Evaluation of Antioxidant Activity, Polyphenolic Compounds, Amino Acids and Mineral Elements of Representative Genotypes of Lonicera edulis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Mineral Compounds


- Group 1: Tomichka, Zolushka, Vasivskaya and Gerda
- Group 2: Nimfa, Morena, Sinoglaska, Sinaja Ptica, Roksana and Altaj
- Group 3: Vasjuganska, Kamchadalka, Viola, Goluboje vreteno and Fialka
- Group 4: Leningradskii velikan, Amur and Amfora.
2.2. Determination of Total Polyphenols Content
| Cultivars | Total Polyphenols | Cultivars | Total Polyphenols |
|---|---|---|---|
| Altaj | 5,542 ± 82 | Nimfa | 6,259 ± 54 |
| Amfora | 5,632 ± 94 | Roksana | 7,896 ± 103 |
| Amur | 7,326 ± 111 | Sinaja Ptica | 7,598 ± 117 |
| Bakcarskaja | 6,859 ± 108 | Sinogalaska | 7,789 ± 124 |
| Fialka | 6,596 ± 71 | Vasilevskaya | 6,985 ± 132 |
| Gerda | 8,236 ± 105 | Vasjuganska | 7,536 ± 135 |
| Goluboje vreteno | 8,659 ± 109 | Viola | 7,159 ± 84 |
| Kamchadalka | 7,798 ± 88 | Tomichka | 8,259 ± 129 |
| Leningradskij velikan | 6,235 ± 81 | Zolushka | 8,569 ± 115 |
| Morena | 6,235 ± 77 |
2.3. Determination of Selected Polyphenolic Compounds
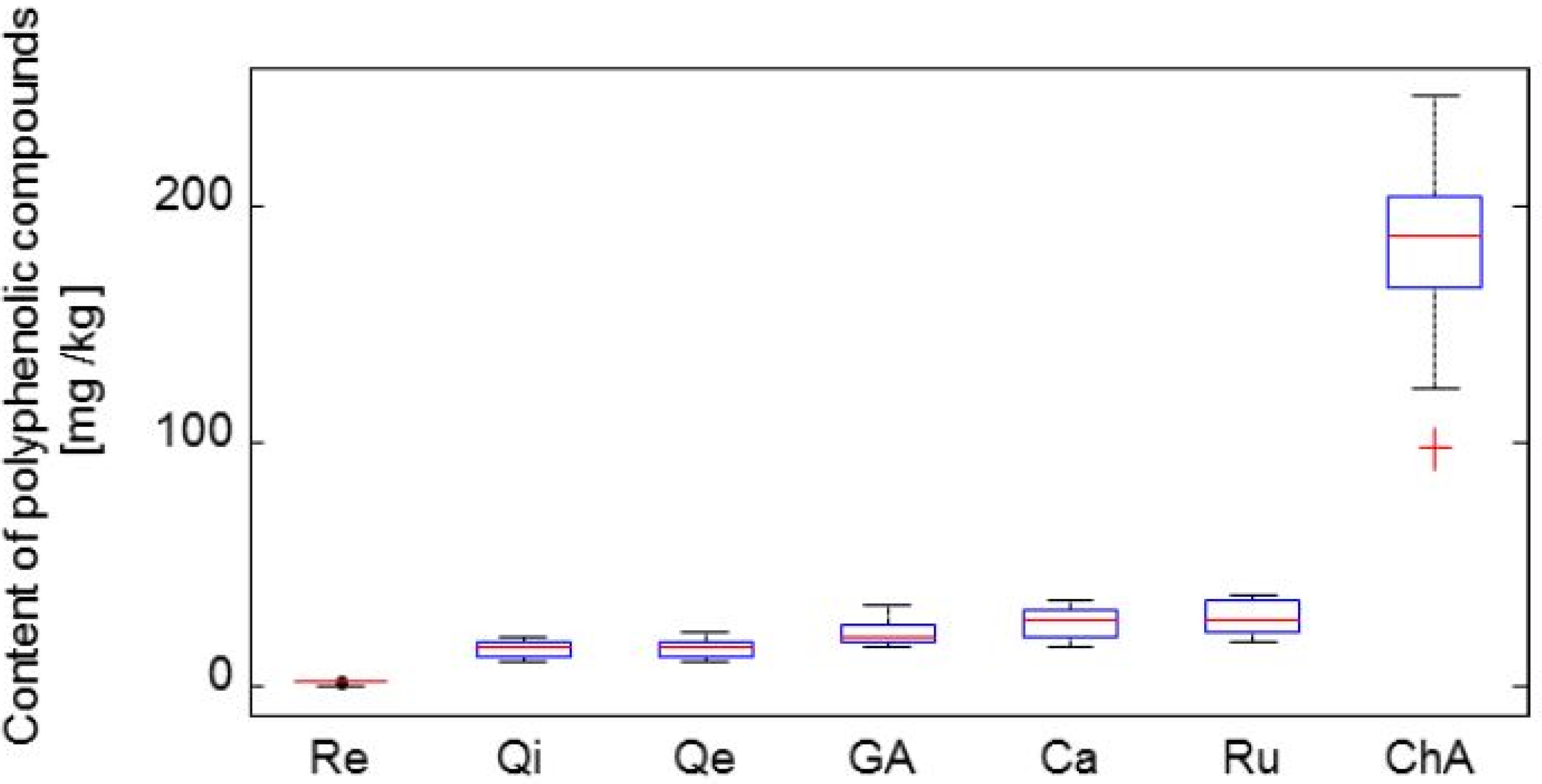
- Group 1: Leningradskij velikan
- Group 2: Vasilevskaya
- Group 3: Tomichka, Viola, Vasjuganska, Zolushka, Morena, Sinoglaska, Kamchadalka
- Group 4: Roksana, Goluboje vreteno, Sinaja Ptica, Nimfa, Gerda
- Group 5: Fialka, Amur, Bakcarskaja, Amfora, Altaj.

2.4. Determination of Antioxidant Activity
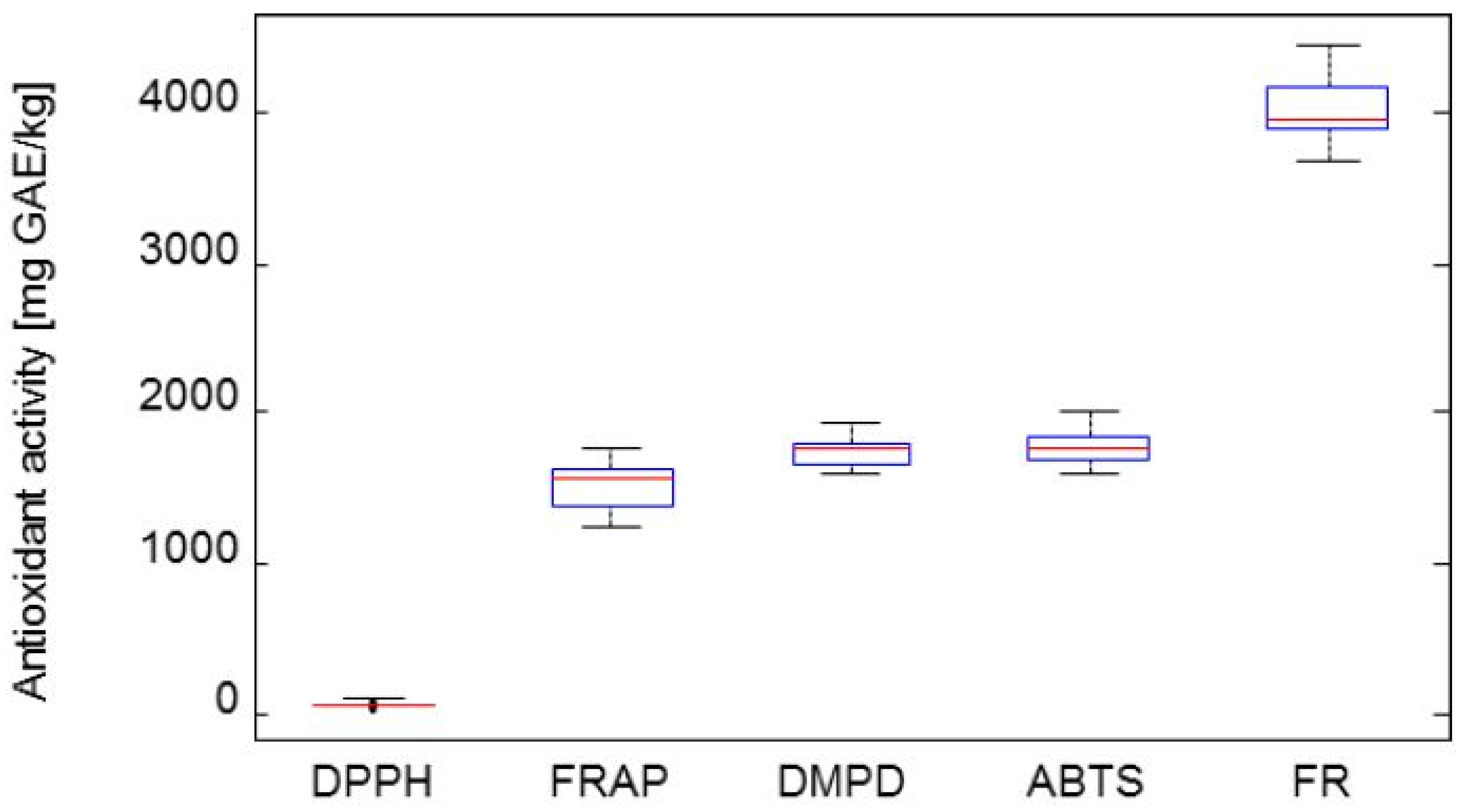
- Group 1: Zolushka, Tomichka
- Group 2: Bakcarskaja, Vasilevskaya, Nimfa, Morena, Fialka, Amfora, Leningradskii velikan, Altaj
- Group3: Vasjuganska, Roksana, Goluboje vreteno, Amur, Kamchadalka, Gerda, Sinaja Ptica, Viola, Sinoglaska.

2.5. Determination of Amino Acids
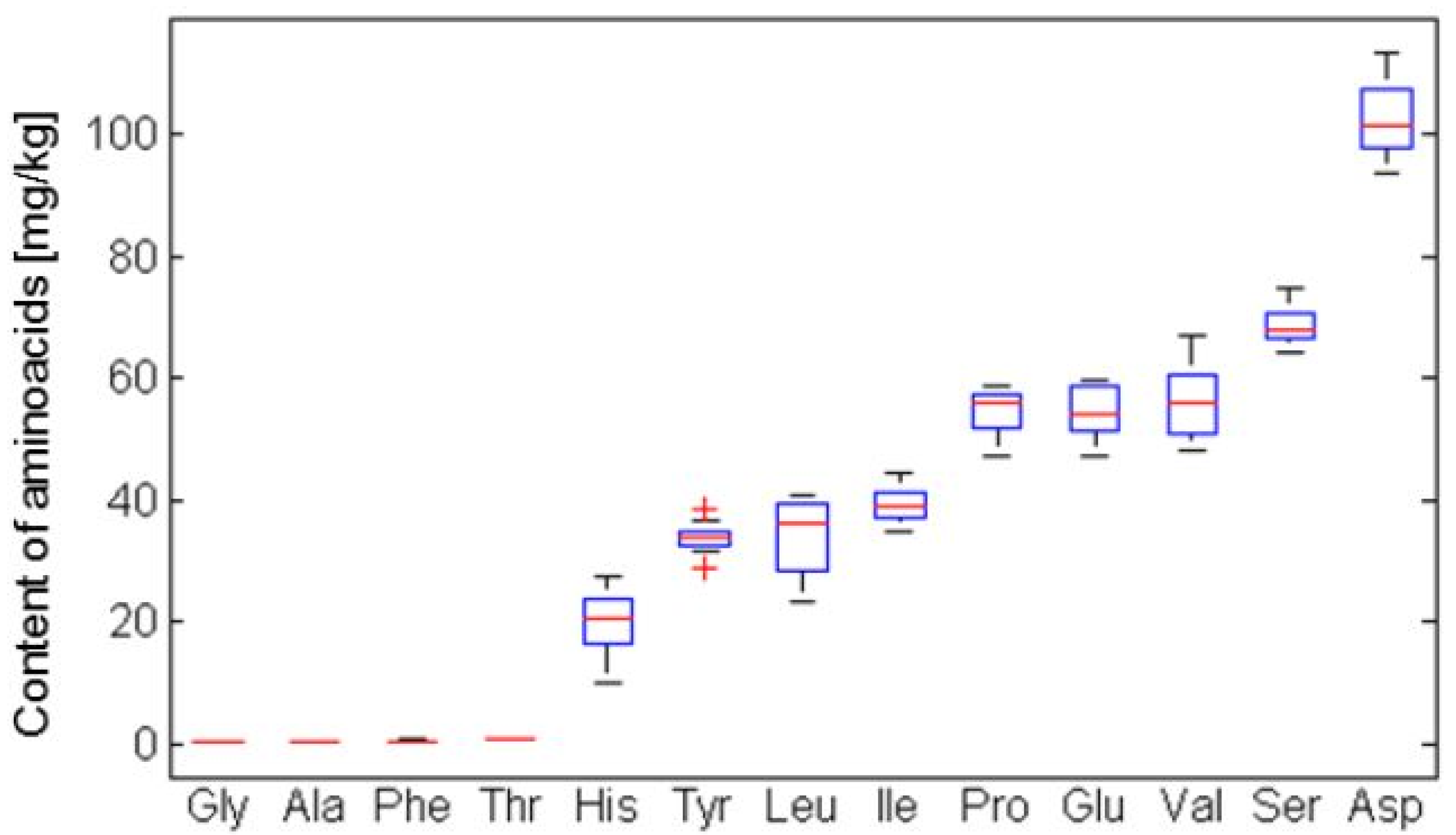
- Group 1: Amfora, Fialka, Amur and Altaj
- Group 2: Zolushka, Tomichka, Viola and Roksana
- Group 3: Morena, Sinoglaska, Gerda, Bakcarskaja, Vasjuganska, Vasilevskaya, Sinaja Ptica, Kamchadalka, Goluboje vreteno, Nimfa, Leningradskij velikan.

3. Experimental
3.1. Biological Material
3.2. Sample Preparation
3.3. Chemicals and PH Measurements
3.4. Determination of Minerals Content
3.5. HPLC Profile of Selected Antioxidants
3.6. Determination of Total Content of Polyphenols
3.7. Determination of Antioxidant Activity
3.7.1. Determination of Antioxidant Activity by the ABTS Test
3.7.2. Determination of Antioxidant Activity by the FRAP Method
3.7.3. Determination of Antioxidant Activity by the Free Radicals Method
3.7.4. Determination of Antioxidant Activity by the DPPH Test
3.7.5. Determination of Antioxidant Activity by the DMPD Method
3.8. Determination of Amino Acids
3.9. Mathematical and Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plekhanova, M.N.; Streltsyna, S.A.; Rostova, N.S. Fruit Chemical Composition of Lonicera subsect Caerulea (Caprifoliaceae species), Genetic Resourses in Russia and Neighbouring Countries; Estonian Agricultural University-Forest Research Institut: Tartu, Estonia, 1998; volume 30, pp. 143–146. [Google Scholar]
- Pokorna-Jurikova, T.; Matuskovic, J. The study of irrigation influence on nutritional value of Lonicera kamtschatica—Cultivar Gerda 25 and Lonicera edulis berries under the Nitra conditions during 2001–2003. Hortic. Sci. 2007, 34, 11–16. [Google Scholar]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (Genus lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Zitny, R.; Zitka, O.; Adam, V.; Kizek, R. Evaluation of polyphenolic profile and nutritional value of non-traditional fruit species in the Czech Republic—A comparative study. Molecules 2012, 17, 8968–8981. [Google Scholar] [CrossRef]
- Gazdik, Z.; Krska, B.; Adam, V.; Saloun, J.; Pokorna, T.; Reznicek, V.; Horna, A.; Kizek, R. Electrochemical determination of the antioxidant potential of some less common fruit species. Sensors 2008, 8, 7564–7570. [Google Scholar] [CrossRef]
- Lefèvre, I.; Ziebel, J.; Guignard, C.; Sorokin, A.; Tikhonova, O.; Dolganova, N.; Hoffmann, L.; Eyzaguirre, P.; Hausman, J.-F. Evaluation and comparison of nutritional quality and bioactive compounds of berry fruits from Lonicera caerulea, Ribes L. species and Rubus idaeus grown in Russia. J. Berry Res. 2011, 1, 159–167. [Google Scholar]
- Rop, O.; Reznicek, V.; Mlcek, J.; Jurikova, T.; Balik, J.; Sochor, J.; Kramarova, D. Antioxidant and radical oxygen species scavenging activities of 12 cultivars of blue honeysuckle fruit. Hortic. Sci. 2011, 38, 63–70. [Google Scholar]
- Myjavcova, R.; Marhol, P.; Kren, V.; Simanek, V.; Ulrichova, J.; Palikova, I.; Papouskova, B.; Lemr, K.; Bednar, P. Analysis of anthocyanin pigments in Lonicera (Caerulea) extracts using chromatographic fractionation followed by microcolumn liquid chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 7932–7941. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Thompson, M.M.; Wrolstad, R.E. Characterization and quantification of Anthocyanins and polyphenolics in blue honeysuckle (Lonicera caerulea L.). J. Agric. Food Chem. 2004, 52, 848–852. [Google Scholar] [CrossRef]
- Palikova, I.; Heinrich, J.; Bednar, P.; Marhol, P.; Kren, V.; Cvak, L.; Valentova, K.; Ruzicka, F.; Hola, V.; Kolar, M.; et al. Constituents and Antimicrobial Properties of Blue Honeysuckle: A Novel Source for Phenolic Antioxidants. J. Agric. Food Chem. 2008, 56, 11883–11889. [Google Scholar] [CrossRef]
- Farcasanu, I.C.; Gruia, M.I.; Paraschivescu, C.; Oprea, E.; Baciu, I. Ethanol extracts of Lonicera caerulea and Sambucus nigra berries exhibit antifungal properties upon heat-stressed Saccharomyces cerevisiae cells. Rev. Chim. 2006, 57, 79–81. [Google Scholar]
- Gruia, M.I.; Oprea, E.; Gruia, I.; Negoita, V.; Farcasanu, I.C. The Antioxidant Response Induced by Lonicera caerulaea Berry Extracts in Animals Bearing Experimental Solid Tumors. Molecules 2009, 14, 893–893. [Google Scholar] [CrossRef]
- Thompson, M.M.; Chaovanalikit, A. A. Preliminary Observations on Adaptation and Nutraceutical Values of Blue Honeysuckle (Lonicera caerulea) in Oregon, USA. In Berry Crop Breeding, Production and Utilization for a New Century; Hicklenton, P., Maas, J., Eds.; 26th International Horticultural Congress: Toronto, ON, Canada, 2003; pp. 65–72. [Google Scholar]
- Zhao, H.T.; Wang, Z.Y.; Cheng, C.L.; Yao, L.; Wang, L.; Lu, W.H.; Yang, X.; Ma, F.M. In-vitro free radical scavenging activities of anthocyanins from three berries. J. Med. Plants Res. 2011, 5, 7036–7042. [Google Scholar]
- Kusznierewicz, B.; Piekarska, A.; Mrugalska, B.; Konieczka, P.; Namiesnik, J.; Bartoszek, A. Phenolic composition and antioxidant properties of polish blue-berried honeysuckle genotypes by HPLC-DAD-MS, HPLC postcolumn derivatization with ABTS or FC, and TLC with DPPH visualization. J. Agric. Food Chem. 2012, 60, 1755–1763. [Google Scholar] [CrossRef]
- Švarcova, I.; Heinrich, J.; Valentova, K. Berry fruits as a source of biologically active compounds: The case of Lonicera caerulea. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 2007, 151, 163–174. [Google Scholar] [CrossRef]
- Skupien, K.; Ochmian, I.; Grajkowski, J. Influence of ripening time on fruit chemical composition of two blue honeysuckle cultigens. J. Fruit Ornam. Plant Res. 2009, 17, 101–111. [Google Scholar]
- Ochmian, I.; Skupien, K.; Grajkowski, J.; Smolik, M.; Ostrowska, K. Chemical Composition and Physical Characteristics of Fruits of Two Cultivars of Blue Honeysuckle (Lonicera caerulea L.) in Relation to their Degree of Maturity and Harvest Date. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 155–162. [Google Scholar]
- Rupasinghe, H.P.V.; Yu, L.J.; Bhullar, K.S.; Bors, B. Short communication: Haskap (Lonicera caerulea): A new berry crop with high antioxidant capacity. Can. J. Plant Sci. 2012, 92, 1311–1317. [Google Scholar] [CrossRef]
- Streltsyna, S.A.; Sorotkin, A.A.; Plekhanova, M.N.; Lobanova, E.V. Sostav biologičeskich aktivnyh fenolyj soedinenii sortov žimolosti v uslovjach everno-zapadnoj zony plodovodstva. Agrar. Ross. 2006, 6, 67–72. [Google Scholar]
- Bakowska-Barczak, A.M.; Marianchuk, M.; Kolodziejczyk, P. Survey of bioactive components in Western Canadian berries. Can. J. Physiol. Pharmacol. 2007, 85, 1139–1152. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Plekhanova, M.N. Blue Honeysuckle (Lonicera caerulea L.)—A New Commercial Berry Crop for Temperate Climate: Genetic Resources and Breeding. In Proceedings of the Eucarpia Symposium on Fruit Breeding and Genetics, Dresden, Germany, 6–10 September 1999; Geibel, M., Fischer, M., Fischer, C., Eds.; International Society Horticultural Science: Leuven, Belgium, 2000; volumes 1 and 2, pp. 159–164. [Google Scholar]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef]
- Orincak, J.; Matuskovic, J.; Jurcak, S. Possibilities of Species Lonicera caerulea in Utilization of the Secondary Metabolism in Food and Pharmaceutical Processing, 1st ed.; SPU: Nitra, Slovakia, 2003; pp. 210–219. [Google Scholar]
- Deineka, V.I.; Sorokopudov, V.N.; Deineka, L.A.; Shaposhnik, E.I.; Koltsov, S.V. Anthocyans from fruit of some plants of the Caprifoliaceae family. Chem. Nat. Compd. 2005, 41, 162–164. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Oszmianski, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Li, H.Y.; Deng, Z.Y.; Wu, T.; Liu, R.H.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Jurikova, T.; Matuskovic, J.; Gazdik, Z. Effect of irrigation on intensity of respiration and study of sugar and organic acids content in different development stages of Lonicera kamtschatica and Lonicera edulis berries. Hortic. Sci. 2009, 36, 14–20. [Google Scholar]
- Gazdik, Z.; Reznicek, V.; Adam, V.; Zitka, O.; Jurikova, T.; Krska, B.; Matuskovic, J.; Plsek, J.; Saloun, J.; Horna, A.; et al. Use of Liquid Chromatography with Electrochemical Detection for the Determination of Antioxidants in Less Common Fruits. Molecules 2008, 13, 2823–2836. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Skupien, K. Field performance, fruit chemical composition and formness under cold storage and stimulated “shelf–life” conditions of tree blue honeysuckle cultigens (Lonicera caerulea). J. Fruit Ornam. Plant Res. 2008, 16, 83–91. [Google Scholar]
- Hakkinen, S.H.; Karenlampi, S.O.; Heinonen, I.M.; Mykkanen, H.M.; Torronen, A.R. HPLC method for screening of flavonoids and phenolic acids in berries. J. Sci. Food Agric. 1998, 77, 543–551. [Google Scholar] [CrossRef]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef]
- Smolik, M.; Ochmian, I.; Grajkowski, J. Genetic variability of Polish and Russian accessions of cultivated blue honeysuckle (Lonicera caerulea). Russ. J. Genet. 2010, 46, 960–966. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.H.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Rop, O.; Posolda, M.; Mlcek, J.; Reznicek, V.; Sochor, J.; Adam, V.; Kizek, R.; Sumczynski, D. Qualities of native apple cultivar juices characteristic of Central Europe. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 222–228. [Google Scholar]
- Rop, O.; Sochor, J.; Jurikova, T.; Zitka, O.; Skutkova, H.; Mlcek, J.; Salas, P.; Krska, B.; Babula, P.; Adam, V.; et al. Effect of five different stages of ripening on chemical compounds in medlar (Mespilus germanica L.). Molecules 2011, 16, 74–91. [Google Scholar]
- Sochor, J.; Skutkova, H.; Babula, P.; Zitka, O.; Cernei, N.; Rop, O.; Krska, B.; Adam, V.; Provaznik, I.; Kizek, R. Mathematical Evaluation of the Amino Acid and Polyphenol Content and Antioxidant Activities of Fruits from Different Apricot Cultivars. Molecules 2011, 16, 7428–7457. [Google Scholar] [CrossRef]
- Rop, O.; Balik, J.; Reznicek, V.; Jurikova, T.; Skardova, P.; Salas, P.; Sochor, J.; Mlcek, J.; Kramarova, D. Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech J. Food Sci. 2011, 29, 65–73. [Google Scholar]
- Rop, O.; Jurikova, T.; Sochor, J.; Mlcek, J.; Kramarova, D. Antioxidant capacity, scavenging radical activity and selected chemical composition of native apple cultivars from central Europe. J. Food Qual. 2011, 34, 187–194. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Sochor, J.; Babula, P.; Krska, B.; Horna, A.; Provaznik, I.; Hubalek, J.; Kizek, R. Evaluation of output signals from CoulArray detector for determination of antioxidant capacity of apricots samples. In Analysis of Biomedical Signals and Images; Jan, J., Jirik, R., Kolar, R., Kolarova, J., Kozumplik, J., Provaznik, I., Eds.; Brno Univ Technology Vut Press: Brno, Czech Republic, 2010; pp. 209–214. [Google Scholar]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Pohanka, M.; Stetina, R.; Svobodova, H.; Ruttkay-Nedecky, B.; Jilkova, M.; Sochor, J.; Sobotka, J.; Adam, V.; Kizek, R. Sulfur mustard causes oxidative stress and depletion of antioxidants in muscles, livers, and kidneys of Wistar rats. Drug Chem. Toxicol. 2013, 36, 270–276. [Google Scholar] [CrossRef]
- Pohanka, M.; Sochor, J.; Ruttkay-Nedecky, B.; Cernei, N.; Adam, V.; Hubalek, J.; Stiborova, M.; Eckschlager, T.; Kizek, R. Automated assay of the potency of natural antioxidants using pipetting robot and spectrophotometry. J. Appl. Biomed. 2012, 10, 155–167. [Google Scholar] [CrossRef]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremae, K.; Pedastsaar, P.; Maesaar, M.; Raal, A.; Laikoja, K.; Pussa, T. The antioxidative and antimicrobial properties of the blue honeysuckle (Lonicera caerulea L.), Siberian rhubarb (Rheum rhaponticum L.) and some other plants, compared to ascorbic acid and sodium nitrite. Food Control 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Paulovicsova, B.; Turianica, I.; Jurikova, T.; Baloghova, M.; Matuskovic, J. Antioxidant properties of selected less common fruit species. Lucrări ştiinţifice Zootehnie şi Biotehnologii 2009, 42, 608–614. [Google Scholar]
- Lee, J.; Schreiner, R.P. Free amino acid profiles from “Pinot noir” grapes are influenced by vine N-status and sample preparation method. Food Chem. 2010, 119, 484–489. [Google Scholar] [CrossRef]
- Wallrauch, S. Amino-acids criteria for the evaluation of fruit juices. Abstr. Pap. Am. Chem. Soc. 1985, 189, 0065–7727. [Google Scholar]
- Zitka, O.; Sochor, J.; Rop, O.; Skalickova, S.; Sobrova, P.; Zehnnalek, J.; Beklova, M.; Krska, B.; Adam, V.; Kizek, R. Comparison of various easy-to-use procedures for extraction of phenols from apricot fruits. Molecules 2011, 16, 2914–2936. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants, Part A; Packer, L., Ed.; Gulf Professional Publishing: San Diego, CA, USA, 1999; pp. 152–178. [Google Scholar]
- Sochor, J.; Ryvolova, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully automated spectrometric protocols for determination of antioxidant activity: Advantages and disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar] [CrossRef]
- Sochor, J.; Salas, P.; Zehnalek, J.; Krska, B.; Adam, V.; Havel, L.; Kizek, R. An assay for spectrometric determination of antioxidant activity of a biological extract. Listy Cukrov. 2010, 126, 416–417. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sochor, J.; Jurikova, T.; Pohanka, M.; Skutkova, H.; Baron, M.; Tomaskova, L.; Balla, S.; Klejdus, B.; Pokluda, R.; Mlcek, J.; et al. Evaluation of Antioxidant Activity, Polyphenolic Compounds, Amino Acids and Mineral Elements of Representative Genotypes of Lonicera edulis. Molecules 2014, 19, 6504-6523. https://doi.org/10.3390/molecules19056504
Sochor J, Jurikova T, Pohanka M, Skutkova H, Baron M, Tomaskova L, Balla S, Klejdus B, Pokluda R, Mlcek J, et al. Evaluation of Antioxidant Activity, Polyphenolic Compounds, Amino Acids and Mineral Elements of Representative Genotypes of Lonicera edulis. Molecules. 2014; 19(5):6504-6523. https://doi.org/10.3390/molecules19056504
Chicago/Turabian StyleSochor, Jiri, Tunde Jurikova, Miroslav Pohanka, Helena Skutkova, Mojmir Baron, Lenka Tomaskova, Stefan Balla, Borivoj Klejdus, Robert Pokluda, Jiri Mlcek, and et al. 2014. "Evaluation of Antioxidant Activity, Polyphenolic Compounds, Amino Acids and Mineral Elements of Representative Genotypes of Lonicera edulis" Molecules 19, no. 5: 6504-6523. https://doi.org/10.3390/molecules19056504