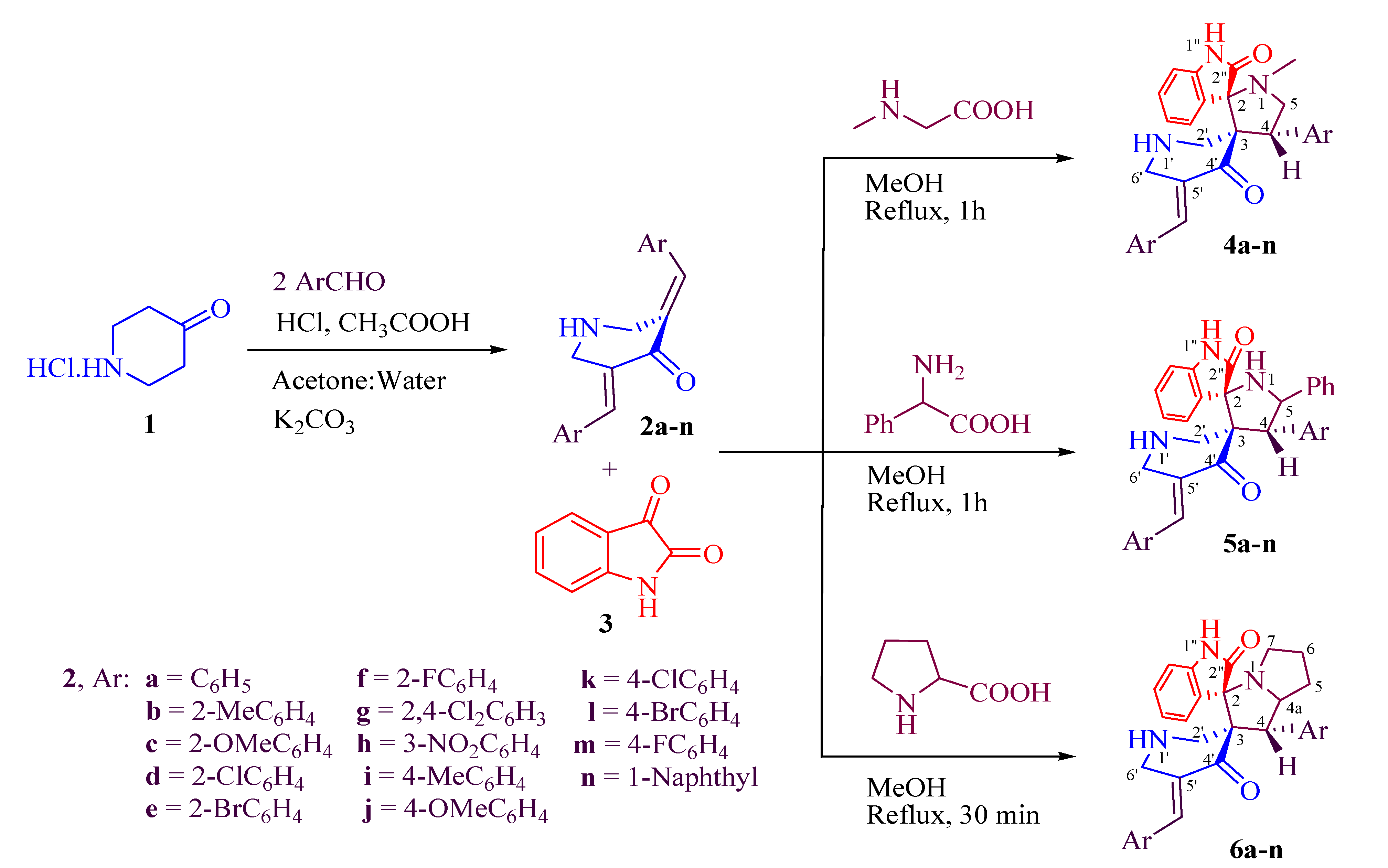
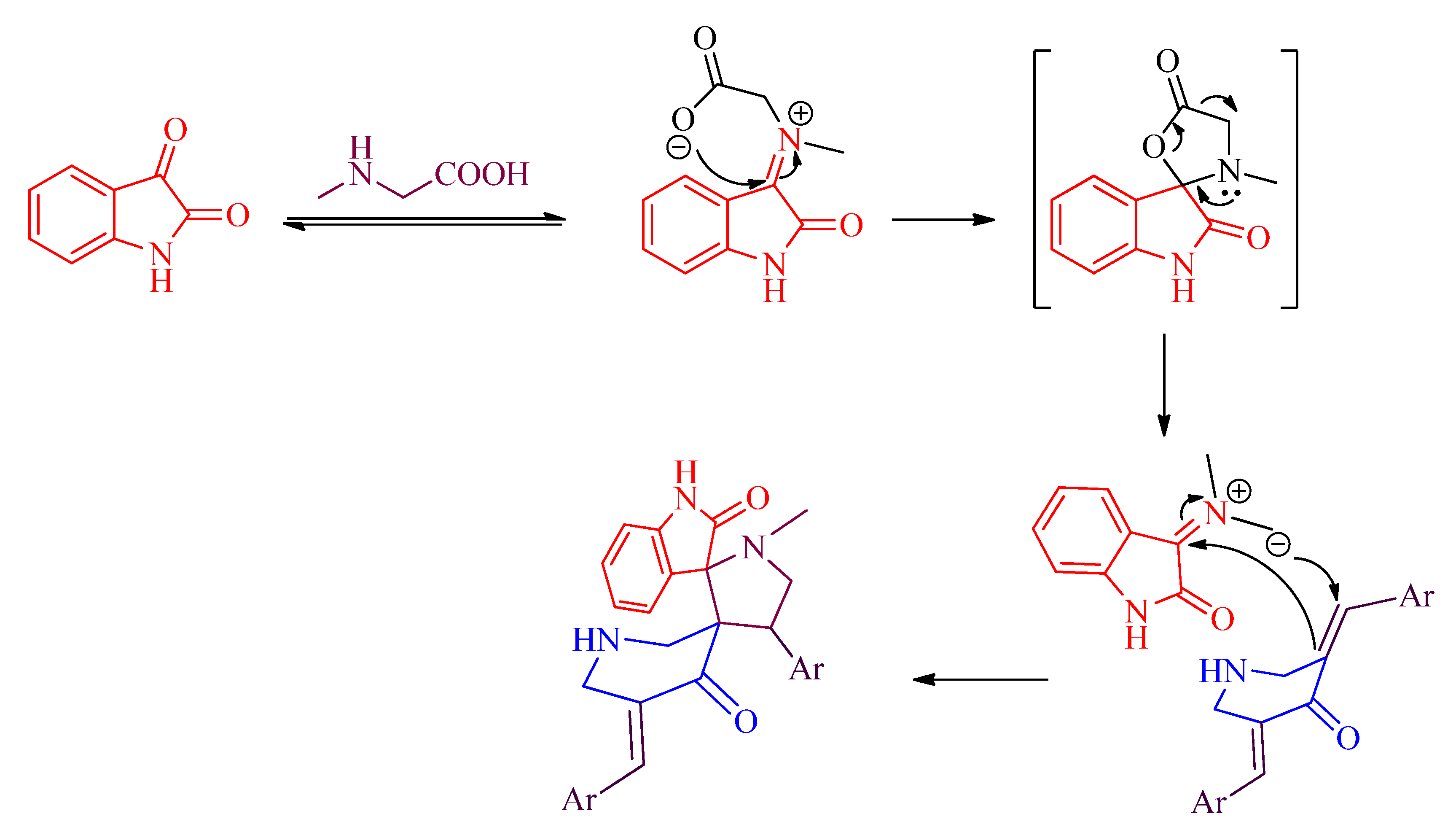
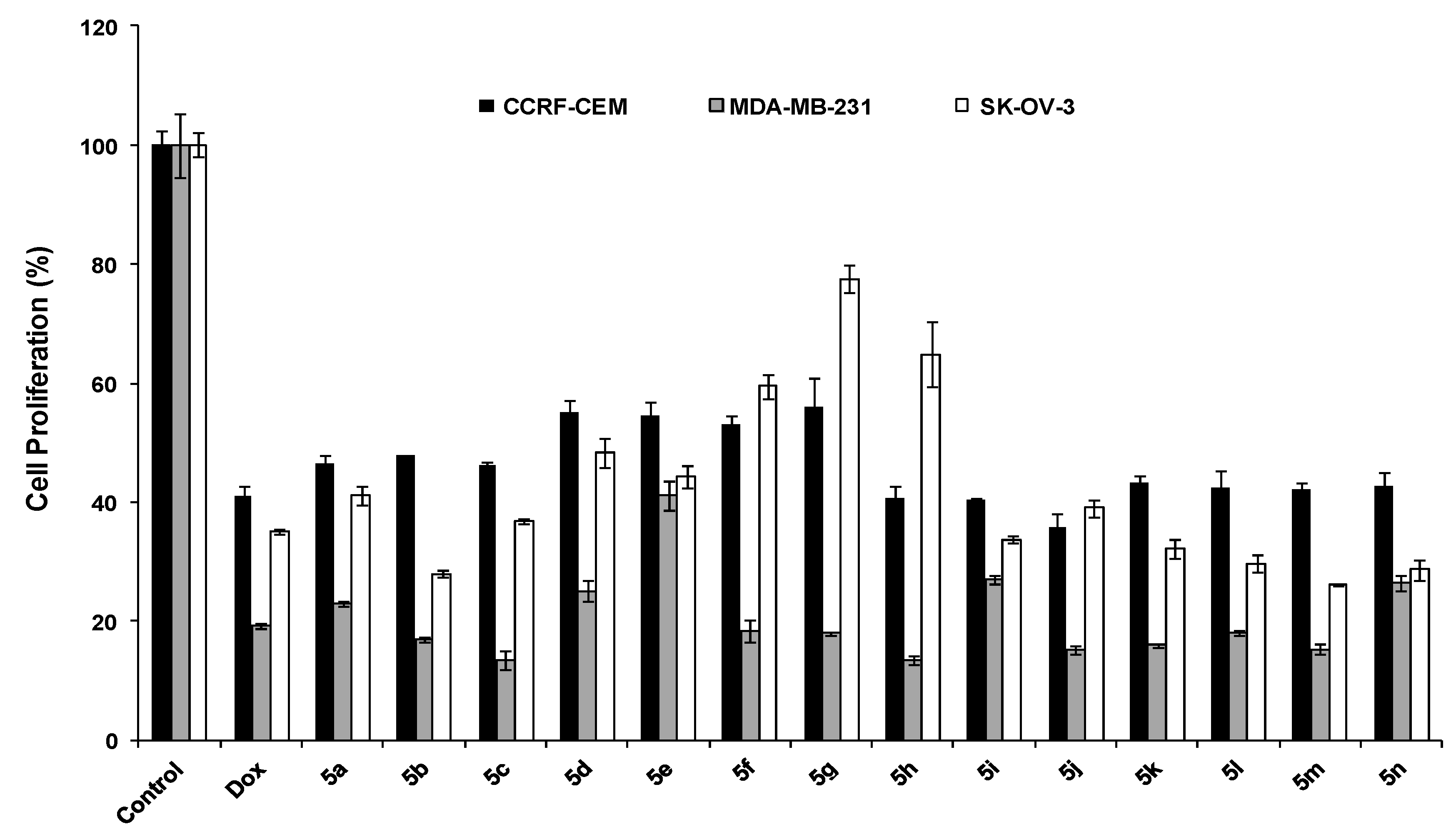
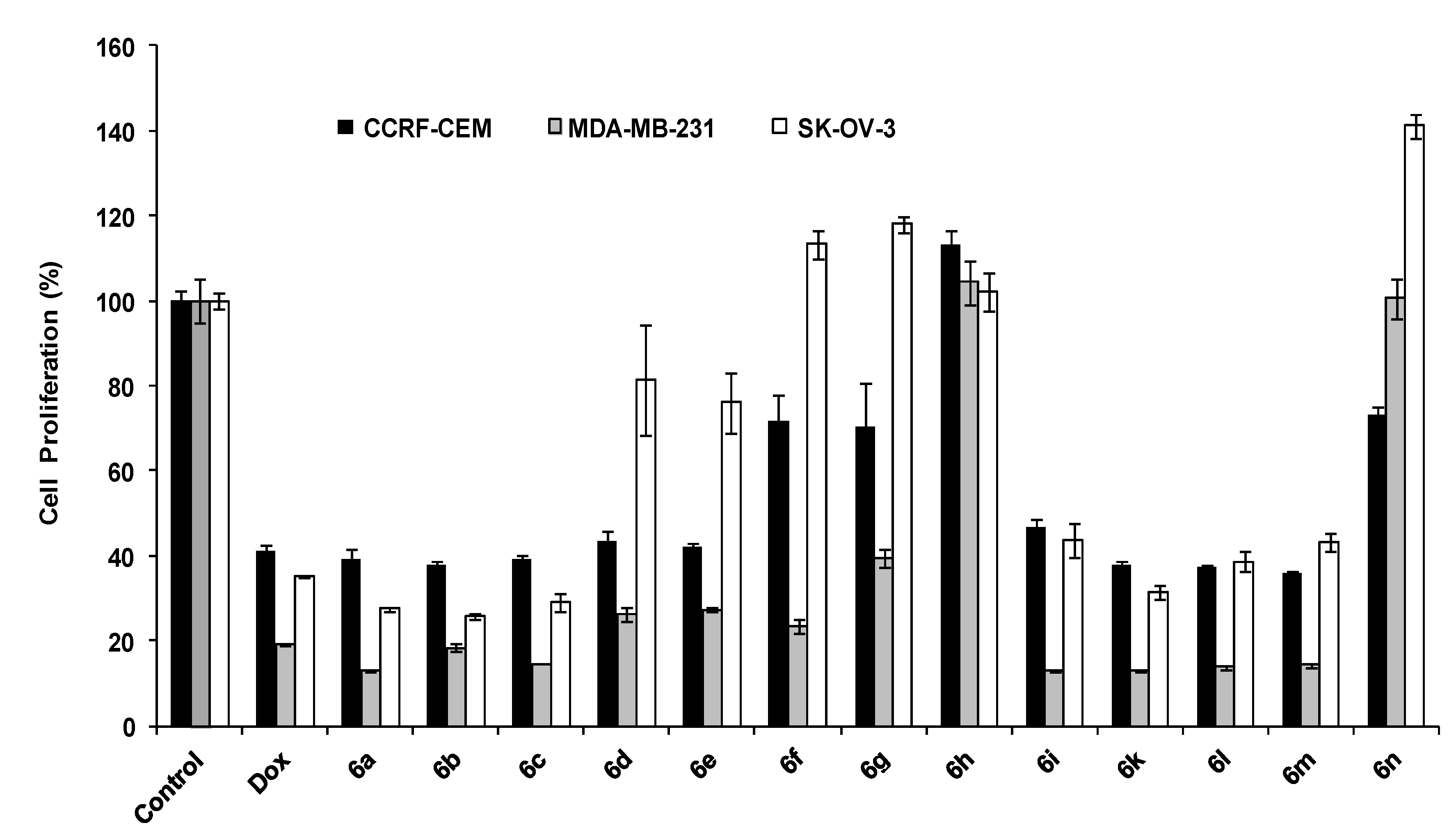
3.4. Spectral Data
1-Methyl-4-(phenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(phenylmethylidene)-piperidin-4′-one (4a). Obtained as a pale yellow solid, (0.150 g, 92%); mp = 169–171°C; IR (KBr): 1604, 1620, 1703, 3407 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.05 (d, 1H, J = 13.2 Hz, 2ꞌ-CH2), 2.14 (s, 1H, N-CH3), 3.35 (dd, 1H, J = 7.2, 7.2 Hz, 5-CH2), 3.50–3.58 (m, 2H, 6ꞌ-CH2), 3.64 (d, 1H, J = 13.2 Hz, 2ꞌ-CH2), 3.94 (dd, 1H, J = 9.0, 8.7 Hz, 5-CH2), 4.85 (dd, 1H, J = 7.2, 7.2 Hz, 4-CH), 6.66–7.43 (m, 15H, Ar-H), 8.41 (s, 1H, 1ꞌꞌ-NH). 13C-NMR (75 MHz, CDCl3): δC 35.07, 46.43, 48.66, 50.38, 57.35, 66.75, 76.29, 109.46, 122.55, 127.22, 127.99, 128.29, 128.59, 128.66, 128.97, 129.26, 130.01, 130.24, 135.16, 135.63, 137.75, 138.97, 142.15, 178.69, 199.98. Anal. calcd for C29H27N3O2: C, 77.48; H, 6.05; N, 9.35; found: C, 77.34; H, 6.23; N, 9.28.
1-Methyl-4-(2-methylphenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(2-methylphenylmethylidene) piperidin-4′-one (4b). Obtained as a pale yellow solid, (0.140 g, 90%); mp = 163–164 °C; IR (KBr): 1602, 1615, 1711, 3405 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.06–2.09 (m, 1H, 2'-CH2), 2.15 (s, 3H, N-CH3), 2.17 (s, 3H, CH3), 2.32 (s, 3H, CH3), 3.25–3.56 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 4.00 (t, 1H, J = 9.2 Hz, 5-CH2), 4.98 (t, 1H, J = 8.0 Hz, 4-CH), 6.72–7.57 (m, 11H, Ar-H), 7.70 (d, 1H, J = 6.8 Hz, Ar-H), 7.83 (d, 1H, J = 8.0 Hz, Ar-H), 8.23 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 20.44, 21.55, 35.06, 42.46, 48.66, 51.44, 58.81, 64.72, 76.04, 109.60, 122.98, 125.69, 126.21, 126.99, 128.38, 128.85, 128.99, 129.18, 129.74, 129.83, 130.47, 130.56, 130.61, 131.15, 134.12, 134.54, 137.53, 138.09, 142.30, 178.21, 199.75. Anal. calcd for C31H31N3O2: C, 77.96; H, 6.54; N, 8.80; found: C, 77.81; H, 6.65; N, 8.69.
1-Methyl-4-(2-methoxyphenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(2-methoxyphenylmethylid ene)piperidin-4′-one (4c). Obtained as a white solid, (0.130 g, 86%); mp = 159–160 °C; IR (KBr): 1599, 1617, 1705, 3410 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.15 (s, 3H, N-CH3), 2.30 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.24–3.71 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 3.73 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 4.10 (dd, 1H, J = 9.3, 9.0 Hz, 5-CH2), 4.93 (dd, 1H, J = 8.1, 7.5 Hz, 4-CH), 6.68–7.65 (m, 13H, Ar-H), 8.37 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 35.28, 40.39, 48.42, 55.07, 55.34, 55.71, 56.28, 64.03, 76.44, 109.66, 110.25, 110.94, 120.18, 120.89,122.50, 122.89, 124.90, 126.47, 127.96, 128.72, 128.98, 129.18, 130.48, 130.63, 133.59, 134.32, 142.28, 158.34, 158.48, 178.51, 199.07. Anal. calcd for C31H31N3O4: C, 73.06; H, 6.13; N, 8.25; found: C, 73.28; H, 6.29; N, 8.32.
1-Methyl-4-(2-chlorophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(2-chlorophenylmethylidene)piperidin-4′-one (4d). Obtained as a white solid, (0.137 g, 91%); mp = 168–169 °C; IR (KBr): 1598, 1615, 1706, 3409 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.03 (d, 1H, J = 14.2 Hz, 2'-CH2), 2.13 (s, 3H, N-CH3), 3.25–3.57 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 3.99 (t, 1H, J = 8.8 Hz, 5-CH2), 5.14 (t, 1H, J = 8.8 Hz, 4-CH), 6.72 (d, 1H, J = 7.6 Hz, Ar-H), 6.87–7.31 (m, 8H, Ar-H), 7.34 (d, 2H, J = 8.0 Hz, Ar-H), 7.64 (s, 1H, C=CH), 8.00 (d, 1H, J = 8.0 Hz, Ar-H), 8.31 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 35.12, 43.14, 48.52, 50.83, 57.97, 64.01, 77.67, 109.62, 123.54, 126.56, 126.83, 127.12, 128.37, 128.58, 129.32, 129.59, 129.64, 130.10, 130.48, 131.29, 134.06, 135.05, 135.30, 135.61, 136.27, 137.30, 141.98, 178.20, 198.60. Anal. calcd for C29H25Cl2N3O2: C, 67.19; H, 4.86; N, 8.11; found: C, 67.30; H, 4.70; N, 8.04.
1-Methyl-4-(2-bromophenyl)pyrrolo-(spiro[2.3ꞌꞌ]oxindole)-spiro[3.3′]-5′-(2-bromophenylmethylidene)piperidin-4′-one (4e). Obtained as a white solid, (0.126 g, 90%); mp = 143–144 °C; IR (KBr): 1605, 1614, 1702, 3412 cm−1; 1H-NMR (400 MHz, CDCl3): δH 1.99 (d, 1H, J = 14.8 Hz, 2'-CH2), 2.13 (s, 3H, N-CH3), 3.24–3.57 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 3.96 (t, 1H, J = 8.8 Hz, 5-CH2), 5.08 (t, 1H, J = 8.4 Hz, 4-CH), 6.74 (d, 1H, J = 7.6 Hz, Ar-H), 6.89 (d, 1H, J = 7.2 Hz, Ar-H), 6.98–7.61 (m, 8H, Ar-H), 7.65 (s, 1H, C=CH), 7.94 (t, 1H, J = 8.8 Hz, Ar-H), 8.05 (d, 1H, J = 7.6 Hz, Ar-H), 8.42 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 35.10, 46.09, 48.27, 50.78, 58.47, 63.85, 76.14, 109.68, 123.64, 125.46, 126.81, 127.19, 127.64, 127.70, 128.59, 128.69, 129.33, 130.24, 130.46, 131.73, 132.95, 133.01, 133.35, 135.85, 137.26, 139.08, 142.05, 178.22, 198.58. Anal. calcd for C29H25Br2N3O2: C, 57.35; H, 4.15; N, 6.92; found: C, 57.52; H, 4.01; N, 6.84.
1-Methyl-4-(2-fluorophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(2-fluorophenylmethylidene)piperidin-4′-one (4f). Obtained as a white solid, (0.143 g, 92%); mp = 165–166 °C; IR (KBr): 1603, 1617, 1705, 3408 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.14 (s, 3H, N-CH3), 2.30 (d, 1H, J = 13.6 Hz, 2'-CH2), 3.27–3.55 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 3.98 (t, 1H, J = 9.6 Hz, 5-CH2), 5.08 (t, 1H, J = 9.6 Hz, 4-CH), 6.68 (d, 1H, J = 7.6 Hz, Ar-H), 6.83–7.32 (m, 11H, Ar-H), 7.76 (t, 1H, J = 7.2 Hz, Ar-H), 8.01 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 35.07, 38.61, 48.80, 50.68, 56.97, 65.03, 76.89, 109.42, 115.45 (2JCF = 22.6 Hz), 116.00 (2JCF = 21.8 Hz), 122.99, 123.92, 123.96, 126.49, 127.15, 128.25, 128.59, 128.73, 129.15, 129.30, 130.68, 130.79, 130.87, 136.23, 141.90, 160.68, 162.23, 178.20, 198.37. Anal. calcd for C29H25F2N3O2: C, 71.74; H, 5.19; N, 8.65; found: C, 71.95; H, 5.08; N, 8.78.
1-Methyl-4-(2,4-dichlorophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(2,4-dichlorophenylmeth ylidene)piperidin-4′-one (4g). Obtained as a white solid, (0.127 g, 90%); mp = 171–172 °C; IR (KBr): 1600, 1619, 1708, 3406 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.11 (s, 3H, N-CH3), 2.22–2.25 (m, 1H, 2'-CH2), 3.27–3.56 (m, 4H, 6'-CH2,2'-CH2 and 5-CH2), 3.91 (t, 1H, J = 9.0 Hz, 5-CH2), 5.07 (t, 1H, J = 8.7 Hz, 4-CH), 6.69–7.44 (m, 8H, Ar-H), 7.54 (s, 1H, Ar-H), 7.86 (d, 1H, J = 8.4 Hz, Ar-H), 7.95 (d, 1H, J = 8.7 Hz, Ar-H), 8.77 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 35.10, 42.65, 48.11, 49.95, 57.10, 63.92, 76.54, 109.20, 123.47, 125.93, 126.55, 127.03, 128.36, 129.34, 129.48, 130.06, 130.87, 132.36, 132.54, 133.46, 133.93, 134.41, 135.45, 135.80, 136.04, 136.78, 142.15, 178.33, 198.39. Anal. calcd for C29H23Cl4N3O2: C, 59.30; H, 3.95; N, 7.15; found: C, 59.45; H, 3.77; N, 7.01.
1-Methyl-4-(3-nitrophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(3-nitrophenylmethylidene)piperidin-4′-one (4h). Obtained as a pale yellow solid, (0.131 g, 89%); mp = 185–186 °C; IR (KBr): 1599, 1617, 1705, 3408 cm−1; 1H-NMR (400 MHz, MeOH): δH 2.09–2.13 (m, 1H, 2'-CH2), 2.17 (s, 3H, N-CH3), 3.44 (t, 1H, J = 7.6 Hz, 5-CH2), 3.51–3.60 (m, 2H, 6'-CH2), 3.64 (d, 1H, J = 12.8 Hz, 2'-CH2) 3.94 (t, 1H, J = 9.6 Hz, 5-CH2), 4.90 (dd, 1H, J = 7.6, 7.2 Hz, 4-CH), 6.74 (d, 1H, J = 7.6 Hz, Ar-H), 6.92–8.12 (m, 11H, Ar-H), 8.34 (s, 1H, 1"-NH). 13C-NMR (100 MHz, MeOH): δC 34.92, 45.92, 48.40, 50.39, 57.54, 66.71, 76.10, 109.75, 122.50, 122.62, 123.56, 124.40, 124.74, 127.41, 128.20, 129.59, 129.68, 129.73, 134.98, 135.67, 136.39, 137.03, 137.12, 141.23, 142.22, 148.50, 148.75, 178.18, 199.03. Anal. calcd for C29H25N5O6: C, 64.56; H, 4.67; N, 12.98; found: C, 64.68; H, 4.59; N, 12.85.
1-Methyl-4-(4-methylphenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(4-methylphenylmethylidene)piperidin-4′-one (4i). Obtained as a white solid, (0.146 g, 93%); mp = 180–181 °C; IR (KBr): 1607, 1621, 1710, 3412 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.14 (s, 3H, N-CH3), 2.20 (d, 1H, J = 13.6 Hz,2'-CH2), 2.30 (s, 3H, CH3), 2.31 (s, 3H, CH3), 3.15–3.63 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 3.91 (t, 1H, J = 9.6 Hz, 5-CH2), 4.80 (t, 1H, J = 9.6 Hz, 4-CH), 6.63–7.32 (m, 13H, Ar-H), 8.08 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 21.43, 21.69, 35.04, 46.18, 48.73, 50.29, 57.51, 66.51, 76.40, 109.33, 122.55, 128.33, 129.19, 129.54, 129.71, 129.89, 130.16, 130.41, 132.84, 134.38, 135.83, 136.77, 137.80, 139.27, 142.02, 178.64, 200.02. Anal. calcd for C31H31N3O2: C, 77.96; H, 6.54; N, 8.80; found: C, 78.10; H, 6.46; N, 8.73.
1-Methyl-4-(4-methoxyphenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(4-methoxyphenylmethylidene)piperidin-4′-one (4j). Obtained as a pale yellow solid, (0.129 g, 85%); mp = 187–188 °C; IR (KBr): 1600, 1615, 1706, 3410 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.14 (s, 3H, N-CH3), 2.21 (d, 1H, J = 13.2 Hz, 2'-CH2), 3.35 (dd, 1H, J = 7.2, 7.2 Hz, 5-CH2), 3.55–3.76 (m, 2H, 6'-CH2), 3.77 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.80–3.91 (m, 2H, 5-CH2 and 2'-CH2), 4.78 (dd, 1H, J = 7.2, 7.2 Hz, 4-CH), 6.65 (d, 1H, J = 7.6 Hz, Ar-H), 6.77–7.09 (m, 8H, Ar-H), 7.13 (d, 2H, J = 7.6 Hz, Ar-H), 7.35 (d, 2H, J = 8.8 Hz, Ar-H), 8.27 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 34.61, 45.52, 48.36, 49.82, 55.18, 55.22, 57.37, 65.85, 76.12, 108.86, 113.60, 113.74, 122.05, 127.61, 127.90, 128.71, 130.58, 131.84, 132.37, 132.72, 137.27, 141.66, 158.45, 159.97, 178.22, 199.52. Anal. calcd for C31H31N3O4: C, 73.06; H, 6.13; N, 8.25; found: C, 73.17; H, 6.01; N, 8.34.
1-Methyl-4-(4-chlorophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(4-chlorophenylmethylidene)piperidin-4′-one (4k). Obtained as a pale yellow solid, (0.140 g, 93%); mp = 169–170°C; IR (KBr): 1602, 1617, 1709, 3410 cm−1; 1H-NMR (500 MHz, CDCl3): δH 2.07–2.10 (m, 1H, 2'-CH2), 2.13 (s, 3H, N-CH3), 3.34 (t, 1H, J = 8.0 Hz, 5-CH2), 3.48–3.54 (m, 2H, 6'-CH2), 3.60 (d, 1H, J = 13.0 Hz, 2'-CH2), 3.86 (t, 1H, J = 10.0 Hz, 5-CH2), 4.79 (dd, 1H, J = 7.5, 7.5 Hz, 4-CH), 6.67 (d, 1H, J = 8.0 Hz, Ar-H), 6.91 (d, 2H, J = 8.0 Hz, Ar-H), 6.97 (t, 1H, J = 7.5 Hz, Ar-H), 7.00 (s, 1H, C=CH), 7.07–7.13 (m, 2H, Ar-H), 7.21–7.28 (m, 4H, Ar-H), 7.35 (d, 2H, J = 8.5 Hz, Ar-H), 8.45 (s, 1H, 1"-NH). 13C-NMR (125 MHz, CDCl3): δC 34.60, 45.32, 48.21, 49.92, 56.99, 66.17, 75.81, 109.17, 122.15, 127.81, 128.42, 128.53, 128.78, 129.00, 130.97, 131.07, 132.69, 133.50, 134.71, 134.98, 136.16, 137.00, 141.77, 178.30, 199.24. Anal. calcd for C29H25Cl2N3O2: C, 67.19; H, 4.86; N, 8.11; found: C, 67.07; H, 4.73; N, 8.19.
1-Methyl-4-(4-bromophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(4-bromophenylmethylidene)piperidin-4′-one (4l). Obtained as a pale yellow solid, (0.127 g, 91%); mp = 172–173 °C; IR (KBr): 1598, 1619, 1710, 3409 cm−1; 1H-NMR (300 MHz, MeOH): δH 2.11 (s, 3H, N-CH3), 2.17 (d, 1H, J = 13.2 Hz, 2'-CH2), 3.32–3.56 (m, 4H, 5-CH2,6'-CH2 and 2'-CH2), 3.90 (dd, 1H, J = 9.0, 9.0 Hz, 5-CH2), 4.64 (s, 1H, 1"-NH), 4.76 (dd, 1H, J = 7.5, 7.2 Hz, 4-CH), 6.72 (d, 1H, J = 7.8 Hz, Ar-H), 6.98–7.18 (m, 6H, Ar-H), 7.34 (d, 2H, J = 8.4 Hz, Ar-H), 7.45–7.48 (m, 4H, Ar-H). 13C-NMR (75 MHz, MeOH): δC 33.86, 45.84, 48.28, 50.01, 57.10, 65.86, 76.31, 109.55, 120.81, 121.91, 122.96, 127.15, 127.74, 129.36, 131.43, 131.56, 131.69, 131.77, 134.43, 135.74, 136.13, 138.06, 143.25, 178.53, 199.78. Anal. calcd for C29H25Br2N3O2: C, 57.35; H, 4.15; N, 6.92; found: C, 57.48; H, 4.27; N, 6.99.
1-Methyl-4-(4-fluorophenyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(4-fluorophenylmethylidene)piperidin-4′-one (4m). Obtained as a white solid, (0.146 g, 94%); mp = 174–175 °C; IR (KBr): 1601, 1618, 1709, 3411 cm−1; 1H-NMR (300 MHz, MeOH): δH 2.10 (s, 3H, N-CH3), 2.18 (d, 1H, J = 13.2 Hz, 2'-CH2), 3.26–3.57 (m, 4H, 5-CH2, 6'-CH2 and 2'-CH2), 3.89 (dd, 1H, J = 9.3, 9.0 Hz, 5-CH2), 4.65 (s, 1H, 1"-NH), 4.78 (dd, 1H, J = 7.5, 7.2 Hz, 4-CH), 6.73 (d, 1H, J = 7.5 Hz, Ar-H), 6.89–7.17 (m, 11H, Ar-H), 7.39–7.44 (m, 1H, Ar-H). 13C-NMR (75 MHz, MeOH): δC 33.90, 45.73, 48.22, 50.02, 57.40, 65.75, 76.42, 109.54, 114.64 (2JCF = 21.2 Hz), 115.30 (2JCF = 21.8 Hz), 121.93, 127.22, 127.75, 129.32, 131.30 (JCF = 77.3 Hz), 131.73 (JCF = 3.3 Hz), 132.25 (JCF = 83.3 Hz), 134.64 (2JCF = 3.2 Hz), 134.87, 136.42, 143.22, 162.32 (1JCF = 242.7 Hz), 163.14 (1JCF = 247.4 Hz), 178.58, 199.98. Anal. calcd for C29H25F2N3O2: C, 71.74; H, 5.19; N, 8.65; found: C, 71.87; H, 5.31; N, 8.59.
1-Methyl-4-(1-naphthyl)pyrrolo-(spiro[2.3″]oxindole)-spiro[3.3′]-5′-(1-naphthylmethylidene)piperidin-4′-one (4n). Obtained as a pale yellow solid, (0.132 g, 90%); mp = 160–161 °C; IR (KBr): 1698, 1620, 1706, 3410 cm−1; 1H-NMR (300 MHz, DMSO): δH 1.66 (d, 1H, J = 12.6 Hz, 2'-CH2), 2.00 (s, 3H, N-CH3), 3.07 (d, 1H, J = 15.8 Hz, 6'-CH2), 3.19 (d, 1H, J = 15.8 Hz, 6'-CH2), 3.35 (t, 1H, J = 7.8 Hz, 5-CH2), 3.55 (d, 1H, J = 12.6 Hz, 2'-CH2), 4.05 (t, 1H, J = 9.3 Hz, 5-CH2), 5.50 (t, 1H, J = 8.4 Hz, 4-CH), 6.66 (d, 1H, J = 6.6 Hz, Ar-H), 7.02–7.96 (m, 17H, Ar-H), 8.12 (d, 1H, J = 8.7 Hz, Ar-H), 10.46 (s, 1H, 1"-NH). 13C-NMR (75 MHz, DMSO): δC 34.95, 40.63, 49.23, 51.52, 58.72, 64.93, 76.59, 109.95, 122.19, 124.48, 125.49, 125.89, 126.17, 126.33, 127.12, 127.32, 127.47, 127.61, 127.79, 128.03, 129.23, 129.36, 129.56, 129.74, 129.80, 131.64, 132.54, 133.63, 133.74, 134.37, 135.14, 135.84, 137.0, 144.41, 177.57, 199.76. Anal. calcd for C37H31N3O2: C, 80.85; H, 5.68; N, 7.64; found: C, 80.96; H, 5.82; N, 7.55.
4,5-Diphenylpyrrolo(spiro[2.3′′]-oxindole)-spiro-[3.3′]-5′-(phenylmethylidene)piperidin-4′-one (5a). Obtained as a white solid, (0.170 g, 93%); mp = 162–163 °C; IR (KBr): 1593, 1613, 1698, 3179, 3344 cm−1; 1H-NMR (500 MHz, CDCl3): δH 2 .31 (d, 1H, J = 13.0 Hz, 2'-CH2), 3.52 (dd, 1H, J = 14.5, 2.5 Hz, 6'-CH2), 3.64 (d, 1H, J = 15.0 Hz, 6'-CH2), 3.77 (dd, 1H, J = 13.0, 1.0 Hz, 2'-CH2), 4.73 (d, 1H, J = 11.0 Hz, 4-CH), 5.43 (d, 1H, J = 11.0 Hz, 5-CH), 6.67 (d, 1H, J = 7.5 Hz, Ar-H), 6.98–7.04 (m, 4H, Ar-H), 7.12–7.28 (m, 11H, Ar-H), 7.41 (d, 2H, J = 7.0 Hz, Ar-H), 7.53 (d, 2H, J = 7.5 Hz, Ar-H), 7.99 (s, 1H, 1"-NH). 13C-NMR (125 MHz, CDCl3): δC48.19, 49.74, 56.85, 64.24, 67.34, 71.82, 109.21, 122.20, 126.85, 127.02, 127.57, 127.66, 128.25, 128.33, 128.70, 128.99, 129.21, 129.89, 129.93, 134.86, 135.12, 137.09, 137.38, 140.74, 141.00, 180.91, 200.14. Anal. calcd for C34H29N3O2: C, 79.82; H, 5.71; N, 8.21; found: C, 79.70; H, 5.85; N, 8.15.
4-(2-Methylphenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(2-methylphenylmethylidene) piperidin-4′-one (5b). Obtained as a pale yellow solid, (0.160 g, 90%); mp = 150–151 °C; IR (KBr): 1595, 1618, 1697, 3168, 3340 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.11 (s, 1H, CH3), 2.20 (s, 1H, CH3), 2.27–2.32 (m, 1H, 2'-CH2), 3.41 (d, 1H, J = 15.3 Hz, 6'-CH2), 3.51 (dd, 1H, J = 15.3, 2.4 Hz, 6'-CH2), 3.58 (d, 1H, J = 13.2 Hz, 2'-CH2), 4.90 (d, 1H, J = 9.9 Hz, 4-CH), 5.54 (d, 1H, J = 9.9 Hz, 5-CH), 6.73 (d, 1H, J = 7.5 Hz, Ar-H), 6.82 (d, 1H, J = 7.5 Hz, Ar-H), 6.92–7.55 (m, 15H, Ar-H), 7.63 (s, 1H, C=CH), 8.42 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 20.49, 21.44, 48.43, 51.42, 54.54, 65.44, 66.53, 74.25, 109.94, 122.91, 125.80, 125.98, 126.29, 126.98, 127.83, 127.91, 128.81, 128.94, 129.42, 129.64, 130.58, 130.78, 134.36, 134.54, 135.46, 135.60, 136.82, 137.44, 138.21, 138.31, 138.63, 141.79, 180.90, 201.06. Anal. calcd for C36H33N3O2: C, 80.12; H, 6.16; N, 7.79; found: C, 80.28; H, 6.27; N, 7.70.
4-(2-Methoxyphenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(2-methoxyphenylmethylidene)piperidin-4′-one (5c). Obtained as a pale yellow solid, (0.144 g, 85%); mp = 155–156 °C; IR (KBr): 1597, 1614, 1695, 3173, 3342 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.43 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.26–3.62 (m, 3H, 6'-CH2 and 2'-CH2), 3.65 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.89 (d, 1H, J = 9.9 Hz, 4-CH), 5.74 (d, 1H, J = 9.9 Hz, 5-CH), 6.67–7.86 (m, 18H, Ar-H and C=CH), 8.02 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.50, 49.76, 54.32, 55.41, 55.80, 63.89, 66.56, 74.29, 110.36, 111.05, 111.12, 120.31, 120.51, 121.09, 122.83, 124.72, 127.33, 127.88, 128.07, 128.31, 128.85, 129.29, 130.53, 130.88, 131.03, 133.42, 141.81, 158.23, 158.76, 180.01, 201.03. Anal. calcd for C36H33N3O4: C, 75.64; H, 5.82; N, 7.35; found: C, 75.50; H, 5.97; N, 7.18.
4-(2-Chlorophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(2-chlorophenylmethylidene)piperidin-4′-one (5d). Obtained as a white solid, (0.155 g, 92%); mp = 151–152 °C; IR (KBr): 1590, 1617, 1692, 3175, 3340 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.97 (br s, 1H, NH), 2.41 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.27 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.42 (d, 1H, J = 15.6 Hz, 6'-CH2), 3.61 (d, 1H, J = 15.6 Hz, 6'-CH2), 5.11 (d, 1H, J = 9.0 Hz, 4-CH), 5.66 (d, 1H, J = 9.0 Hz, 5-CH), 6.69 (d, 1H, J = 7.5 Hz, Ar-H), 6.97–7.39 (m, 12H, Ar-H), 7.58–7.66 (m, 3H, Ar-H), 7.84 (s, 1H, C=CH), 8.15 (d, 1H, J = 7.2 Hz, Ar-H), 8.68 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.52, 50.71, 55.41, 64.74, 65.74, 75.18, 109.92, 123.44, 126.66, 127.17, 127.29, 127.72, 128.12, 128.18, 128.47, 129.00, 129.51, 129.71, 130.19, 130.32, 130.52, 131.09, 133.87, 135.11, 135.33, 135.58, 136.52, 136.70, 141.22, 141.64, 179.14, 200.63. Anal. calcd for C34H27Cl2N3O2: C, 70.35; H, 4.69; N, 7.24; found: C, 70.47; H, 4.61; N, 7.16.
4-(2-Bromophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(2-bromophenylmethylidene)piperidin-4′-one (5e). Obtained as a white solid, (0.137 g, 89%); mp = 153–154 °C; IR (KBr): 1590, 1611, 1694, 3184, 3337 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.91 (br s, 1H, NH), 2.45 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.23 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.40 (d, 1H, J = 15.6 Hz, 6'-CH2), 3.62 (dd, 1H, J = 15.6, 2.1 Hz, 6'-CH2), 5.06 (d, 1H, J = 9.3 Hz, 4-CH), 5.67 (d, 1H, J = 9.3 Hz, 5-CH), 6.71 (d, 1H, J = 7.8 Hz, Ar-H), 6.95–7.44 (m, 8H, Ar-H), 7.48 (d, 2H, J = 7.5 Hz, Ar-H), 7.58 (d, 2H, J = 7.2 Hz, Ar-H), 7.67 (d, 2H, J = 7.2 Hz, Ar-H), 7.84 (s, 1H, C=CH), 8.21(d, 1H, J = 7.8 Hz, Ar-H), 8.67 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.28, 50.66, 58.56, 64.64, 66.37, 75.47, 110.01, 123.57, 125.76, 127.16, 127.32, 127.59, 127.93, 128.13, 128.22, 128.54, 128.83, 129.04, 129.55, 130.54, 131.49, 133.07, 133.45, 135.14, 135.62, 137.38, 138.44, 141.13, 141.68, 178.95, 200.93. Anal. calcd for C34H27Br2N3O2: C, 61.00; H, 4.07; N, 6.28; found: C, 61.13; H, 4.26; N, 6.40.
4-(2-Fluorophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(2-fluorophenylmethylidene)piperidin-4′-one (5f). Obtained as a white solid, (0.158 g, 90%); mp = 148–149 °C; IR (KBr): 1592, 1618, 1694, 3178, 3345 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.20 (br s, 1H, NH), 2.43 (d, 1H, J = 13.5 Hz, 2'-CH2), 3.31–3.66 (m, 3H, 2'-CH2 and 6'-CH2), 5.00 (d, 1H, J = 9.9 Hz, 4-CH), 5.57 (d, 1H, J = 9.9 Hz, 5-CH), 6.68 (d, 1H, J = 7.5 Hz, Ar-H), 6.90–7.63 (m, 16H, Ar-H), 7.87 (s, 1H, C=CH), 8.65 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.79, 50.39, 63.10, 64.48, 65.97, 73.65, 109.92, 115.64 (2JCF = 23.3 Hz), 116.08 (2JCF = 21.7 Hz), 122.94, 123.39, 123.57, 124.07 (JCF = 3.0 Hz), 124.48 (JCF = 3.0 Hz), 127.28, 128.01, 128.18, 128.31, 128.92, 129.41, 129.56, 130.16, 130.61, 130.80, 131.16, 136.04, 140.98, 141.59, 161.15 (1JCF = 250.6 Hz), 161.96 (1JCF = 244.1 Hz), 180.24, 199.77. Anal. calcd for C34H27F2N3O2: C, 74.57; H, 4.97; N, 7.67; found: C, 74.45; H, 4.88; N, 7.74.
4-(2,4-Dichlorophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(2,4-dichlorophenyl methylidenedichlorophenylmethylidene)piperidin-4′-one (5g). Obtained as a white solid, (0.144 g, 92%); mp = 160–161 °C; IR (KBr): 1599, 1618, 1704, 3182, 3338 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.35 (d, 1H, J = 13.5 Hz, 2'-CH2), 3.23–3.42 (m, 2H, 2'-CH2 and 6'-CH2), 3.56 (d, 1H, J = 15.3 Hz, 6'-CH2), 5.04 (d, 1H, J = 9.3 Hz, 4-CH), 5.58 (d, 1H, J = 9.3 Hz, 5-CH), 6.69 (d, 1H, J = 7.2 Hz, Ar-H), 6.88 (d, 1H, J = 8.1 Hz, Ar-H), 6.97 (d, 1H, J = 7.5 Hz, Ar-H), 7.12–7.29 (m, 5H, Ar-H), 7.40 (s, 1H, Ar-H), 7.54 (d, 2H, J = 7.2 Hz, Ar-H), 7.61 (d, 2H, J = 7.8 Hz, Ar-H), 7.76 (s, 1H, C=CH), 7.88 (d, 1H, J = 7.2 Hz, Ar-H), 8.09 (d, 1H, J = 8.1 Hz, Ar-H), 8.90 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.49, 50.62, 54.85, 64.54, 65.89, 75.09, 110.16, 123.42, 127.13, 127.47, 127.64, 128.02, 128.43, 129.12, 129.42, 129.52, 130.18, 131.16, 131.93, 132.21, 133.64, 134.12, 134.92, 135.27, 135.51, 135.71, 136.34, 137.08, 140.77, 141.75, 179.13, 200.24. Anal. calcd for C34H25Cl4N3O2: C, 62.88; H, 3.88; N, 6.47; found: C, 62.75; H, 3.97; N, 6.40.
4-(3-Nitrophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(3-nirophenylmethylidene) piperidin-4′-one (5h). Obtained as a white solid, (0.149 g, 91%); mp = 154–155 °C; IR (KBr): 1596, 1612, 1702, 3186, 3336 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.26 (d, 1H, J = 12.9 Hz, 2'-CH2), 3.43–3.85 (m, 3H, 2'-CH2 and 6'-CH2), 4.78 (d, 1H, J = 10.5 Hz, 4-CH), 5.43 (d, 1H, J = 10.5 Hz, 5-CH), 6.70 (m, 11H, Ar-H), 7.80 (s, 1H, C=CH), 7.87–8.13 (m, 4H, Ar-H), 8.20 (s, 1H, Ar-H), 8.35 (s, 1H, Ar-H), 8.76 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.33, 56.82, 63.88, 65.01, 67.37, 72.16, 110.16, 122.62, 123.71, 124.55, 125.02, 127.31, 127.95, 128.50, 129.01, 129.42, 129.71, 129.84, 130.17, 134.77, 135.81, 136.76, 136.88, 137.20, 140.01, 140.29, 141.81, 148.46, 148.64, 181.44, 199.69. Anal. calcd for C34H27N5O6: C, 67.88; H, 4.52; N, 11.64; found: C, 67.73; H, 4.73; N, 11.46.
4-(4-Methylphenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(4-methylphenylmethylidene) piperidin-4′-one (5i). Obtained as a pale yellow solid, (0.164 g, 92%); mp = 157–158 °C; IR (KBr): 1597, 1615, 1690, 3181, 3340 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.25 (s, 1H, CH3), 2.29 (s, 1H, CH3), 2.34 (d, 1H, J = 13.2 Hz, 2'-CH2), 3.50 (d, 1H, J = 15.0 Hz, 6'-CH2), 3.60 (d, 1H, J = 15.0 Hz, 6'-CH2), 3.70 (d, 1H, J = 13.2 Hz, 2'-CH2), 4.68 (d, 1H, J = 10.5 Hz, 4-CH), 5.40 (d, 1H, J = 10.5 Hz, 5-CH), 6.65 (d, 1H, J = 7.5 Hz, Ar-H), 6.76 (d, 1H, J = 7.8 Hz, Ar-H), 6.91 (d, 2H, J = 8.1 Hz, Ar-H), 6.97–7.30 (m, 12H, Ar-H), 7.52 (d, 2H, J = 6.9 Hz, Ar-H), 8.72 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 21.49, 21.77, 48.63, 50.03, 57.33, 64.90, 67.44, 72.62, 109.86, 122.53, 127.31, 127.95, 128.11, 128.76, 129.24, 129.42, 129.46, 130.17, 130.58, 132.73, 134.46, 134.73, 135.52, 136.76, 137.66, 139.52, 141.30, 141.69, 181.55, 200.83. Anal. calcd for C36H33N3O2: C, 80.12; H, 6.16; N, 7.79; found: C, 80.03; H, 6.24; N, 7.85.
4-(4-Methoxyphenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(4-methoxyphenylmethyl idene)piperidin-4′-one (5j). Obtained as a pale yellow solid, (0.146 g, 86%); mp = 159–160 °C; IR (KBr): 1598, 1620, 1710, 3179, 3347 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.44 (d, 1H, J = 13.8 Hz, 2'-CH2), 3.28–3.63 (m, 3H, 6'-CH2 and 2'-CH2), 3.66 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 4.89 (d, 1H, J = 9.9 Hz, 4-CH), 5.74 (d, 1H, J = 9.9 Hz, 5-CH), 6.69–7.87 (m, 18H, Ar-H), 8.20 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.53, 50.07, 55.42, 55.79, 57.46, 64.93, 67.51, 72.69, 110.35, 111.05, 111.11, 120.30, 120.50, 121.09, 122.86, 124.74, 127.38, 127.87, 128.07, 128.31, 128.84, 129.41, 130.52, 130.85, 131.03, 141.81, 158.52, 158.76, 181.22, 199.93. Anal. calcd for C36H33N3O4: C, 75.64; H, 5.82; N, 7.35; found: C, 75.78; H, 5.93; N, 7.42.
4-(4-Chlorophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(4-chlorophenylmethylidene)piperidin-4′-one (5k). Obtained as a pale yellow solid, (0.151 g, 90%); mp = 154–155 °C; IR (KBr): 1598, 1618, 1711, 3179, 3338 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.09 (s, 1H, NH), 2.27 (d, 1H, J = 13.2 Hz, 2'-CH2), 3.46–3.60 (m, 2H, 6'-CH2), 3.72 (d, 1H, J = 13.2 Hz, 2'-CH2), 4.67 (d, 1H, J = 10.8 Hz, 4-CH), 5.35 (d, 1H, J = 10.8 Hz, 5-CH), 6.67 (d, 1H, J = 7.2 Hz, Ar-H), 6.77 (d, 1H, J = 7.2 Hz, Ar-H), 6.90–7.54 (m, 16H, Ar-H), 8.49 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.52, 50.03, 56.65, 64.81, 67.43, 72.25, 109.85, 122.58, 124.35, 127.32, 127.98, 128.34, 128.76, 128.87, 129.00, 129.31, 129.53, 131.57, 132.07, 133.16, 133.79, 135.25, 135.42, 136.33, 140.72, 141.61, 181.42, 200.18. Anal. calcd for C34H27Cl2N3O2: C, 70.35; H, 4.69; N, 7.24; found: C, 70.51; H, 4.80; N, 7.31.
4-(4-Bromophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(4-bromophenylmethylidene)piperidin-4′-one (5l). Obtained as a white solid, (0.142 g, 92%); mp = 166–167 °C; IR (KBr): 1597, 1615, 1705, 3174, 3340 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.08 (s, 1H, NH), 2.27 (d, 1H, J = 12.9 Hz, 2'-CH2), 3.46–3.79 (m, 3H, 6'-CH2 and 2'-CH2), 4.66 (d, 1H, J = 10.8 Hz, 4-CH), 5.34 (d, 1H, J = 10.8 Hz, 5-CH), 6.66–6.72 (m, 2H, Ar-H), 6.84 (d, 2H, J = 8.1 Hz, Ar-H), 6.93 (s, 1H, C=CH), 6.97–7.51 (m, 13H, Ar-H), 8.39 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.52, 50.03, 56.67, 64.73, 67.43, 72.21, 109.84, 121.36, 122.59, 123.59, 127.98, 128.24, 128.88, 129.54, 129.77, 130.18, 131.77, 131.85, 131.97, 132.27, 134.23, 135.53, 136.37, 136.79, 140.69, 141.58, 181.33, 200.14. Anal. calcd for C34H27Br2N3O2: C, 61.00; H, 4.07; N, 6.28; found: C, 61.17; H, 4.21; N, 6.35.
4-(4-Fluorophenyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(4-fluorophenylmethylidene) piperidin-4′-one (5m). Obtained as a pale yellow solid, (0.164 g, 93%); mp = 159–160 °C; IR (KBr): 1596, 1617, 1700, 3181, 3346 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.13 (s, 1H, NH), 2.28 (d, 1H,J = 13.2 Hz, 2'-CH2), 3.48 (d, 1H, J = 14.7 Hz, 6'-CH2), 3.58 (d, 1H, J = 14.7 Hz, 6'-CH2), 3.70 (d, 1H, J = 13.2 Hz, 2'-CH2), 4.67 (d, 1H, J = 10.5 Hz, 4-CH), 5.34 (d, 1H, J = 10.5 Hz, 5-CH), 6.68 (d, 2H, J = 7.5 Hz, Ar-H), 6.89–7.55 (m, 16H, Ar-H), 8.58 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.50, 50.05, 56.79, 65.10, 67.27, 72.40, 109.86, 115.56 (2JCF = 21.0 Hz), 115.88 (2JCF = 21.5 Hz), 122.56, 127.33, 127.99, 128.15, 128.83, 129.41, 129.46, 130.17, 131.76, 132.30, 133.43, 134.77, 136.55, 140.93, 141.65, 162.23 (1JCF = 244.1 Hz), 163.13 (1JCF = 248.9 Hz), 181.43, 200.42. Anal. calcd for C34H27F2N3O2: C, 74.57; H, 4.97; N, 7.67; found: C, 74.69; H, 4.90; N, 7.76.
4-(1-Naphthyl)-5-phenylpyrrolo(spiro[2.3″]oxindole)spiro[3.3′]-5′-(1-naphthylmethylidene)piperidin-4′-one (5n). Obtained as a yellow solid, (0.142 g, 87%); mp = 164–165 °C; IR (KBr): 1590, 1619, 1704, 3174, 3335 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.94 (s, 1H, NH), 2.07 (d, 1H, J = 12.9 Hz, 2'-CH2), 3.18–3.73 (m, 3H, 6'-CH2 and 2'-CH2), 5.58 (d, 1H, J = 9.9 Hz, 4-CH), 5.80 (d, 1H, J = 9.9 Hz, 5-CH), 6.71–8.06 (m, 24H, Ar-H), 8.50 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 48.70, 50.15, 56.53, 64.71, 67.46, 72.20, 109.83, 122.96, 124.46, 125.04, 125.41, 126.45, 126.73, 127.12, 127.24, 127.58, 127.84, 127.98, 128.61, 128.82, 129.05, 129.20, 129.41, 129.99, 130.16, 132.41, 132.64, 133.24, 133.84, 134.84, 135.15, 135.62, 136.91, 137.01, 144.40, 181.38, 200.13. Anal. calcd for C42H33N3O2: C, 82.46; H, 5.44; N, 6.87; found: C, 82.39; H, 5.56; N, 6.80.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(phenylmethylidene)-tetrahydro-4′(1H)-pyridinone-4-(phenyl) hexahydro-1H-pyrrolizine (6a). Obtained as a white solid, (0.165 g, 94%); mp = 182–188 °C; IR (KBr): 1610, 1618, 1706, 3390 cm−1; 1H-NMR (400 MHz, CDCl3): δH 1.60–1.69 (m, 1H, 5-CH2), 1.82–2.01 (m, 2H, 6-CH2), 2.04–2.14 (m, 1H, 5-CH2), 2.33 (d, 1H, J = 13.2 Hz, 2'-CH2), 2.60 (td, 1H, J = 8.8, 2.4 Hz, 7-CH2), 3.07–3.13 (m, 1H, 7-CH2), 3.39 (d, 1H, J = 14.8 Hz, 6'-CH2), 3.62 (d, 1H, J = 14.8 Hz, 6'-CH2), 3.96 (d, 1H, J = 13.2 Hz, 2'-CH2), 4.38 (d, 1H, J = 11.2 Hz, 4-CH), 4.62–4.70 (m, 1H, 4a-CH), 6.73 (d, 1H, J = 6.8 Hz, Ar-H), 6.94–7.02 (m, 3H, Ar-H), 7.15–7.31 (m, 9H, Ar-H and arylmethylidene), 7.37 (d, 2H, J = 7.6 Hz, Ar-H), 8.87 (s, 1H, 1"-NH). 13C-NMR (100 MHz, CDCl3): δC 26.28, 29.29, 48.13, 48.35, 49.30, 53.64, 66.52, 71.88, 74.81, 109.97, 121.78, 127.23, 128.62, 128.69, 128.93, 129.36, 129.39, 130.03, 130.14, 135.58, 136.18, 136.30, 137.95, 142.06, 180.06, 200.63. Anal. calcd for C31H29N3O2: C, 78.29; H, 6.15; N, 8.84%; found: C, 78.12; H, 6.33; N, 8.95%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(2-methylphenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(2-methylphenyl)hexahydro-1H-pyrrolizine (6b). Obtained as a white solid, (0.154 g, 93%); mp = 184–185 °C; IR (KBr): 1605, 1622, 1705, 3387 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.58–1.70 (m, 1H, 5-CH2), 1.78–2.04 (m, 3H, 6-CH2 and 5-CH2), 2.30 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.42 (d, 1H, J = 12.9 Hz, 2'-CH2), 2.64 (td, 1H, J = 8.1, 3.0 Hz, 7-CH2), 3.09–3.17 (m, 1H, 7-CH2), 3.61 (dd, 1H, J = 15.0, 2.1 Hz, 6'-CH2), 3.97 (d, 1H, J = 15.0 Hz, 6'-CH2), 4.19 (d, 1H, J = 12.9 Hz, 2'-CH2), 4.62 (d, 1H, J = 10.8 Hz, 4-CH), 4.82–4.86 (m, 1H, 4a-CH), 6.62 (d, 1H, J = 7.5 Hz, Ar-H), 6.87–7.25 (m, 10H, Ar-H), 7.53–7.63 (m, 2H, Ar-H and arylmethylidene), 8.03 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 20.39, 21.12, 26.08, 28.59, 47.19, 48.92, 49.22, 53.22, 65.83, 70.05, 74.73, 110.12, 122.47, 125.78, 125.95, 127.34, 128.61, 129.30, 129.42, 129.59, 130.62, 130.80, 131.13, 133.45, 134.52, 135.11, 136.61, 138.26, 138.77, 141.61, 180.74, 202.27. Anal. calcd for C33H33N3O2: C, 78.70; H, 6.60; N, 8.34%; found: C, 78.59; H, 6.76; N, 8.25%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(2-methoxyphenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(2-methoxyphenyl)hexahydro-1H-pyrrolizine (6c). Obtained as a brown solid, (0.140 g, 88%); mp = 187–188 °C; IR (KBr): 1603, 1619, 1702, 3389 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.69–2.00 (m, 4H, 5-CH2 and 6-CH2), 2.62 (d, 1H, J = 12.9 Hz, 2'-CH2), 2.83 (td, 1H, J = 8.1, 3.0 Hz, 7-CH2), 3.30–3.43 (m, 1H, 7-CH2), 3.65 (s, 3H, OCH3), 3.71–3.81 (m, 1H, 6'-CH2), 3.85 (s, 3H, OCH3), 3.99–4.15 (m, 2H, 6'-CH2 and 2'-CH2), 4.64 (d, 1H, J = 10.8 Hz, 4-CH), 4.82–4.87 (m, 1H, 4a-CH), 6.62–7.37 (m, 12H, Ar-H and arylmethylidene), 7.51 (d, 1H, J = 7.2 Hz, Ar-H), 8.02 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 25.31, 27.97, 48.65, 49.36, 50.51, 54.02, 54.97, 55.88, 65.45, 70.05, 74.18, 110.51, 111.11, 111.17, 120.49, 120.90, 122.44, 125.24, 126.17, 128.17, 128.33, 129.61, 130.69, 130.84, 131.03, 132.16, 135.25, 135.58, 142.01, 158.02, 158.79, 180.27, 201.51. Anal. calcd for C33H33N3O4: C, 74.00; H, 6.21; N, 7.84%; found: C, 74.21; H, 6.10; N, 7.98%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(2-chlorophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(2-chlorophenyl)hexahydro-1H-pyrrolizine (6d). Obtained as a pale yellow solid, (0.145 g, 92%); mp = 172–173 °C; IR (KBr): 1605, 1618, 1702, 3389 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.79–2.19 (m, 4H, 5-CH2 and 6-CH2), 2.39 (d, 1H, J = 12.9 Hz, 2'-CH2), 2.59–2.64 (m, 1H, 7-CH2), 3.22–3.28 (m, 1H, 7-CH2), 3.45–3.66 (m, 2H, 6'-CH2), 3.95 (d, 1H, J = 12.9 Hz, 2'-CH2), 4.55–4.60 (m, 1H, 4-CH), 4.82–4.87 (m, 1H, 4a-CH), 6.61–7.74 (m, 13H, Ar-H and arylmethylidene), 8.72 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 25.81, 28.35, 48.10, 48.27, 48.81, 52.20, 64.19, 70.19, 74.78, 110.10, 122.62, 126.78, 126.86, 127.54, 128.74, 130.14, 130.54, 130.85, 131.06, 132.04, 132.92, 133.34, 133.60, 133.85, 135.47, 136.42, 136.66, 137.46, 141.69, 180.46, 201.23. Anal. calcd for C31H27Cl2N3O2: C, 68.38; H, 5.00; N, 7.72%; found: C, 68.23; H, 5.17; N, 7.61%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(2-bromophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(2-bromophenyl)hexahydro-1H-pyrrolizine (6e). Obtained as a light brown solid, (0.134 g, 92%); mp = 164–165 °C; IR (KBr): 1604, 1619, 1700, 3391 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.67–2.14 (m, 4H, 5-CH2 and 6-CH2), 2.24–2.28 (m, 1H, 2'-CH2), 2.52–2.65 (m, 1H, 7-CH2), 3.22–3.29 (m, 1H, 7-CH2), 3.46–3.61 (m, 2H, 6'-CH2), 3.92 (d, 1H, J = 12.9 Hz, 2'-CH2), 4.55–4.60 (m, 1H, 4-CH), 4.80–4.90 (m, 1H, 4a-CH), 6.60 (d, 1H, J = 7.2 Hz, Ar-H), 6.76–7.655 (m, 11H, Ar-H and arylmethylidene), 7.75 (d, 1H, J = 7.2 Hz, Ar-H), 8.99 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 25.75, 28.46, 47.89, 48.09, 48.61, 51.55, 64.19, 70.91, 75.32, 110.23, 122.47, 125.66, 127.39, 127.50, 127.61, 129.09, 130.68, 130.87, 131.06, 133.32, 133.54, 134.05, 134.64, 135.58, 135.68, 135.93, 136.27, 137.16, 141.84, 180.50, 201.21. Anal. calcd for C31H27Br2N3O2: C, 58.79; H, 4.30; N, 6.63%; found: C, 58.93; H, 4.12; N, 6.75%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(2-fluorophenylmethylidene)-tetrahydro-4′(1H)-pyridinone-4-(2-fluorophenyl)hexahydro-1H-pyrrolizine (6f). Obtained as a white solid; mp = 198–199 °C; IR (KBr): 1606, 1620, 1703, 3392 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.65–1.72 (m, 1H, 5-CH2), 1.82–1.88 (m, 1H, 6-CH2), 1.99–2.13 (m, 2H, 6-CH2 and 5-CH2), 2.35 (d, 1H, J = 13.0 Hz, 2'-CH2), 2.62 (td, 1H, J = 8.0, 2.0 Hz, 7-CH2), 3.31–3.36 (m, 1H, 7-CH2), 3.42–3.50 (m, 2H, 6'-CH2), 3.87 (d, 1H, J = 13.0 Hz, 2'-CH2), 4.64 (d, 1H, J = 10.5 Hz, 4-CH), 4.81–4.85 (m, 1H, 4a-CH), 6.72 (d, 1H, J = 7.5 Hz, Ar-H), 6.88 (t, 1H, J = 7.0 Hz, Ar-H), 6.96–7.04 (m, 4H, Ar-H), 7.12 (d, 1H, J = 8.0 Hz, Ar-H), 7.15 (d, 1H, J = 8.0 Hz, Ar-H), 7.18 (s, 1H, arylmethylidene), 7.20–7.25 (m, 3H, Ar-H), 7.29 (d, 1H, J = 8.0 Hz, Ar-H), 7.59 (t, 1H, J = 7.0 Hz, Ar-H), 8.74 (s, 1H, 1"-NH). 13C-NMR (125 MHz, CDCl3): δC 25.16, 28.59, 47.30, 47.87, 48.32, 49.88, 65.78, 69.35, 74.86, 109.50, 115.45 (2JCF = 18.0 Hz), 115.59 (2JCF = 17.0 Hz), 121.73, 123.10 (JCF = 10.0 Hz), 123.58 (JCF = 3.0 Hz), 123.96 (JCF = 3.0 Hz), 124.87 (JCF = 12.0 Hz), 126.08, 128.36 (123.10 (JCF = 7.0 Hz), 128.95, 129.22, 129.65 (JCF = 3.0 Hz), 130.36 (JCF =2.0 Hz), 130.48 (JCF =8.0 Hz), 130.76 (JCF = 4.0 Hz), 136.29, 141.62, 160.60 (1JCF = 200.0 Hz), 161.86 (1JCF = 196.0 Hz), 179.57, 199.06. Anal. calcd for C31H27F2N3O2: C, 72.78; H, 5.32; N, 8.21%; found: C, 72.96; H, 5.51; N, 8.09%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(2,4-chlorophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(2,4-chlorophenyl)hexahydro-1H-pyrrolizine (6g). Obtained as a pale yellow solid, (0.141 g, 95%); mp = 210–211 °C; IR (KBr): 1604, 1620, 1702, 3391 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.73–2.29 (m, 4H, 5-CH2 and 6-CH2), 2.60 (d, 1H, J = 12.3 Hz, 2'-CH2), 2.64–2.79 (m, 1H, 7-CH2), 3.21–3.30 (m, 1H, 7-CH2), 3.52–3.68 (m, 1H, 6'-CH2), 3.97 (d, 1H, J = 15.6 Hz, 6'-CH2), 4.14 (d, 1H, J = 12.3 Hz, 2'-CH2), 4.54–4.60 (m, 1H, 4-CH), 4.83–4.89 (m, 1H, 4a-CH), 6.62–7.92 (m, 11H, Ar-H and arylmethylidene), 8.75 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 25.82, 28.51, 48.14, 48.54, 49.25, 52.23, 65.17, 70.21, 74.73, 110.29, 122.77, 127.23, 127.31, 128.37, 130.10, 130.30, 130.73, 130.87, 131.48, 131.68, 132.26, 132.59, 133.93, 135.49, 135.90, 136.34, 137.15, 141.75, 180.37, 201.14. Anal. calcd for C31H25Cl4N3O2: C, 60.70; H, 4.11; N, 6.85%; found: C, 60.87; H, 4.23; N, 6.64%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(3-nitrophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(3-nitrophenyl)hexahydro-1H-pyrrolizine (6h). Obtained as a pale yellow solid, (0.144 g, 93%); mp = 204–205 °C; IR (KBr): 1608, 1621, 1707, 3386 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.58–2.02 (m, 4H, 5-CH2 and 6-CH2), 2.27 (d, 1H, J = 12.6 Hz, 2'-CH2), 2.60–2.63 (m, 1H, 7-CH2), 3.06–3.15 (m, 1H, 7-CH2), 3.46–3.51 (m, 1H, 6'-CH2), 3.62 (d, 1H, J = 14.7 Hz, 6'-CH2), 4.01 (d, 1H, J = 12.6 Hz, 2'-CH2), 4.48 (d, 1H, J = 10.8 Hz, 4-CH), 4.65–4.73 (m, 1H, 4a-CH), 6.70–8.25 (m, 13H, Ar-H and arylmethylidene), 9.10 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 26.10, 29.10, 48.13, 48.86, 49.07, 51.06, 63.20, 71.89, 74.04, 110.72, 122.78, 123.02, 123.99, 124.68, 125.03, 125.41, 125.90, 127.47, 129.68, 130.04, 133.91, 135.68, 136.30, 136.93, 137.33, 137.90, 141.92, 148.45, 148.61, 180.54, 201.06. Anal. calcd for C31H27N5O6: C, 65.83; H, 4.81; N, 12.38%; found: C, 65.69; H, 4.94; N, 12.47%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(4-methylphenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(4-methylphenyl)hexahydro-1H-pyrrolizine (6i). Obtained as a brown solid, (0.156 g, 94%); mp = 168–169 °C; IR (KBr): 1611, 1623, 1704, 3392 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.60–2.03 (m, 4H, 5-CH2 and 6-CH2), 2.29 (d, 1H, J = 12.9 Hz, 2'-CH2), 2.36 (s, 3H, CH3), 2.38 (s, 3H, CH3), 2.58–2.63 (m, 1H, 7-CH2), 3.10–3.16 (m, 1H, 7-CH2), 3.49 (d, 1H, J = 15.3 Hz, 6'-CH2), 3.78 (d, 1H, J = 15.3 Hz, 6'-CH2), 3.99–4.03 (m, 1H, 2'-CH2), 4.36 (d, 1H, J = 11.7 Hz, 4-CH), 4.50–4.55 (m, 1H, 4a-CH), 6.66 (d, 1H, J = 7.5 Hz, Ar-H), 6.84–7.54 (m, 12H, Ar-H and arylmethylidene), 8.56 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 21.3, 21.8, 26.07, 28.83, 48.50, 48.78, 49.35, 53.42, 65.96, 71.84, 74.06, 110.25, 122.61, 126.02, 129.33, 129.66, 129.74, 130.51, 131.01, 131.04, 132.11, 132.78, 135.12, 136.49, 136.97, 139.82, 141.96, 181.00, 202.06. Anal. calcd for C33H33N3O2: C, 78.70; H, 6.60; N, 8.34%; found: C, 78.84; H, 6.42; N, 8.42%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(4-methoxyphenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(4-methoxyphenyl)hexahydro-1H-pyrrolizine (6j). Obtained as a brown solid, (0.135 g, 85%); mp = 204–205 °C; IR (KBr): 1610, 1623, 1703, 3394 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.58–2.05 (m, 4H, 5-CH2 and 6-CH2), 2.32 (d, 1H, J = 12.6 Hz, 2'-CH2), 2.57–2.65 (m, 1H, 7-CH2), 3.08–3.15 (m, 1H, 7-CH2), 3.48 (d, 1H, J = 15.3 Hz, 6'-CH2), 3.62 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 3.82 (d, 1H, J = 15.3 Hz, 6'-CH2), 3.97–4.06 (m, 1H, 2'-CH2), 4.38 (d, 1H, J = 11.4 Hz, 4-CH), 4.52–4.56 (m, 1H, 4a-CH), 6.65–7.54 (m, 13H, Ar-H and arylmethylidene), 8.52 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 26.12, 28.86, 47.50, 48.18, 49.63, 53.13, 55.20, 55.31, 64.41, 70.34, 74.39, 110.12, 113.45, 113.92, 122.64, 127.42, 127.86, 129.51, 130.57, 132.21, 132.98, 137.44, 141.53, 158.33, 159.85, 181.03, 201.06. Anal. calcd for C33H33N3O4: C, 74.00; H, 6.21; N, 7.84%; found: C, 74.26; H, 6.14; N, 7.76%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(4-chlorophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(4-chlorophenyl)hexahydro-1H-pyrrolizine (6k). Obtained as a pale yellow solid, (0.150 g, 95%); mp = 175–176 °C; IR (KBr): 1610, 1621, 1703, 3387 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.56–2.04 (m, 4H, 5-CH2 and 6-CH2), 2.29 (d, 1H, J = 12.9 Hz, 2'-CH2), 2.58–2.62 (m, 1H, 7-CH2), 3.07–3.15 (m, 1H, 7-CH2), 3.49 (d, 1H, J = 15.6 Hz, 6'-CH2), 3.76 (dd, 1H, J = 15.6, 2.1 Hz, 6'-CH2), 4.05–4.16 (m, 1H, 2'-CH2), 4.35 (d, 1H, J = 11.4 Hz, 4-CH), 4.51–4.56 (m, 1H, 4a-CH), 6.68 (d, 1H, J = 7.8 Hz, Ar-H), 6.88–7.55 (m, 12H, Ar-H and arylmethylidene), 8.66 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 26.08, 28.96, 48.29, 48.85, 49.20, 53.41, 63.99, 71.86, 74.04, 110.43, 122.84, 125.96, 128.03, 128.82, 129.21, 129.29, 131.73, 132.07, 132.11, 133.63, 133.88, 135.37, 135.49, 136.05, 141.85, 180.74, 201.55. Anal. calcd for C31H27Cl2N3O2: C, 68.38; H, 5.00; N, 7.72%; found: C, 68.59; H, 5.15; N, 7.60%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(4-bromophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(4-bromophenyl)hexahydro-1H-pyrrolizine (6l). Obtained as a pale yellow solid, (0.136 g, 93%); mp = 207–208 °C; IR (KBr): 1612, 1619, 1702, 3389 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.52–2.07 (m, 4H, 5-CH2 and 6-CH2), 2.29 (d, 1H, J = 13.2 Hz, 2'-CH2), 2.58–2.62 (m, 1H, 7-CH2), 3.06–3.14 (m, 1H, 7-CH2), 3.47 (d, 1H, J = 15.6 Hz, 6'-CH2), 3.75 (dd, 1H, J = 15.6, 2.1 Hz, 6'-CH2), 4.05–4.15 (m, 1H, 2'-CH2), 4.33 (d, 1H, J = 11.1 Hz, 4-CH), 4.51–4.56 (m, 1H, 4a-CH), 6.69 (d, 1H, J = 7.8 Hz, Ar-H), 6.81–7.55 (m, 12H, Ar-H and arylmethylidene), 8.60 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 26.10, 28.98, 48.35, 48.76, 49.25, 53.28, 64.98, 71.85, 74.12, 110.41, 121.80, 122.84, 123.92, 128.06, 129.83, 131.64, 131.77, 131.92, 132.17, 132.25, 132.39, 134.32, 135.44, 136.23, 141.81, 180.70, 201.52. Anal. calcd for C31H27Br2N3O2: C, 58.79; H, 4.30; N, 6.63%; found: C, 58.96; H, 4.47; N, 6.51%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(4-fluorophenylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(4-fluorophenyl)hexahydro-1H-pyrrolizine (6m). Obtained as a pale yellow solid, (0.154 g, 94%); mp = 192–193 °C; IR (KBr): 1611, 1620, 1701, 3390 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.50–2.03 (m, 4H, 5-CH2 and 6-CH2), 2.30 (d, 1H, J = 13.5 Hz, 2'-CH2), 2.57–2.65 (m, 1H, 7-CH2), 3.03–3.12 (m, 1H, 7-CH2), 3.37 (d, 1H, J = 15.0 Hz, 6'-CH2), 3.77 (d, 1H, J = 15.0, 2.1 Hz, 6'-CH2), 4.06–4.13 (m, 1H, 2'-CH2), 4.36 (d, 1H, J = 11.0 Hz, 4-CH), 4.52–4.55 (m, 1H, 4a-CH), 6.61–7.54 (m, 13H, Ar-H and arylmethylidene), 8.62 (s, 1H, 1"-NH). 13C-NMR (125 MHz, CDCl3): δC 25.67, 28.54, 47.84, 48.47, 48.70, 52.83, 64.51, 69.60, 74.93, 109.99, 115.12 (2JCF = 20.0 Hz), 115.80 (2JCF = 21.25 Hz), 122.41, 125.59, 127.73, 128.70, 130.40, 131.71, 132.13, 132.52, 133.61, 135.17, 141.52, 161.95 (1JCF = 245.0 Hz), 163.01 (1JCF =250.0 Hz), 180.62, 201.19. Anal. calcd for C31H27F2N3O2: C, 72.78; H, 5.32; N, 8.21%; found: C, 72.92; H, 5.54; N, 8.04%.
Spiro[2.3″]oxindole-spiro[3.3′]-5′-(1-naphthylmethylidene)tetrahydro-4′(1H)-pyridinone-4-(1-naphthyl)hexahydro-1H-pyrrolizine (6n). Obtained as a yellow solid, (0.139 g, 91%); mp = 158–159 °C; IR (KBr): 1607, 1619, 1705, 3391 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.82–2.09 (m, 4H, 5-CH2 and 6-CH2), 2.65–2.68 (m, 1H, 7-CH2), 2.78 (d, 1H, J = 12.9 Hz, 2'-CH2), 3.30–3.35 (m, 1H, 7-CH2), 3.44 (d, 1H, J = 15.6 Hz, 6'-CH2), 3.87 (d, 1H, J = 15.6 Hz, 6'-CH2), 4.22 (d, 1H, J = 12.6 Hz, 2'-CH2), 4.44–4.50 (m, 1H, 4-CH), 4.68–4.73 (m, 1H, 4a-CH), 6.27 (s, 1H, arylmethylidene), 6.41–8.07 (m, 18H, Ar-H), 9.02 (s, 1H, 1"-NH). 13C-NMR (75 MHz, CDCl3): δC 25.99, 28.74, 48.00, 48.68, 49.30, 51.14, 64.58, 70.98, 75.04, 110.30, 122.63, 124.62, 125.05, 125.30, 125.45, 125.75, 126.77, 127.16, 127.59, 127.91, 128.20, 128.85, 129.08, 129.22, 129.39, 129.64, 130.05, 132.45, 132.48, 133.64, 133.70, 133.96, 134.46, 134.90, 136.90, 137.73, 138.82, 141.09, 181.13, 202.63. Anal. calcd for C39H33N3O2: C, 81.37; H, 5.78; N, 7.30%; found: C, 81.59; H, 5.60; N, 7.43%.