Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry
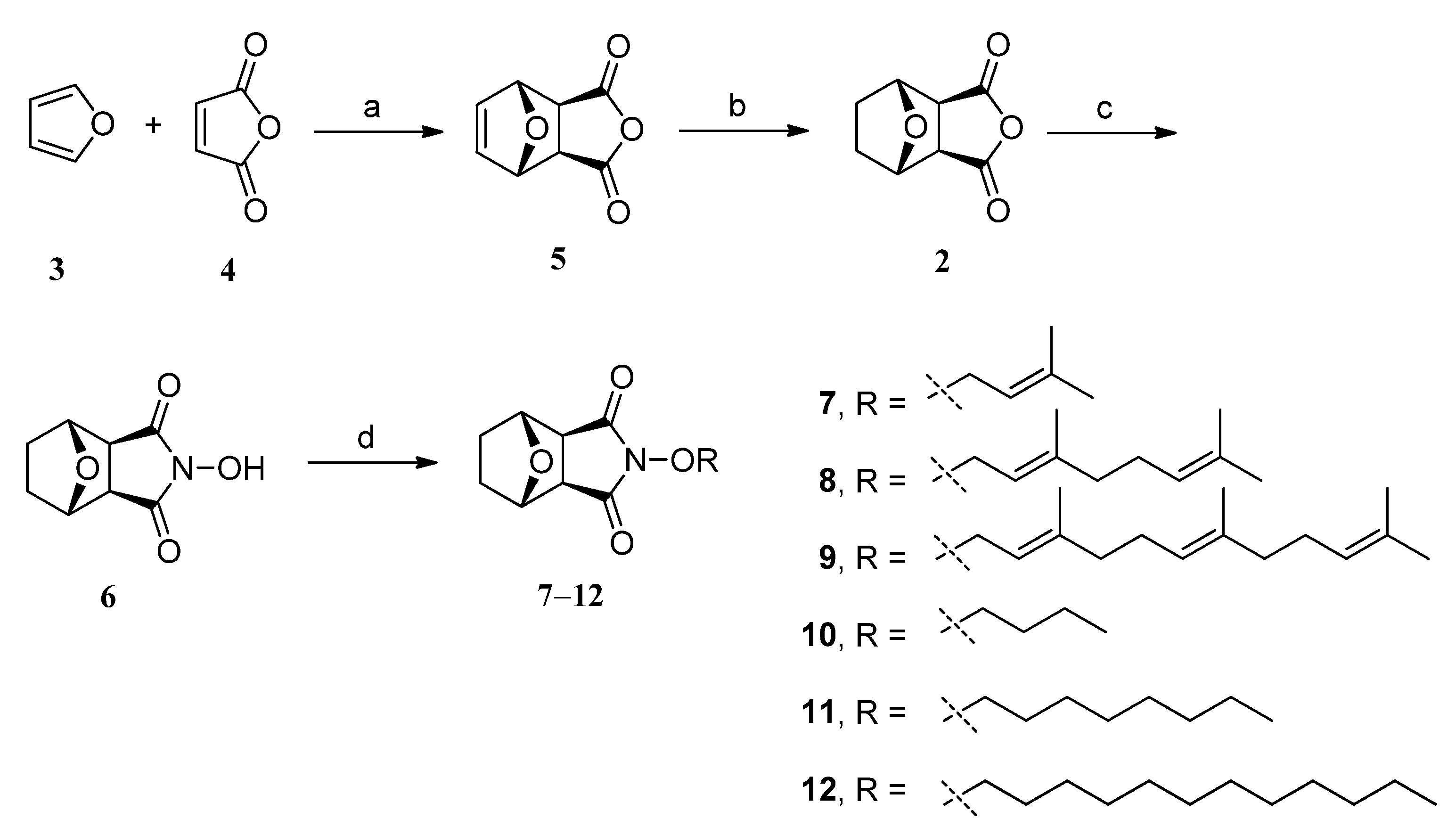
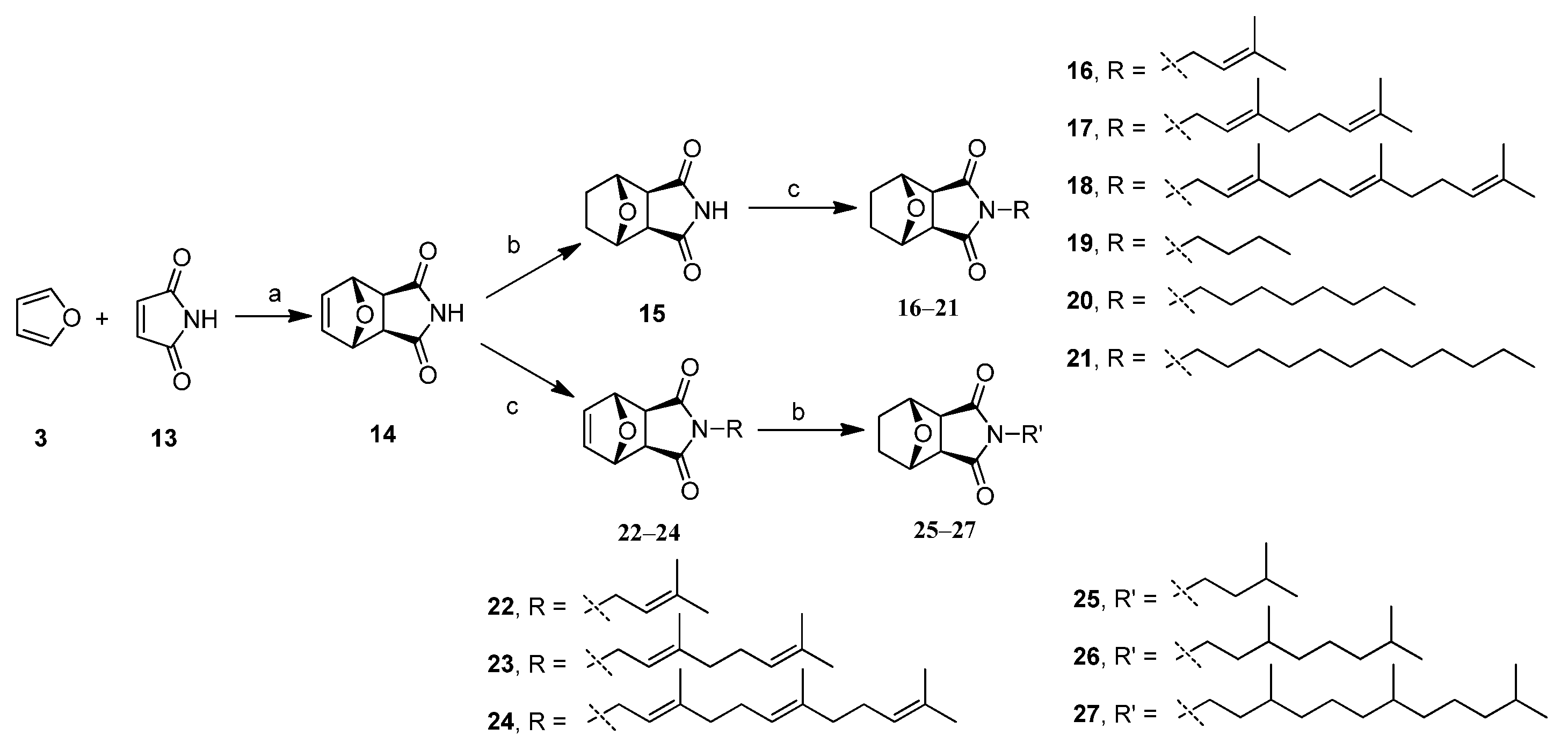
2.2. In Vitro Anti-Proliferative Activity by MTT Assay
| Compound | clogP a | IC50 (μM) (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| HepG2 b | BFTC905 c | HT-29 d | SW480 d | HL-60 e | ||
| NCTD (2) | −0.86 | 42.0 ± 1.8 | 18.9 ± 0.3 | 19.5 ± 0.2 | 49.1 ± 8.4 | ND f |
| 5 | −1.14 | ND | 75.3 ± 2.4 | ND | ND | ND |
| 6 | −1.64 | ND | ND | ND | ND | ND |
| 7 | 1.23 | 83.2 ± 2.2 | 31.7 ± 2.6 | 38.5 ± 5.9 | 66.3 ± 2.4 | ND |
| 8 | 3.26 | 32.5 ± 1.4 | 24.7 ± 1.8 | 22.0 ± 0.1 | 44.7 ± 2.3 | 90.2 ± 2.2 |
| 9 | 5.29 | 8.3 ± 1.3 | 11.3 ± 1.0 | 9.7 ± 1.7 | 18.5 ± 2.3 | 39.0 ± 1.1 |
| 10 | 1.11 | ND | ND | ND | ND | ND |
| 11 | 3.23 | ND | ND | ND | ND | ND |
| 12 | 5.35 | ND | ND | ND | ND | ND |
| 14 | −1.35 | ND | ND | ND | ND | ND |
| 15 | −1.67 | ND | ND | ND | ND | ND |
| 16 | 1.05 | ND | ND | ND | ND | ND |
| 17 | 3.09 | 34.3 ± 4.9 | 15.5 ± 3.9 | 26.9 ± 2.2 | 86.6 ± 2.6 | ND |
| 18 | 5.12 | 16.4 ± 1.2 | 9.3 ± 0.6 | 14.8 ± 1.9 | 33.1 ± 1.0 | 79.8 ± 1.1 |
| 19 | 0.94 | ND | ND | ND | ND | ND |
| 20 | 3.06 | 67.2 ± 8.5 | 25.8 ± 1.2 | 57.4 ± 1.6 | 84.6 ± 1.3 | ND |
| 21 | 5.17 | 34.0 ± 2.7 | 21.9 ± 0.7 | 21.9 ± 0.9 | 46.8 ± 3.0 | 81.0 ± 2.1 |
| 22 | 0.77 | ND | ND | ND | ND | ND |
| 23 | 2.80 | 39.1 ± 2.5 | 53.0 ± 3.4 | 54.9 ± 1.5 | ND | ND |
| 24 | 4.83 | 16.5 ± 2.5 | 10.2 ± 2.8 | 20.2 ± 3.3 | 42.8 ± 0.4 | ND |
| 25 | 1.34 | ND | ND | ND | ND | ND |
| 26 | 3.85 | 36.4 ± 1.5 | ND | 28.6 ± 2.1 | ND | ND |
| 27 | 6.37 | 28.1 ± 8.3 | 13.5 ± 1.9 | 27.8 ± 4.5 | 33.8 ± 1.9 | ND |
| 5-Fu | −1.72 | 40.2 ± 7.6 | ND | ND | 32.7 ± 8.3 | ND |
| Cisplatin | −2.50 | 36.1 ± 3.1 | - g | 24.1 ± 0.1 | 40.7 ± 1.2 | ND |
| Doxorubicin | 0.87 | 0.3 ± 0.0 | - g | 1.7 ± 0.2 | 0.5 ± 0.1 | 14.3 ± 0.9 |
| Compound | IC50 (μM) a (Mean ± SD) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| NCTD (2) | 48.0 ± 0.7 | 42.0 ± 1.8 | 22.8 ± 0.6 |
| 9 | 10.7 ± 0.2 *** | 8.3 ± 1.3 *** | 8.2 ± 0.6 *** |
| 18 | 19.4 ± 2.1 ** | 16.4 ± 1.2 *** | 12.8 ± 2.5 *** |
2.3. Nuclear Morphological Changes of HepG2 Cells Treated with Norcantharidin (2), Compounds 9 and 18
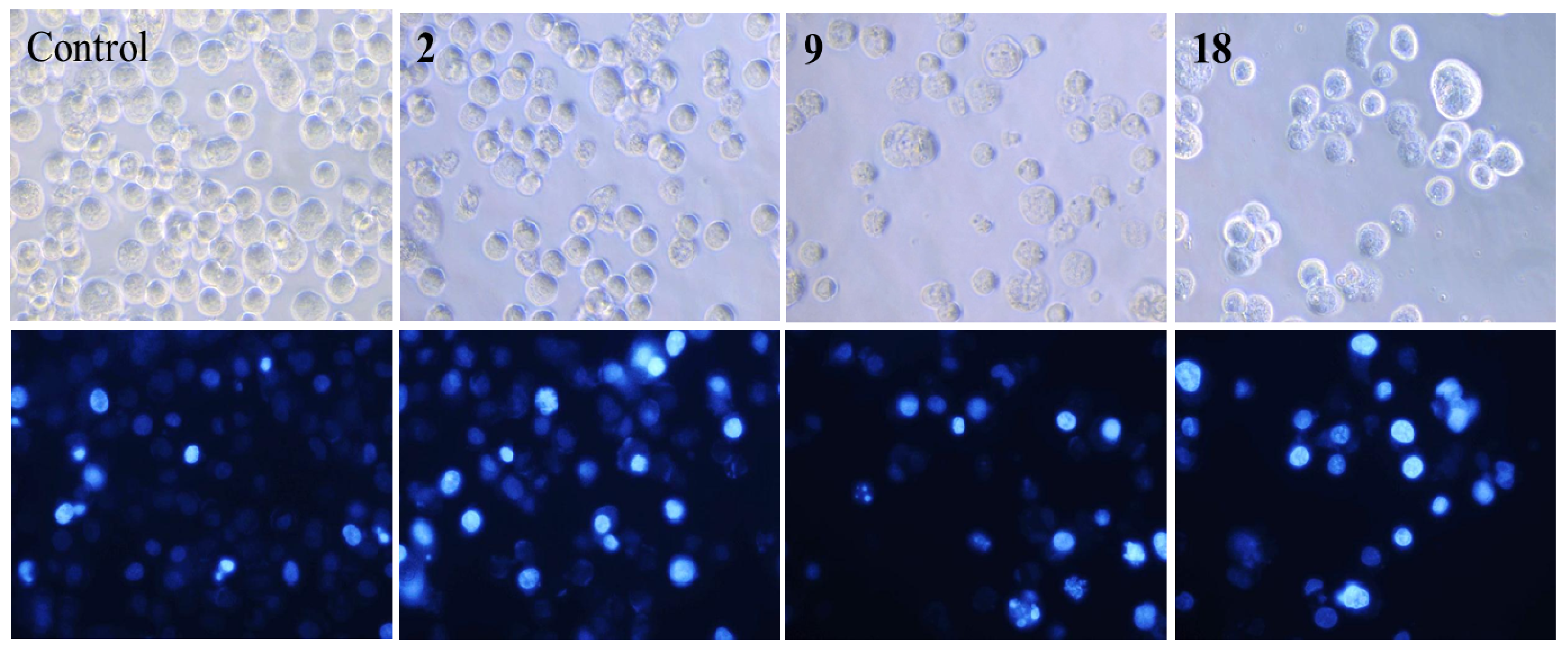
2.4. Cell Cycle Distribution Analysis Using Flow Cytometry
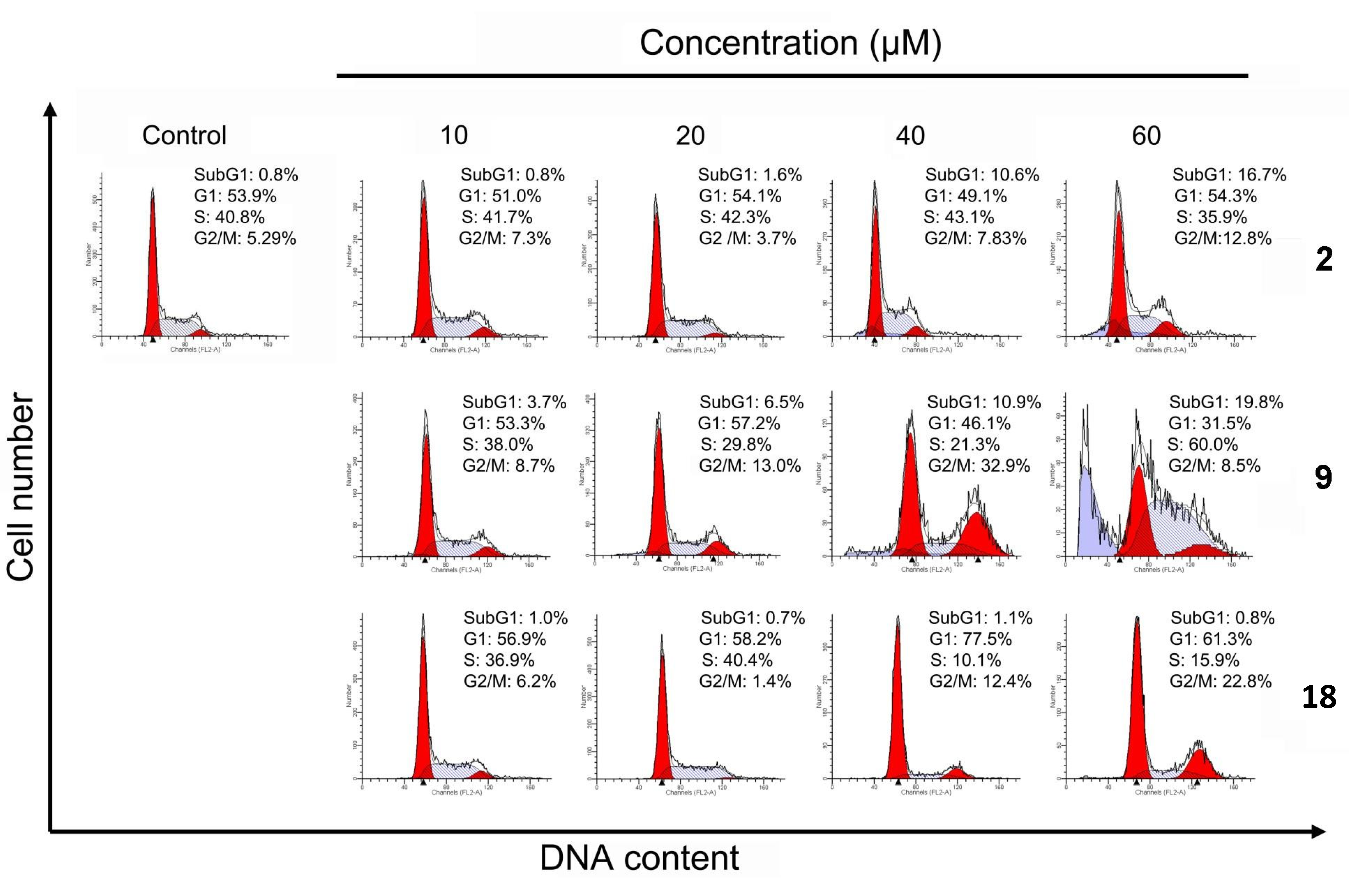
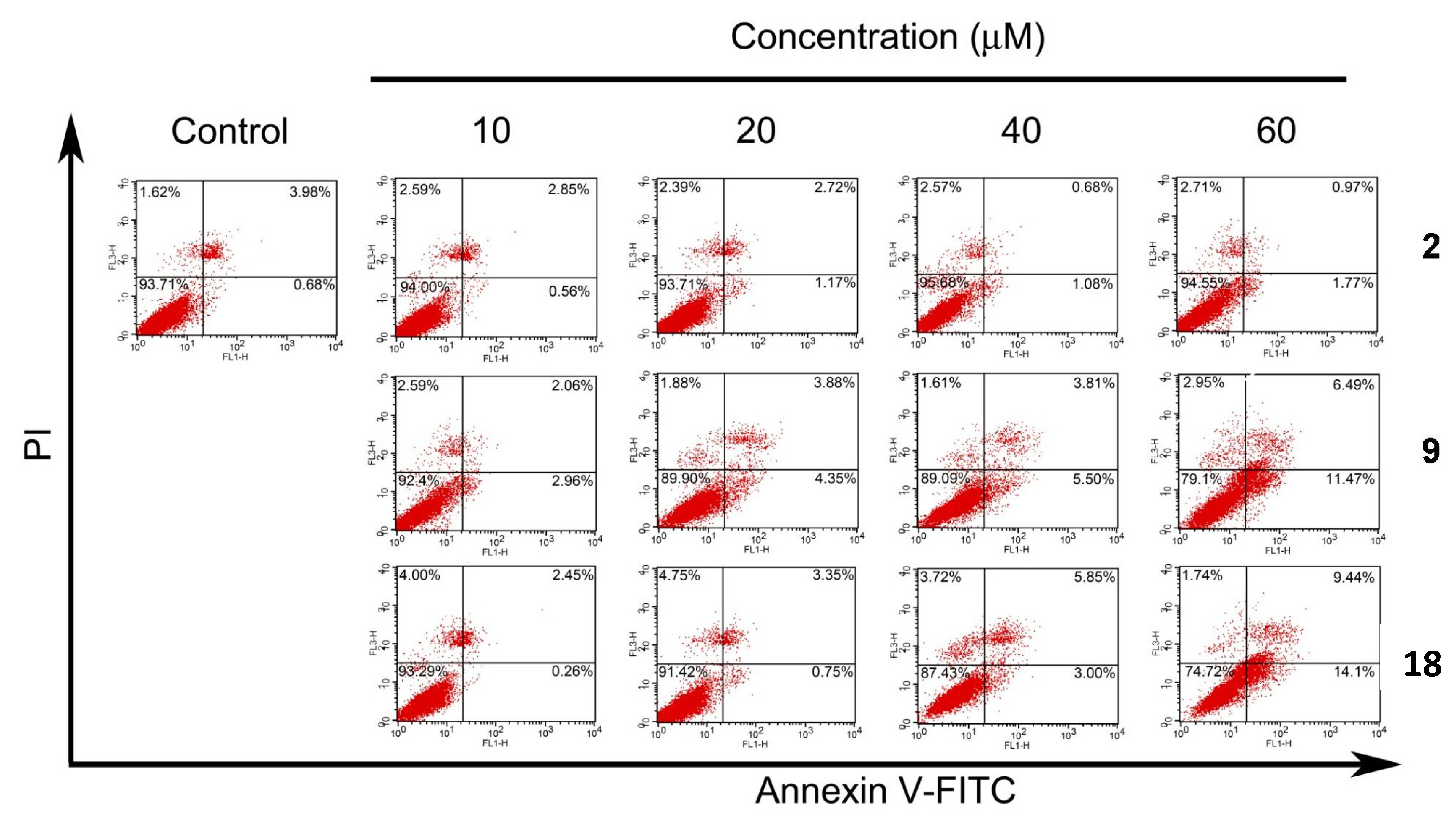
2.5. Apoptotic Analyses-Annexin V-FITC/PI Double Staining and Flow Cytometry Analyses
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of Compounds 2, 5 and 6
3.3. General Procedure for Synthesis of Target Compounds 7‒12
3.4. General Procedure for Synthesis of Target Compounds 16–24
3.5. General Procedure for Synthesis of Target Compounds 25–27
3.6. In Vitro Pharmacology
3.6.1. Cell Culture and Stock Solutions
3.6.2. Cell Cytotoxicity Assay Using MTT Assay
3.6.3. Morphological Observations of Nuclear Change with Hoechst 33,258 Staining
3.6.4. Flow Cytometry Analysis
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, G.S. Medical uses of mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, C.H.; Hsieh, K.J.; Ten, K.Y.; Yang, L.L. Cytotoxic effects of cantharidin on the growth of normal and carcinoma cells. Toxicology 2000, 147, 77–87. [Google Scholar] [CrossRef]
- Karras, D.J.; Farrell, S.E.; Harrigan, R.A.; Henretig, F.M.; Gealt, L. Poisoning from “Spanish fly” (Cantharidin). Am. J. Emerg. Med. 1996, 14, 478–483. [Google Scholar]
- Chen, R.T.; Hua, Z.; Yang, J.L.; Han, J.X.; Zhang, S.Y.; Lü, F.L.; Xü, B. Studies on antitumor actions of cantharidin. Chin. Med. J. 1980, 93, 183–187. [Google Scholar]
- Hsia, T.C.; Lin, J.H.; Hsu, S.C.; Tang, N.Y.; Lu, H.F.; Wu, S.H.; Lin, J.G.; Chung, J.G. Cantharidin induces DNA damage and inhibits DNA repair-associated protein levels in NCI-H460 human lung cancer cells. Environ. Toxicol. 2014. [Google Scholar] [CrossRef]
- Huang, Y.P.; Ni, C.H.; Lu, C.C.; Chiang, J.H.; Yang, J.S.; Ko, Y.C.; Lin, J.P.; Kuo, J.H.; Chang, S.J.; Chung, J.G. Suppressions of migration and invasion by cantharidin in TSGH-8301 human bladder carcinoma cells through the inhibitions of matrix metalloproteinase-2/-9 signaling. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Huan, S.K.-H.; Lee, H.-H.; Liu, D.-Z.; Wu, C.-C.; Wang, C.-C. Cantharidin-induced cytotoxicity and cyclooxygenase 2 expression in human bladder carcinoma cell line. Toxicology 2006, 223, 136–143. [Google Scholar] [CrossRef]
- Kok, S.H.L.; Chui, C.H.; Lam, W.S.; Chen, J.; Tang, J.C.O.; Lau, F.Y.; Cheng, G.Y.M.; Wong, R.S.M.; Chan, A.S.C. Induction of apoptosis on carcinoma cells by two synthetic cantharidin analogues. Int. J. Mol. Med. 2006, 17, 151–157. [Google Scholar]
- Laidley, C.W.; Cohen, E.; Casida, J.E. Protein phosphatase in neuroblastoma cells: [3H]cantharidin binding site in relation to cytotoxicity. J. Pharmacol. Exp. Ther. 1997, 280, 1152–1158. [Google Scholar]
- McCluskey, A.; Ackland, S.P.; Bowyer, M.C.; Baldwin, M.L.; Garner, J.; Walkom, C.C.; Sakoff, J.A. Cantharidin analogues: Synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorg. Chem. 2003, 31, 68–79. [Google Scholar] [CrossRef]
- Massicot, F.; Dutertre-Catella, H.; Pham-Huy, C.; Liu, X.H.; Duc, H.T.; Warnet, J.M. In vitro assessment of renal toxicity and inflammatory events of two protein phosphatase inhibitors cantharidin and nor-cantharidin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 26–32. [Google Scholar] [CrossRef]
- Honkanen, R.E. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 1993, 330, 283–286. [Google Scholar] [CrossRef]
- McCluskey, A.; Walkom, C.; Bowyer, M.C.; Ackland, S.P.; Gardiner, E.; Sakoff, J.A. Cantharimides: A new class of modified cantharidin analogues inhibiting protein phosphatases 1 and 2A. Bioorg. Med. Chem. Lett. 2001, 11, 2941–2946. [Google Scholar] [CrossRef]
- Baba, Y.; Hirukawa, N.; Sodeoka, M. Optically active cantharidin analogues possessing selective inhibitory activity on Ser/Thr protein phosphatase 2B (calcineurin): Implications for the binding mode. Bioorg. Med. Chem. Lett. 2005, 13, 5164–5170. [Google Scholar] [CrossRef]
- Goldfarb, M.T.; Gupta, A.K.; Gupta, M.A.; Sawchuk, W.S. Office therapy for human papillomavirus infection in nongenital sites. Dermatol. Clin. 1991, 9, 287–296. [Google Scholar]
- Chen, Y.-N.; Chen, J.-C.; Yin, S.-C.; Wang, G.-S.; Tsauer, W.; Hsu, S.-F.; Hsu, S.-L. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int. J. Cancer 2002, 100, 158–165. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Shieh, C.-J.; Tsai, T.-H.; Kuo, C.-D.; Ho, L.-T.; Liu, T.-Y.; Liao, H.-F. Inhibitory effect of norcantharidin, a derivative compound from blister beetles, on tumor invasion and metastasis in CT26 colorectal adenocarcinoma cells. Anti-Cancer Drugs 2005, 16, 293–299. [Google Scholar] [CrossRef]
- Lin, L.-H.; Huang, H.-S.; Lin, C.-C.; Lee, L.-W.; Lin, P.-Y. Effects of cantharidinimides on human carcinoma cells. Chem. Pharm. Bull. 2004, 52, 855–857. [Google Scholar] [CrossRef]
- An, W.-W.; Wang, M.-W.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Mitogen-activated protein kinase-dependent apoptosis in norcantharidin-treated A375-S2 cells is proceeded by the activation of protein kinase C. Chin. Med. J. 2005, 118, 198–203. [Google Scholar]
- An, W.-W.; Gong, X.-F.; Wang, M.-W.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Norcantharidin induces apoptosis in HeLa cells through caspase, MAPK, and mitochondrial pathways. Acta Pharmacol. Sin. 2004, 25, 1502–1508. [Google Scholar]
- Liao, H.-F.; Su, S.-L.; Chen, Y.-J.; Chou, C.-H.; Kuo, C.-D. Norcantharidin preferentially induces apoptosis in human leukemic Jurkat cells without affecting viability of normal blood mononuclear cells. Food Chem. Toxicol. 2007, 45, 1678–1687. [Google Scholar] [CrossRef]
- Liao, H.-F.; Chen, Y.-J.; Chou, C.-H.; Wang, F.-W.; Kuo, C.-D. Norcantharidin induces cell cycle arrest and inhibits progression of human leukemic Jurkat T cells through mitogen-activated protein kinase-mediated regulation of interleukin-2 production. Toxicol. In Vitro 2011, 25, 206–212. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Chen, M.-F.; Kao, Y.-H.; Hu, D.-N.; Chang, F.-R.; Wu, Y.-C. Involvement of caspase and MAPK activities in norcantharidin-induced colorectal cancer cell apoptosis. Toxicol. In Vitro 2010, 24, 766–775. [Google Scholar] [CrossRef]
- Peng, C.; Liu, X.; Liu, E.; Xu, K.; Niu, W.; Chen, R.; Wang, J.; Zhang, Z.; Lin, P.; Wang, J.; et al. Norcantharidin induces HT-29 colon cancer cell apoptosis through the alphavbeta6-extracellular signal-related kinase signaling pathway. Cancer Sci. 2009, 100, 2302–2308. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, W.-M.; Liu, Y.-W.; Lee, C.-Y.; Jang, Y.-H.; Kuo, C.-D.; Liao, H.-F. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem. Biol. Interact. 2009, 181, 440–446. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Tsai, Y.-M.; Kuo, C.-D.; Ku, K.-L.; Shie, H.-S.; Liao, H.-F. Norcantharidin is a small-molecule synthetic compound with anti-angiogenesis effect. Life Sci. 2009, 85, 642–651. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Liu, K.; Yagasaki, K.; Zhang, G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology 2009, 59, 201–208. [Google Scholar] [CrossRef]
- Yeh, C.-B.; Hsieh, M.-J.; Hsieh, Y.-H.; Chien, M.-H.; Chiou, H.-L.; Yang, S.-F. Antimetastatic effects of norcantharidin on hepatocellular carcinoma by transcriptional inhibition of MMP-9 through modulation of NF-κB activity. PLoS One 2012, 7, e31055. [Google Scholar]
- Li, X.Q.; Shao, S.H.; Fu, G.L.; Han, X.H.; Gao, H. Study on norcantharidin- induced apoptosis in SMMC-7721 cells through mitochondrial pathways. Chin. J. Integr. Med. 2010, 16, 448–452. [Google Scholar] [CrossRef]
- Chang, C.; Zhu, Y.; Tang, X.; Tao, W. The anti-proliferative effects of norcantharidin on human HepG2 cells in cell culture. Mol. Biol. Rep. 2011, 38, 163–169. [Google Scholar]
- Chen, Y.-N.; Cheng, C.-C.; Chen, J.-C.; Tsauer, W.; Hsu, S.-L. Norcantharidin-induced apoptosis is via the extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase signaling pathways in human hepatoma HepG2 cells. Br. J. Pharmacol. 2003, 140, 461–470. [Google Scholar]
- Chang, C.; Zhu, Y.-Q.; Mei, J.-J.; Liu, S.-Q.; Luo, J. Involvement of mitochondrial pathway in NCTD-induced cytotoxicity in human hepG2 cells. J. Exp. Clin. Cancer Res. 2010, 29, 145. [Google Scholar] [CrossRef]
- McCluskey, A.; Bowyer, M.C.; Collins, E.; Sim, A.T.R.; Sakoff, J.A.; Baldwin, M.L. Anhydride modified cantharidin analogues: Synthesis, inhibition of protein phosphatases 1 and 2A and anticancer activity. Bioorg. Med. Chem. Lett. 2000, 10, 1687–1690. [Google Scholar] [CrossRef]
- Sodeoka, M.; Baba, Y.; Kobayashi, S.; Hirukawa, N. Structure-activity relationship of cantharidin derivatives to protein phosphatases 1, 2A1, and 2B. Bioorg. Med. Chem. Lett. 1997, 7, 1833–1836. [Google Scholar] [CrossRef]
- Hill, T.A.; Stewart, S.G.; Gordon, C.P.; Ackland, S.P.; Gilbert, J.; Sauer, B.; Sakoff, J.A.; McCluskey, A. Norcantharidin analogues: Synthesis, anticancer activity and protein phosphatase 1 and 2A inhibition. ChemMedChem 2008, 3, 1878–1892. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.-Y.; Wang, X.-X.; Yan, D.-M.; Kong, L.-C.; Lin, Q.-Y. Synthesis, antiproliferative activity and DNA-binding properties of nitrogen and sulfur heterocyclic norcantharidin acylamide acid. Chin. J. Chem. 2011, 29, 473–477. [Google Scholar] [CrossRef]
- Robertson, M.J.; Gordon, C.P.; Gilbert, J.; McCluskey, A.; Sakoff, J.A. Norcantharimide analogues possessing terminal phosphate esters and their anti-cancer activity. Bioorg. Med. Chem. 2011, 19, 5734–5741. [Google Scholar] [CrossRef]
- McCuskey, A.; Keane, M.A.; Mudgee, L.-M.; Sim, A.R.T.; Sakoff, J.; Quinn, R.J. Anhydride modified cantharidin analogues. Is ring opening important in the inhibition of protein phosphatase 2A? Eur. J. Med. Chem. 2000, 35, 957–964. [Google Scholar] [CrossRef]
- Hill, T.A.; Stewart, S.G.; Ackland, S.P.; Gilbert, J.; Sauer, B.; Sakoff, J.A.; McCluskey, A. Norcantharimides, synthesis and anticancer activity: Synthesis of new norcantharidin analogues and their anticancer evaluation. Bioorg. Med. Chem. 2007, 15, 6126–6134. [Google Scholar] [CrossRef]
- Campbell, B.E.; Tarleton, M.; Gordon, C.P.; Sakoff, J.A.; Gilbert, J.; McCluskey, A.; Gasser, R.B. Norcantharidin analogues with nematocidal activity in Haemonchus. contortus. Bioorg. Med. Chem. Lett. 2011, 21, 3277–3281. [Google Scholar] [CrossRef]
- Luan, J.; Duan, H.; Liu, Q.; Yagasaki, K.; Zhang, G. Inhibitory effects of norcantharidin against human lung cancer cell growth and migration. Cytotechnology. 2010, 62, 349–355. [Google Scholar]
- van Engeland, M.; Nieland, L.J.W.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, C.P.M. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2, 5–27 are available from the authors. |
© 2014 by MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, J.-Y.; Kuo, C.-D.; Chu, C.-Y.; Chen, M.-S.; Lin, J.-H.; Chen, Y.-J.; Liao, H.-F. Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities. Molecules 2014, 19, 6911-6928. https://doi.org/10.3390/molecules19066911
Wu J-Y, Kuo C-D, Chu C-Y, Chen M-S, Lin J-H, Chen Y-J, Liao H-F. Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities. Molecules. 2014; 19(6):6911-6928. https://doi.org/10.3390/molecules19066911
Chicago/Turabian StyleWu, Jin-Yi, Cheng-Deng Kuo, Chien-Yu Chu, Min-Shin Chen, Jia-Hua Lin, Yu-Jen Chen, and Hui-Fen Liao. 2014. "Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities" Molecules 19, no. 6: 6911-6928. https://doi.org/10.3390/molecules19066911
APA StyleWu, J.-Y., Kuo, C.-D., Chu, C.-Y., Chen, M.-S., Lin, J.-H., Chen, Y.-J., & Liao, H.-F. (2014). Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities. Molecules, 19(6), 6911-6928. https://doi.org/10.3390/molecules19066911







