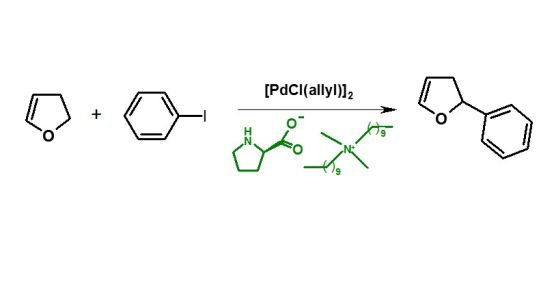
Palladium Catalyzed Heck Arylation of 2,3-Dihydrofuran—Effect of the Palladium Precursor
Abstract
:1. Introduction
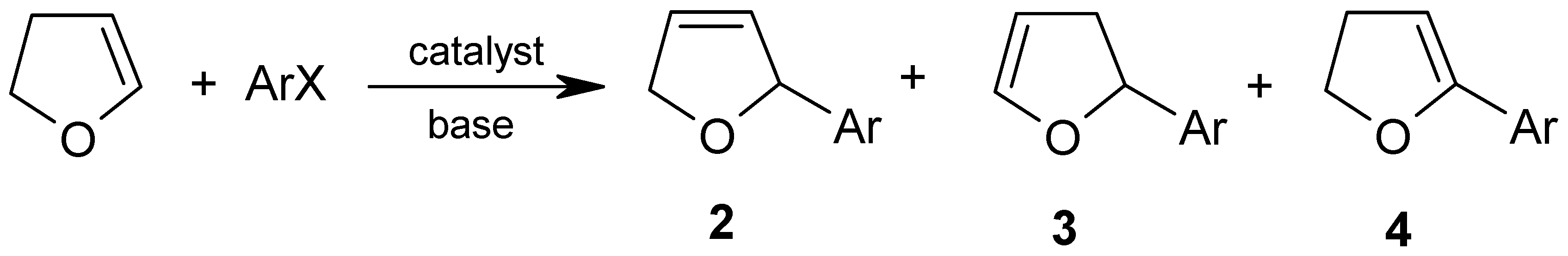
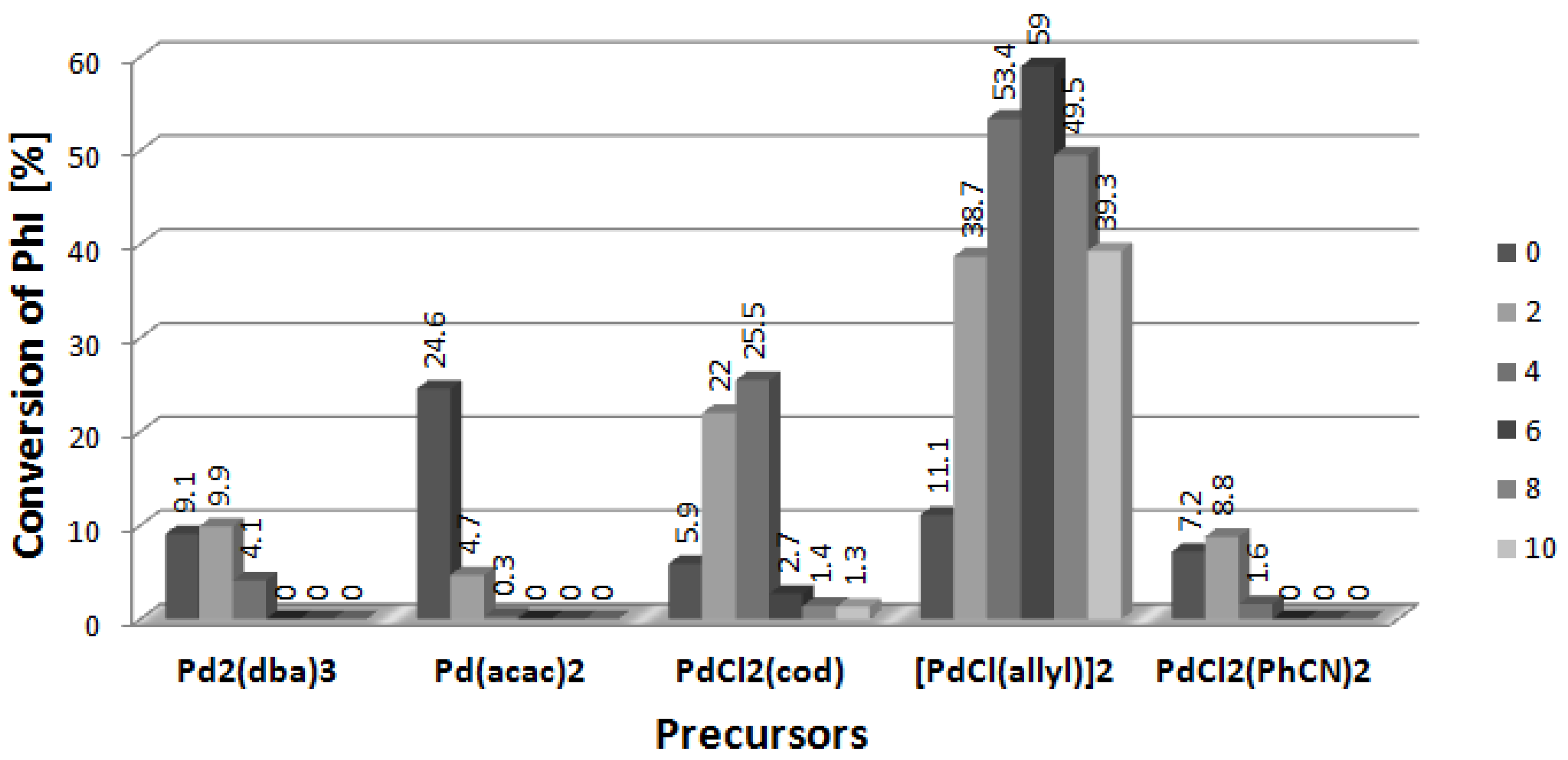
2. Results and Discussion
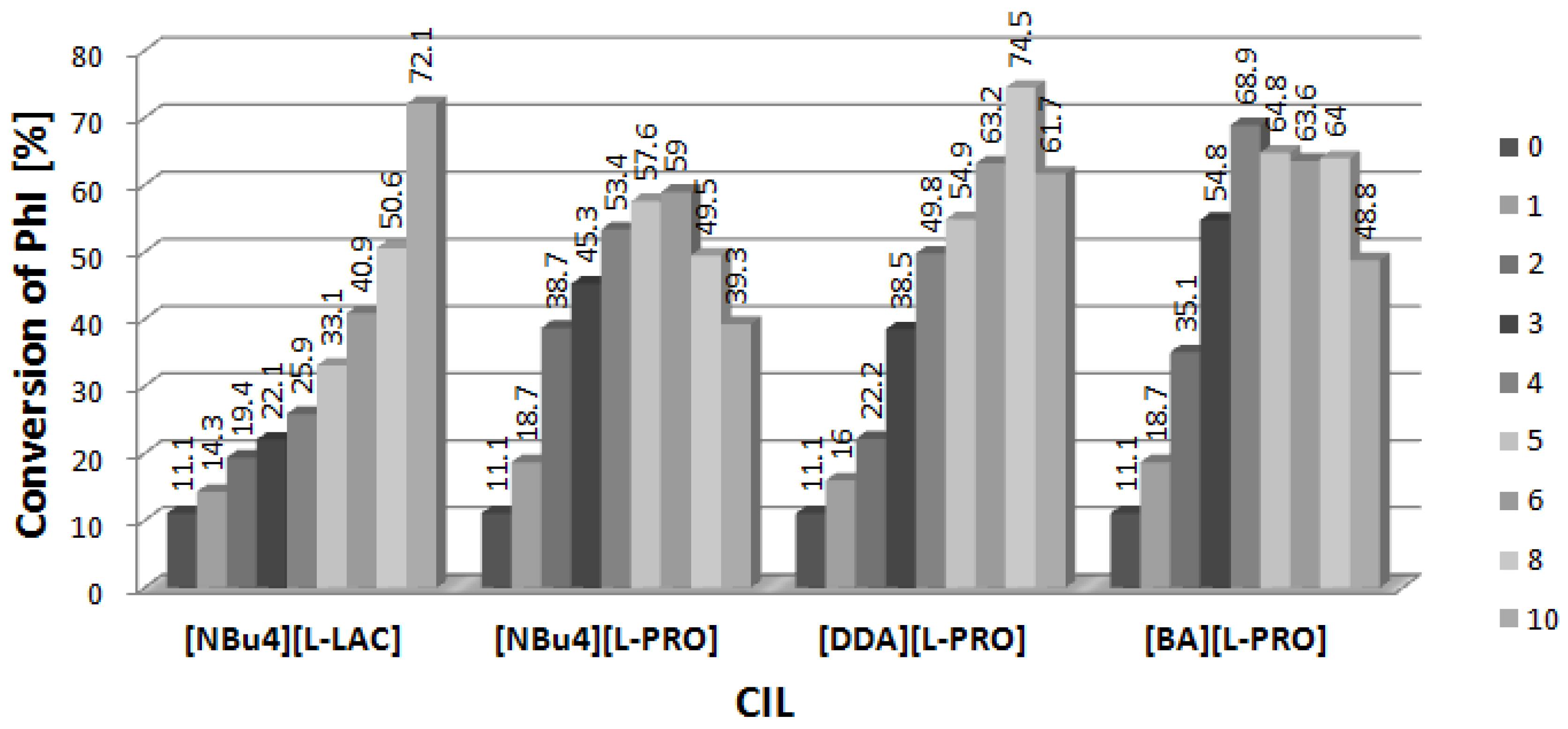
2.1. Arylation of DHF at the Presence of [DDA][L-PRO]
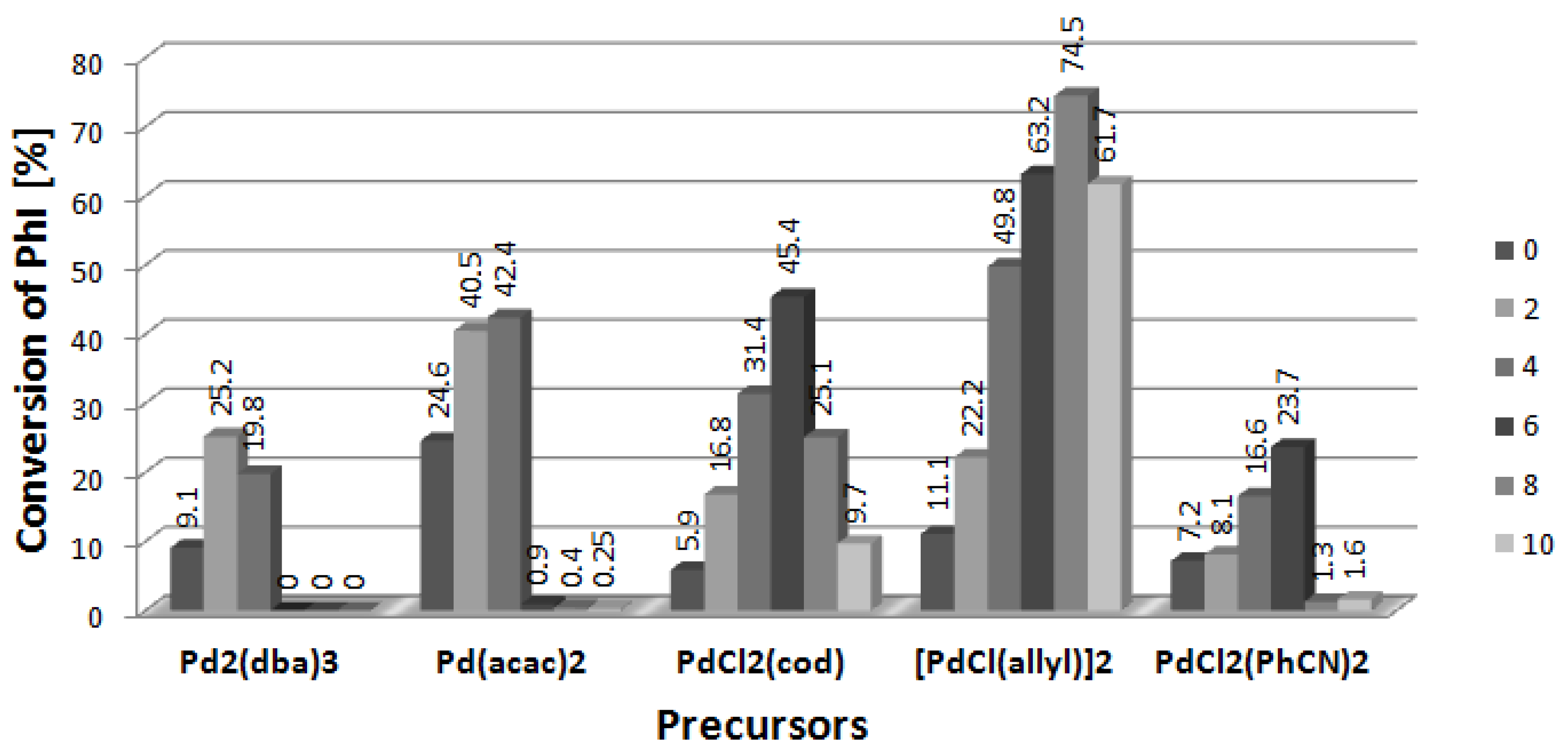
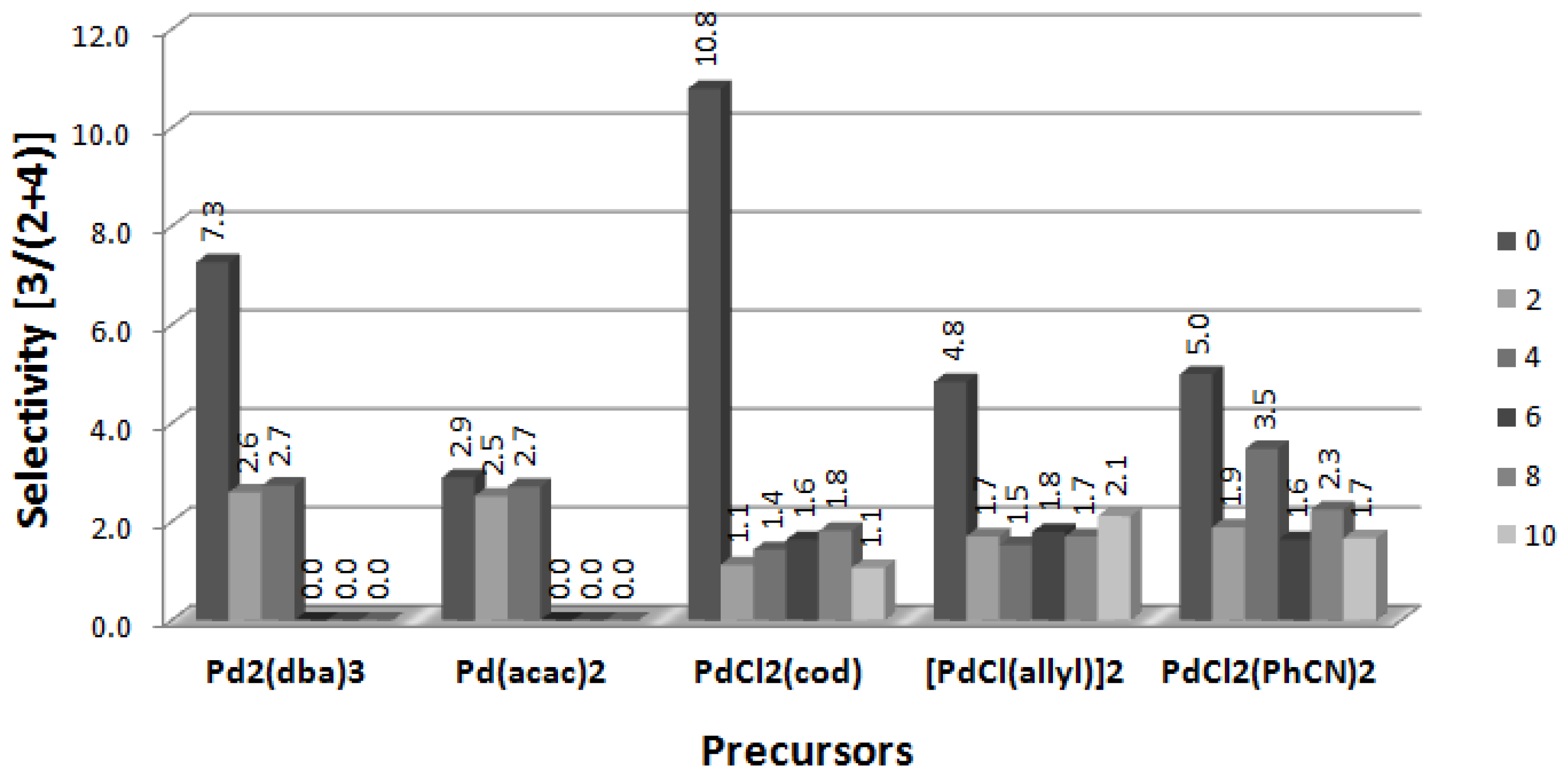
2.2. Arylation of DHF at the Presence of [BA][L-PRO]

2.3. Arylation of DHF at the Presence of [NBu4][L-PRO]
2.4. Arylation of DHF at the Presence of [NBu4][L-LAC]

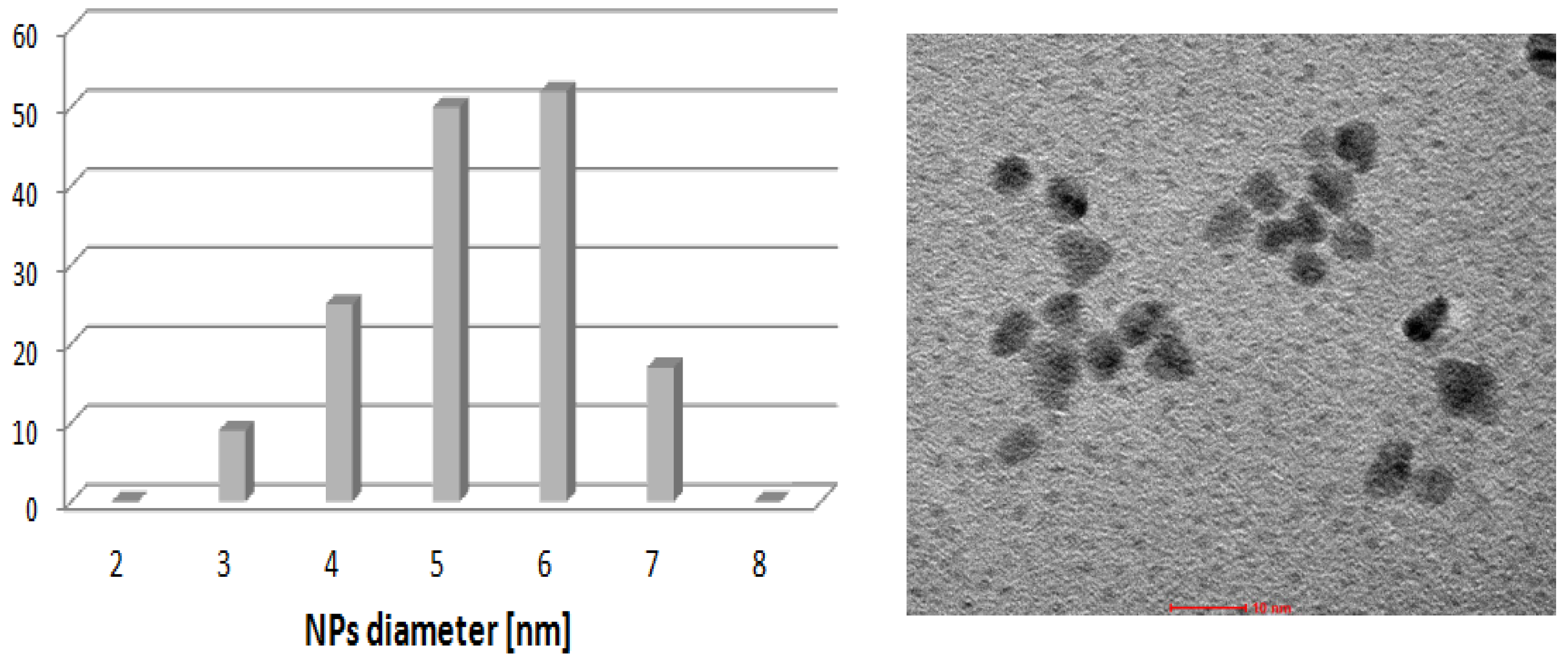
2.5. Arylation of DHF with [PdCl(allyl)]2 Precursor
| (a) | (b) | ||||
|---|---|---|---|---|---|
 |  | ||||
| L-proline | Pd(L-PRO)2 | [BA][L-PRO] | [BA][L-PRO]/[PdCl(allyl)]2 | ||
| Proton | δ (ppm) | δ (ppm) | δ (ppm) | δ (ppm) | |
| CH-2 | 4.04 t (J = 7.8 Hz) | 3.90 t (J = 8.4 Hz) | 3.70 m | 3.71 m | |
| CH2-3a | 3.32 m | 3.14 m | 3.10 m | 2.19 m | |
| CH2-3b | 3.26 m | 2.93 m | |||
| CH2- 4a | 2.26 m | 2.22 m | 2.08 m | 2.00 m | |
| CH2-4b | 1.99 m | 1.95 m | 1.92 m | 1.85 m | |
| CH2-5 | 1.92 m | 2.04 m; 1.71 m | 1.70 m | 1.75 m | |

3. Experimental
General Information
Heck Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Whitcombe, N.J.; Hii, K.K.; Gibson, S.E. Advances in the Heck chemistry of aryl bromides and chlorides. Tetrahedron 2001, 57, 7449–7449. [Google Scholar] [CrossRef]
- Palladium Catalyzed Coupling Reactions; Molnar, A. (Ed.) Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013.
- Tsuji, I. Palladium reagents and catalysts. In New Perspectives for the 21st Century; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Bedford, R.B.; Cazin, C.S.J.; Holder, D. The development of palladium catalysts for C-C and C-heteroatom bond forming reactions of aryl chloride substrates. Coord. Chem. Rev. 2004, 248, 2283–2321. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Ziółkowski, J.J. Structural and mechanistic studies of Pd-catalyzed C-C bond formation: The case of carbonylation and Heck reaction. Coord. Chem. Rev. 2005, 249, 2308–2322. [Google Scholar] [CrossRef]
- Mc Cartney, D.; Guiry, P.J. The asymmetric Heck and related reactions. Chem. Soc. Rev. 2011, 40, 5122–5150. [Google Scholar] [CrossRef]
- Hayashi, T.; Kubo, A.; Ozawa, F. Catalytic asymmetric arylation of olefins. Pure Appl. Chem. 1992, 64, 421–427. [Google Scholar]
- Ozawa, F.; Kobatake, Y.; Hayashi, T. Palladium-catalyzed asymmetric alkenylation of cyclic olefins. Tetrahedron Lett. 1993, 34, 2505–2508. [Google Scholar] [CrossRef]
- Mieczyńska, E.; Trzeciak, A.M. Selective Heck arylation of cyclohexene with homogeneous and heterogeneous palladium catalysts. Molecules 2000, 15, 2166–2177. [Google Scholar]
- Oliveira, C.C.; dos Santos, E.A.F.; Bormio Nunes, J.H.; Correia, C.R.D. Stereoselective arylation of substituted cyclopentenes by substrate-directable Heck-Matsuda reactions: A concise total synthesis of the sphingosine 1-phosphate receptor (S1P1) agonist VPC01091. J. Org. Chem. 2012, 77, 8182–8190. [Google Scholar] [CrossRef]
- Correira, C.R.D.; Oliveira, C.C.; Salles, A.G., Jr.; Santos, E.A.F. The first example of the enantioselective Heck-Matsuda reaction: Arylation of unactivated cyclic olefins using chiral bisoxazolines. Tetrahedron Lett. 2012, 53, 3325–3328. [Google Scholar] [CrossRef]
- Mazuela, J.; Tolstoy, P.; Pàmies, O.; Andersson, P.G.; Diéguez, M. Phosphite-oxazole/imidazole ligands in asymmetric intermolecular Heck reaction. Org. Biomol. Chem. 2011, 9, 941–946. [Google Scholar] [CrossRef]
- Ozawa, F.; Kubo, A.; Matsumoto, Y.; Hayashi, T. Palladium-catalyzed asymmetric arylation of 2,3-dihydrofuran with phenyl triflate. A novel asymmetric catalysis involving a kinetic resolution proces. Organometallics 1993, 12, 4188–4196. [Google Scholar] [CrossRef]
- Jeffery, T.; David, M. [Pd/Base/QX] catalyst system for directing Heck-type reactions. Tetrahedron Lett. 1998, 39, 5751–5754. [Google Scholar] [CrossRef]
- Wöste, T.H.; Oestreich, M. BINAP versus BINAP(o) in asymmetric intermolecular Mizoroki-Heck reactions: Substantial effects on selectivities. Chem. Eur. J. 2011, 17, 11914–11918. [Google Scholar] [CrossRef]
- Roszak, R.; Trzeciak, A.M.; Pernak, J.; Borucka, N. Effect of chiral ionic liquids on palladium catalyzed Heck arylation of 2,3-dihydrofuran. Appl. Catal. A: Gen. 2011, 409–410, 148–155. [Google Scholar] [CrossRef]
- Ozawa, F.; Kubo, A.; Hayashi, T. Catalytic asymmetric arylation of 2,3-dihydrofuran with aryl triflates. J. Am. Chem. Soc. 1991, 113, 1417–1419. [Google Scholar] [CrossRef]
- Rankic, D.A.; Lucciola, D.; Keay, B.A. Application of 3,3'-disubstituted xylBINAP derivatives in inter- and intramolecular asymmetric Heck/Mizoroki reactions. Tetrahedron Lett. 2010, 51, 5724–5727. [Google Scholar] [CrossRef]
- Machado, A.H.L.; de Sousa, M.A.; Patto, D.C.S.; Azevedo, L.F.S.; Bombonato, F.I.; Correia, C.R.D. The scope of the Heck arylation of enol ethers with arenediazonium salts: A new approach to the synthesis of flavonoids. Tetrahedron Lett. 2009, 50, 1222–1225. [Google Scholar] [CrossRef]
- Morel, A.; Silarska, E.; Trzeciak, A.M.; Pernak, J. Palladium-catalyzed asymmetric Heck arylation of 2,3-dihydrofuran—Effect of prolinate salts. Dalton Trans. 2013, 42, 1215–1222. [Google Scholar] [CrossRef]
- Dodd, D.W.; Toews, H.E.; Carneiro, F.D.S.; Jennings, M.C.; Jones, N.D. Model intermolecular asymmetric Heck reactions catalyzed by chiral pyridyloxazoline palladium(II) complexes. Inorg. Chim. Acta 2006, 359, 2850–2858. [Google Scholar] [CrossRef]
- Hu, J.; Lu, Y.; Li, Y.; Zhou, J. (Steve). Highly active catalysts of bisphosphine oxides for asymmetric Heck reaction. Chem. Commun. 2013, 49, 9425–9427. [Google Scholar] [CrossRef]
- Shibasaki, M.; Vogl, E.M.; Ohshima, T. Asymmetric Heck reaction. Adv. Synth. Catal. 2004, 346, 1533–1552. [Google Scholar] [CrossRef]
- Hii, K.K. (Mimi); Claridge, T.D.W.; Brown, J.M. Intermediates in the intermolecular, asymmetric Heck arylation of dihydrofuran. Angew. Chem. Int. Ed. Engl. 1997, 36, 984–987. [Google Scholar] [CrossRef]
- Wheatley, B.M.M.; Keay, B.A. Use of deuterium labelling studies to determine the stereochemical outcome of palladium migration during as asymmetric intermolecular Heck reaction. J. Org. Chem. 2007, 72, 7253–7259. [Google Scholar] [CrossRef]
- Hendriksen, S.T.; Norrby, P.-O.; Kaukoranta, P.; Andersson, P.G. Combined experimental and theoretical study of the mechanism and enantioselectivity of palladium-catalyzed intermolecular Heck coupling. J. Am. Chem. Soc. 2008, 130, 10414–10421. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Fu, H.-Y.; Li, R.-X.; Chen, H.; Li, X.-J. Palladium nanoparticles generated from Allylpalladium chloride in situ: A simple and highly efficient catalytic system for Mizoroki-Heck reactions. J. Organomet. Chem. 2012, 697, 1–5. [Google Scholar]
- Trzeciak, A.M.; Ziółkowski, J.J. Monomolecular, nanosized and heterogenized palladium catalysts for the Heck reaction. Coord. Chem. Rev. 2007, 9–10, 1281–1293. [Google Scholar] [CrossRef]
- Szulmanowicz, M.S.; Gniewek, A.; Gil, W.; Trzeciak, A.M. Palladium(II) complexes with small N-heteryclic carbene ligands as highly active catalysts for the Suzuki-Miyaura cross-coupling reaction. ChemCatChem 2013, 13, 1043–1049. [Google Scholar]
- Cotugno, P.; Monopoli, A.; Ciminale, F.; Cioffi, N.; Nacci, A. Pd nanoparticle catalysed one-pot sequential Heck and Suzuki couplings of bromo-chloroarenes in ionic liquids and water. Org. Biomol. Chem. 2012, 10, 808–813. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Towards the ideal catalyst: From atomic centers to “cocktail” of catalysts. Chem. Rev. 2011, 11, 1596–1604. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Ananikov, V.P. Pd2(dba)3 as a precursor of soluble metal complexes and nanoparticles: Determination of palladium active species for catalysis and synthesis. Organometallics 2012, 31, 2302–2309. [Google Scholar] [CrossRef]
- Tsvelikhovsky, D.; Popov, I.; Gutkin, V.; Rozin, A.; Shvartsman, A.; Blum, J. On the involvement of palladium nanoparticles in the Heck and Suzuki reactions. Eur. J. Org. Chem. 2009, 98–102. [Google Scholar] [CrossRef]
- Sanhes, D.; Rauy, E.; Retory, S.; Saffon, N.; Teuma, E.; Gomez, M. Unexpected activation of carbon-bromide bond promoted by palladium nanoparticles in Suzuki C-C coupling. Dalton Trans. 2010, 39, 9719–9726. [Google Scholar] [CrossRef]
- Jin, C.-J.; Schüth, F. Colloidal metal nanoparticles as a component of designed catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2457–2487. [Google Scholar] [CrossRef]
- Jansat, S.; Durand, J.; Favier, I.; Malbosc, F.; Pradel, C.; Teuma, E.; Gomez, M. A single catalyst for sequential reactions: Dual homogeneous and heterogeneous behaviour of palladium nanoparticles in solution. ChemCatChem 2009, 1, 244–246. [Google Scholar] [CrossRef]
- De Vries, J.G. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans. 2006, 421–429. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, K.N. An efficient phosphine-free Heck reaction in water using Pd(L-proline)2 as the catalyst under microwave irradiation. Synthesis 2011, 7, 1125–1131. [Google Scholar]
- Canseco-Gonzalez, D.; Gniewek, A.; Szulmanowicz, M.; Müller-Bunz, H.; Trzeciak, A.M.; Albrecht, M. PEPPSI-type palladium complexes containing basic 1,2,3-triazolylidene ligands and their role in Suzuki-Miyaura catalysis. Chem. Eur. J. 2012, 18, 6055–6062. [Google Scholar] [CrossRef]
- Cybulski, J.; Wiśniewska, A.; Kulig-Adamiak, A.; Dąbrowski, Z.; Praczyk, T.; Michalczyk, A.; Walkiewicz, F.; Materna, K.; Pernak, J. Mandelate and prolinate ionic liquids: Synthesis, characterization, catalytic and biological activity. Tetrahedron Lett. 2011, 52, 1325–1328. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the ionic liquids are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morel, A.; Trzeciak, A.M.; Pernak, J. Palladium Catalyzed Heck Arylation of 2,3-Dihydrofuran—Effect of the Palladium Precursor. Molecules 2014, 19, 8402-8413. https://doi.org/10.3390/molecules19068402
Morel A, Trzeciak AM, Pernak J. Palladium Catalyzed Heck Arylation of 2,3-Dihydrofuran—Effect of the Palladium Precursor. Molecules. 2014; 19(6):8402-8413. https://doi.org/10.3390/molecules19068402
Chicago/Turabian StyleMorel, Adam, Anna M. Trzeciak, and Juliusz Pernak. 2014. "Palladium Catalyzed Heck Arylation of 2,3-Dihydrofuran—Effect of the Palladium Precursor" Molecules 19, no. 6: 8402-8413. https://doi.org/10.3390/molecules19068402