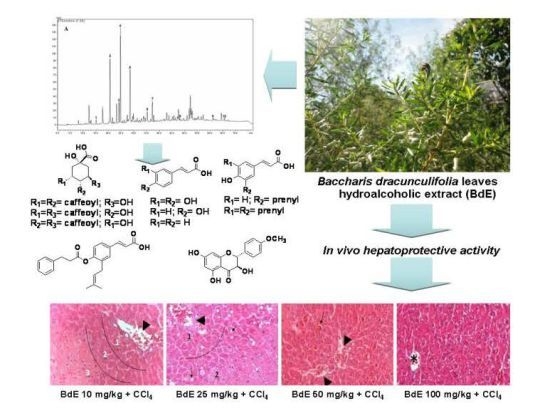
Protective Effects of Baccharis dracunculifolia Leaves Extract against Carbon Tetrachloride- and Acetaminophen-Induced Hepatotoxicity in Experimental Animals
Abstract
:1. Introduction
2. Results and Discussion
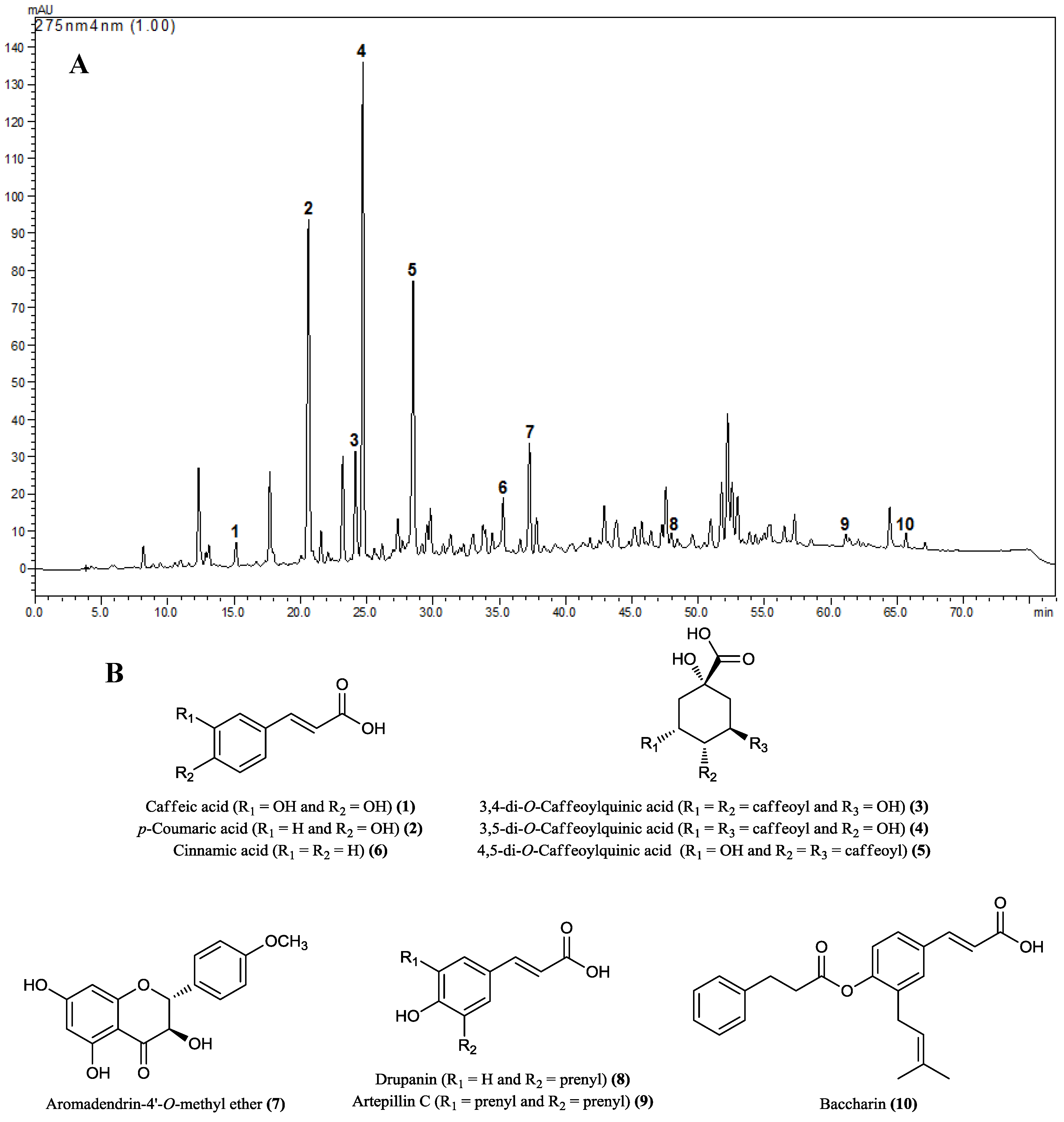
2.1. HPLC Analysis of Hydroalcoholic Extract of B. dracunculifolia (BdE)
2.2. DPPH Free Radical Scavenging Assay, Total Phenolic Content and Total Flavonoid Content
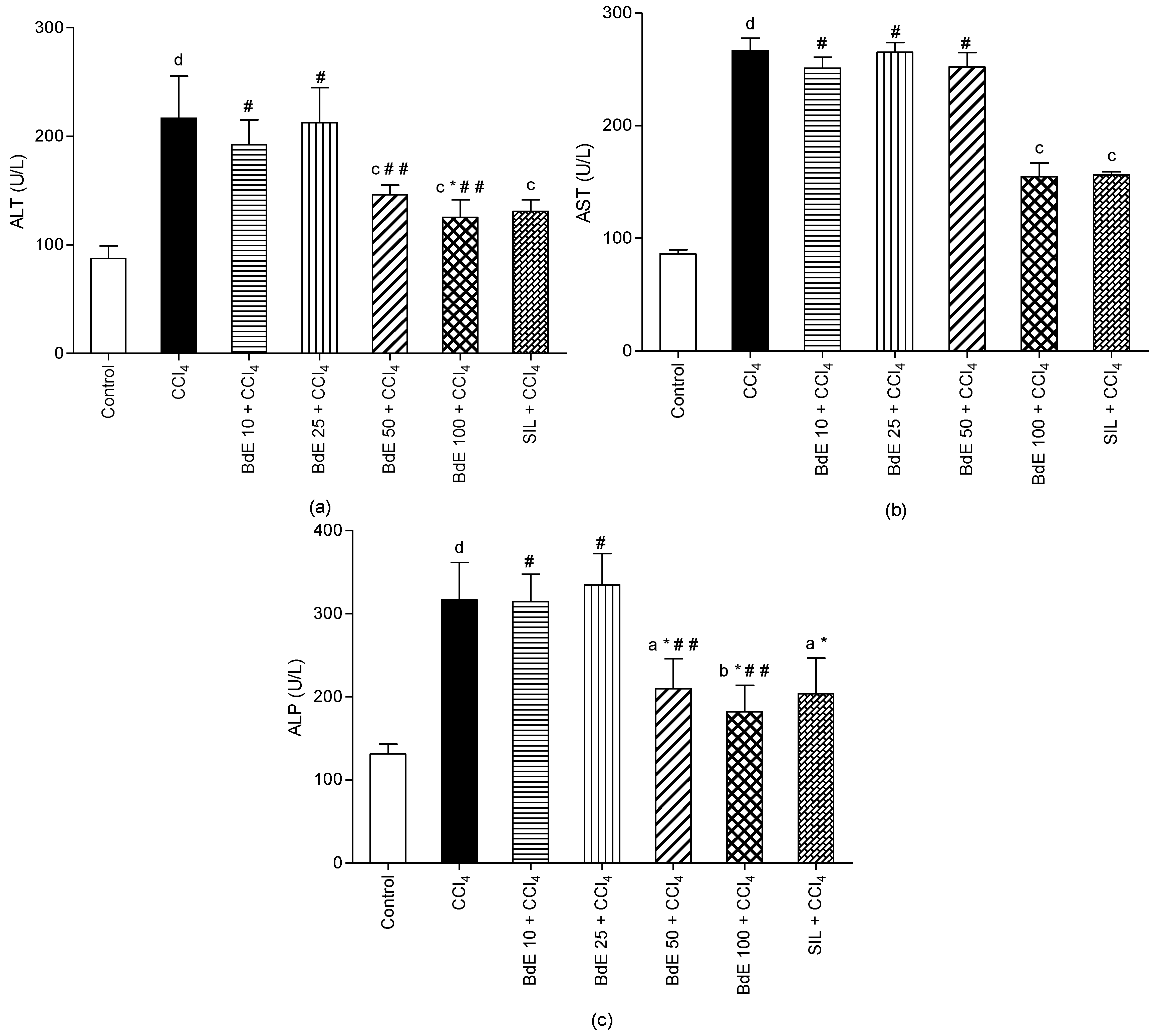
2.3. CCl4-Induced Hepatotoxicity
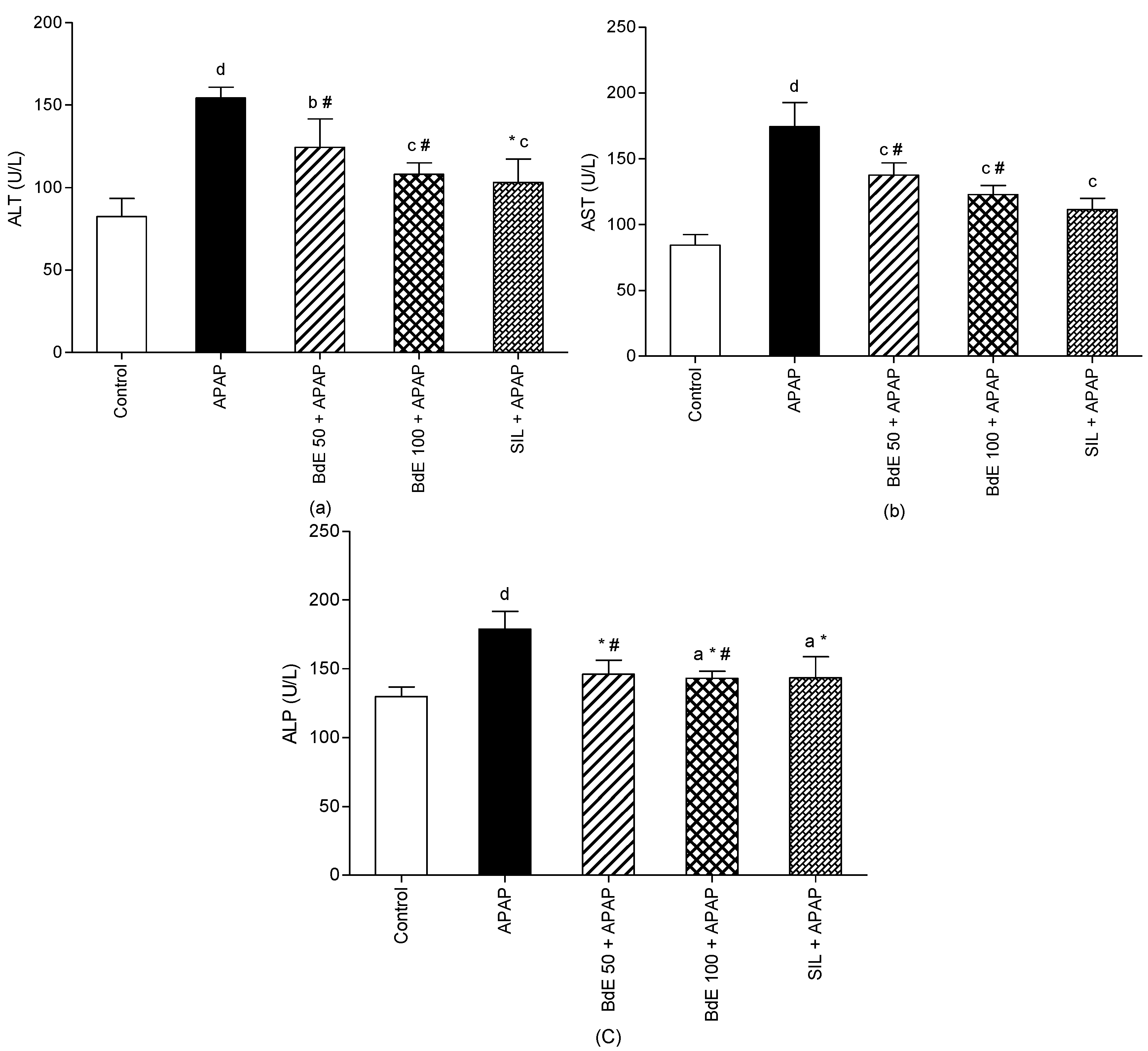
2.4. APAP-Induced Hepatotoxicity
2.5. Histopathological Studies
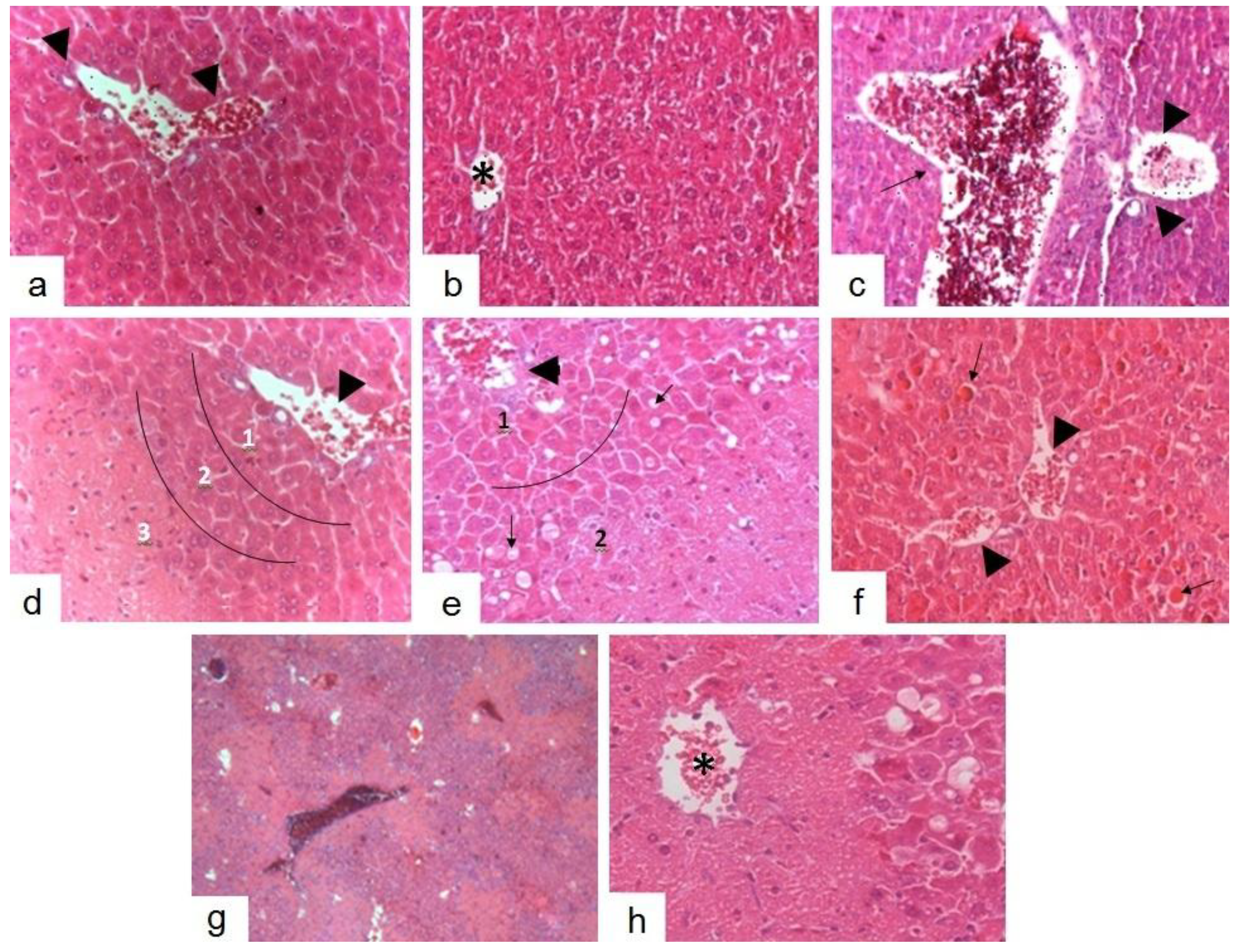
2.6. Discussion
3. Experimental
3.1. Chemicals
3.2. Plant Material
3.3. Preparation of the Hydroalcoholic Extract of B. dracunculifolia (BdE)
3.4. HPLC Analysis of BdE
3.5. DPPH Radical Scavenging Activity
3.6. Total Phenolic Content
3.7. Total Flavonoid Content
3.8. Animals
3.9. CCl4-Induced Hepatotoxicity Model
3.10. APAP-Induced Hepatotoxicity Model
3.11. Assessment of Liver Function
3.12. Liver Histopathological Examinations
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sabir, S.M.; Ahmad, S.D.; Hamid, A.; Khan, M.Q.; Athayde, M.L.; Santos, D.B.; Boligon, A.A.; Rocha, J.B.T. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012, 131, 741–747. [Google Scholar] [CrossRef]
- Guimarães, N.S.; Mello, J.C.; Paiva, J.S.; Bueno, P.C.; Berretta, A.A.; Torquato, R.J.; Nantes, I.L.; Rodrigues, T. Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem. Toxicol. 2012, 50, 1091–1097. [Google Scholar] [CrossRef]
- León-González, A.J.; Mateos, R.; Ramos, S.; Martín, M.A.; Sarriá, B.; Martín-Cordero, C.; López-Lázaro, M.; Bravo, L.; Goya, L. Chemo-protective activity and characterization of phenolics extracts from Corema album. Food Res. Int. 2012, 49, 728–738. [Google Scholar] [CrossRef]
- Shankar, N.L.G.; Manavalan, R.; Venkappayya, D.; David Raj, C. Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl4-induced oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3182–3185. [Google Scholar] [CrossRef]
- Fasalu, R.O.M.; Rupesh, K.M.; Tamizh, M.T.; Mohamed Niyas, K. A review of hepatoprotective natural products. Int. J. Pharm. Sci. Rev. Res. 2011, 8, 80–84. [Google Scholar]
- Santos, D.A.; Fukui Mde, J.; Nanayakkara, N.P.D.; Khan, S.I.; Sousa, J.P.; Bastos, J.K.; Andrade, S.F.; da Silva Filho, A.A.; Quintão, N.L. Anti-inflammatory and antinociceptive effects of Baccharis dracunculifolia D.C. (Asteraceae) in different experimental models. J. Ethnopharmacol. 2010, 127, 543–550. [Google Scholar] [CrossRef]
- Fernandez, E.C.; Sandi, Y.E.; Kokoska, L. Ethnobotanical inventory of medicinal plants used in the Bustillo Province of the Potosi Department, Bolivia. Fitoterapia 2003, 74, 407–416. [Google Scholar] [CrossRef]
- Sousa, J.P.B.; Jorge, R.F.; Leite, M.F.; Furtado, N.A.J.C.; Bastos, J.K.; da Silva Filho, A.A.; Queiroga, C.L.; Magalhães, P.M.; Soares, A.E.E. Seasonal variation of the (E)-nerolidol and other volatile compounds within ten different cultivated populations of Baccharis dracunculifolia D.C. (Asteraceae). J. Essent. Oil Res. 2009, 21, 308–314. [Google Scholar] [CrossRef]
- Kumazawa, S.; Yoneda, M.; Shibata, I.; Kanaeda, J.; Hamasaka, T.; Nakayama, T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem. Pharm. Bull. 2003, 51, 740–742. [Google Scholar] [CrossRef]
- Simões, L.M.; Gregório, L.E.; da Silva Filho, A.A.; Souza, M.L.; Azzolini, A.E.; Bastos, J.K.; Lucisano-Valim, Y.M. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. J. Ethnopharmacol. 2004, 94, 59–65. [Google Scholar] [CrossRef]
- Jorge, R.; Furtado, N.A.J.C.; Sousa, J.P.B.; da Silva Filho, A.A.; Gregório Junior, L.E.; Martins, C.H.G.; Soares, A.E.E.; Bastos, J.K.; Cunha, W.R.; Silva, M.L.A. Brazilian propolis: Seasonal variation of the prenylated p-coumaric acids and antimicrobial activity. Pharm. Biol. 2008, 46, 889–893. [Google Scholar]
- Gonzalez, R.; Corcho, I.; Remirez, D.; Rodriguez, S.; Ancheta, O.; Merino, N.; Gonzalez, A.; Pascual, C. Hepatoprotective effects of propolis extract on carbon tetrachloride-induced liver-injury in rats. Phytother. Res. 1995, 9, 114–117. [Google Scholar] [CrossRef]
- Gonzalez, R.; Remirez, D.; Rodriguez, S.; Gonzalez, A.; Ancheta, O.; Merino, N.; Pascual, C. Hepatoprotective effects of propolis extract on paracetamol-induced liver-damage in mice. Phytother. Res. 1994, 8, 229–232. [Google Scholar] [CrossRef]
- Da Silva Filho, A.A.; Resende, D.O.; Fukui, M.J.; Santos, F.F.; Pauletti, P.M.; Cunha, W.R.; Silva, M.L.; Gregorio, L.E.; Bastos, J.K.; Nanayakkara, N.P.D. In vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D.C. (Asteraceae). Fitoterapia 2009, 80, 478–482. [Google Scholar] [CrossRef]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Da Silva Filho, A.A.; Sousa, J.P.B.; Soares, S.; Furtado, N.A.J.C.; Andrade e Silva, M.L.; Cunha, W.R.; Gregório, L.E.; Nanayakkara, N.P.D.; Bastos, J.K. Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia D.C. (Asteraceae). Z. Naturforsch. C 2008, 63, 40–46. [Google Scholar]
- Missima, F.; da Silva Filho, A.A.; Nunes, G.A.; Bueno, P.C.P.; Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. Effect of Baccharis dracunculifolia D.C. (Asteraceae) extracts and its isolated compounds on macrophage activation. J. Pharm. Pharmacol. 2007, 59, 463–468. [Google Scholar] [CrossRef]
- Sousa, J.P.B.; da Silva Filho, A.A.; Bueno, P.C.; Gregório, L.E.; Furtado, N.A.J.C.; Jorge, R.F.; Bastos, J.K. A validated reverse-phase HPLC analytical method for the quantification of phenolic compounds in Baccharis dracunculifolia. Phytochem. Anal. 2009, 20, 24–32. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Adnyana, I.K.; Ishii, E.; Midorikawa, K.; Matsushige, K.; Kadota, S. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine 2001, 8, 16–23. [Google Scholar] [CrossRef]
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol. 2008, 46, 3417–3421. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A., Jr.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Wu, J.; Danielsson, A.; Zern, M.A. Toxicity of hepatotoxins: New insights into mechanisms and therapy. Expert Opin. Investig. Drugs 1999, 8, 585–607. [Google Scholar] [CrossRef]
- Gellert, J.; Goldermann, L.; Teschke, R. Effect of CO2-induced hyperventilation on carbon tetrachloride (CCl4) levels following acute CCl4 poisoning. Intens. Care Med. 1983, 9, 333–337. [Google Scholar] [CrossRef]
- Goldermann, L.; Gellert, J.; Teschke, R. Quantitative assessment of carbon tetrachloride levels in human blood by head-space gas chromatography: Application in a case of suicidal carbon tetrachloride intoxication. Intens. Care Med. 1983, 9, 131–135. [Google Scholar] [CrossRef]
- Jaeschke, H.; Williams, C.D.; McGill, M.R.; Xie, Y.; Ramachandran, A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem. Toxicol. 2013, 55, 279–289. [Google Scholar] [CrossRef]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 387–394. [Google Scholar] [CrossRef]
- Kim, H.P.; Kim, Y.C. Revisiting hepatoprotective natural products from a biological point of view. Nat. Prod. Sci. 2011, 17, 165–174. [Google Scholar]
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef]
- Basnet, P.; Matsushige, K.; Hase, K.; Kadota, S.; Namba, T. Four di-O-caffeoyl quinic acid derivatives from propolis. Potent hepatoprotective activity in experimental liver injury models. Biol. Pharm. Bull. 1996, 19, 1479–1484. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Rao, A.S.; Ahemad, S.R.; Ibrahim, M. Phytochemical studies and hepatoprotective activity of Melia azedarach Linn, against CCl4 induced hepatotoxicity in rats. J. Pharm. Res. 2012, 5, 2664–2667. [Google Scholar]
- Bhadauria, M. Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Exp. Toxicol. Pathol. 2010, 62, 627–635. [Google Scholar] [CrossRef]
- Nolan, J.P.; Leibowitz, A.I. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b - an antiendotoxin. Gastroenterol. 1978, 75, 445–449. [Google Scholar]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; Mac Graw Hill: New York, NY, USA, 1968; p. 258. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezende, T.P.; Corrêa, J.O.d.A.; Aarestrup, B.J.V.; Aarestrup, F.M.; De Sousa, O.V.; Da Silva Filho, A.A. Protective Effects of Baccharis dracunculifolia Leaves Extract against Carbon Tetrachloride- and Acetaminophen-Induced Hepatotoxicity in Experimental Animals. Molecules 2014, 19, 9257-9272. https://doi.org/10.3390/molecules19079257
Rezende TP, Corrêa JOdA, Aarestrup BJV, Aarestrup FM, De Sousa OV, Da Silva Filho AA. Protective Effects of Baccharis dracunculifolia Leaves Extract against Carbon Tetrachloride- and Acetaminophen-Induced Hepatotoxicity in Experimental Animals. Molecules. 2014; 19(7):9257-9272. https://doi.org/10.3390/molecules19079257
Chicago/Turabian StyleRezende, Túlio P., José Otávio do A. Corrêa, Beatriz J. V. Aarestrup, Fernando M. Aarestrup, Orlando V. De Sousa, and Ademar A. Da Silva Filho. 2014. "Protective Effects of Baccharis dracunculifolia Leaves Extract against Carbon Tetrachloride- and Acetaminophen-Induced Hepatotoxicity in Experimental Animals" Molecules 19, no. 7: 9257-9272. https://doi.org/10.3390/molecules19079257
APA StyleRezende, T. P., Corrêa, J. O. d. A., Aarestrup, B. J. V., Aarestrup, F. M., De Sousa, O. V., & Da Silva Filho, A. A. (2014). Protective Effects of Baccharis dracunculifolia Leaves Extract against Carbon Tetrachloride- and Acetaminophen-Induced Hepatotoxicity in Experimental Animals. Molecules, 19(7), 9257-9272. https://doi.org/10.3390/molecules19079257