Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages
Abstract
:1. Introduction
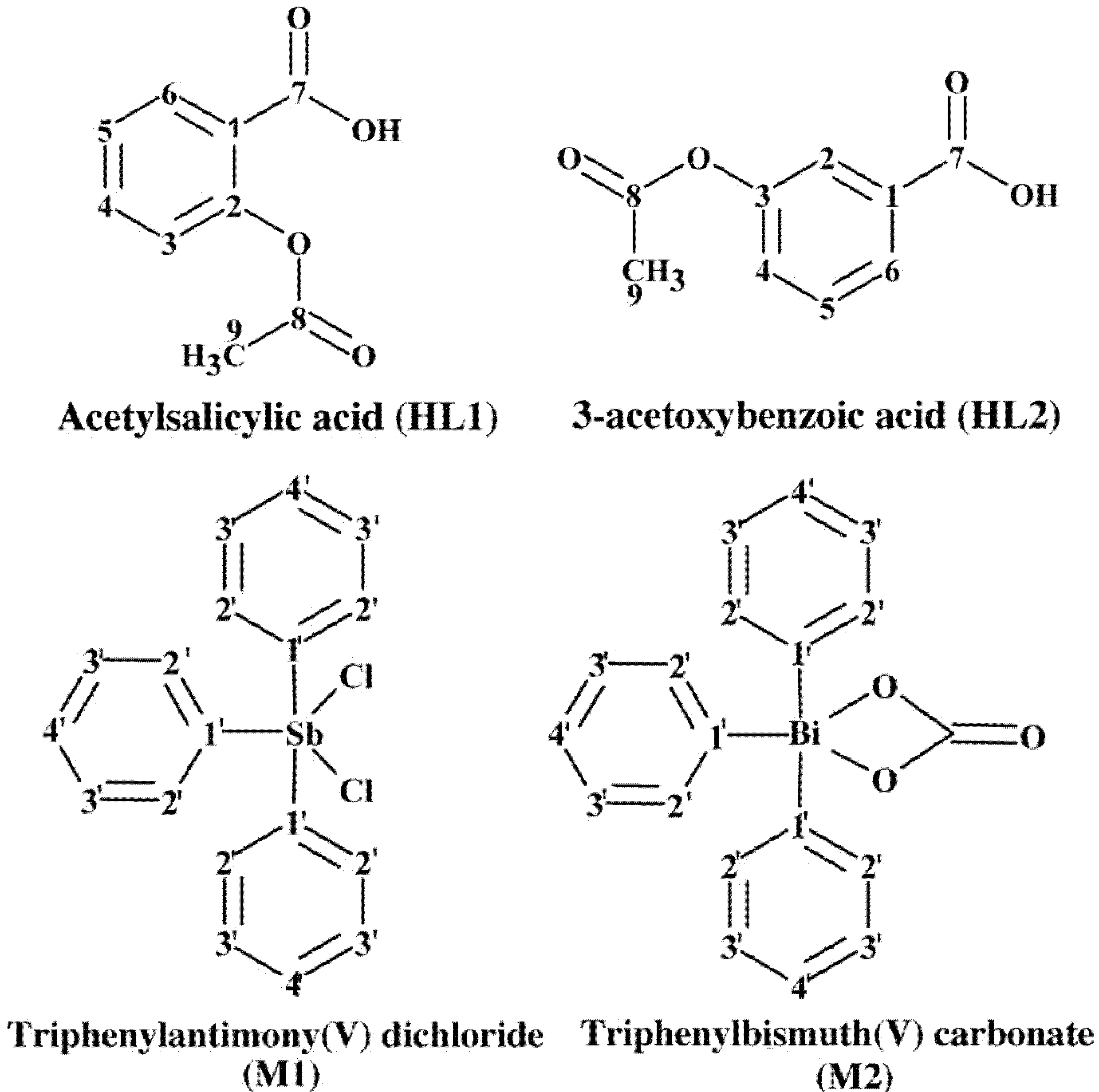
2. Results and Discussion
2.1. Characterization of Complexes 1–4
2.1.1. Infrared Spectra
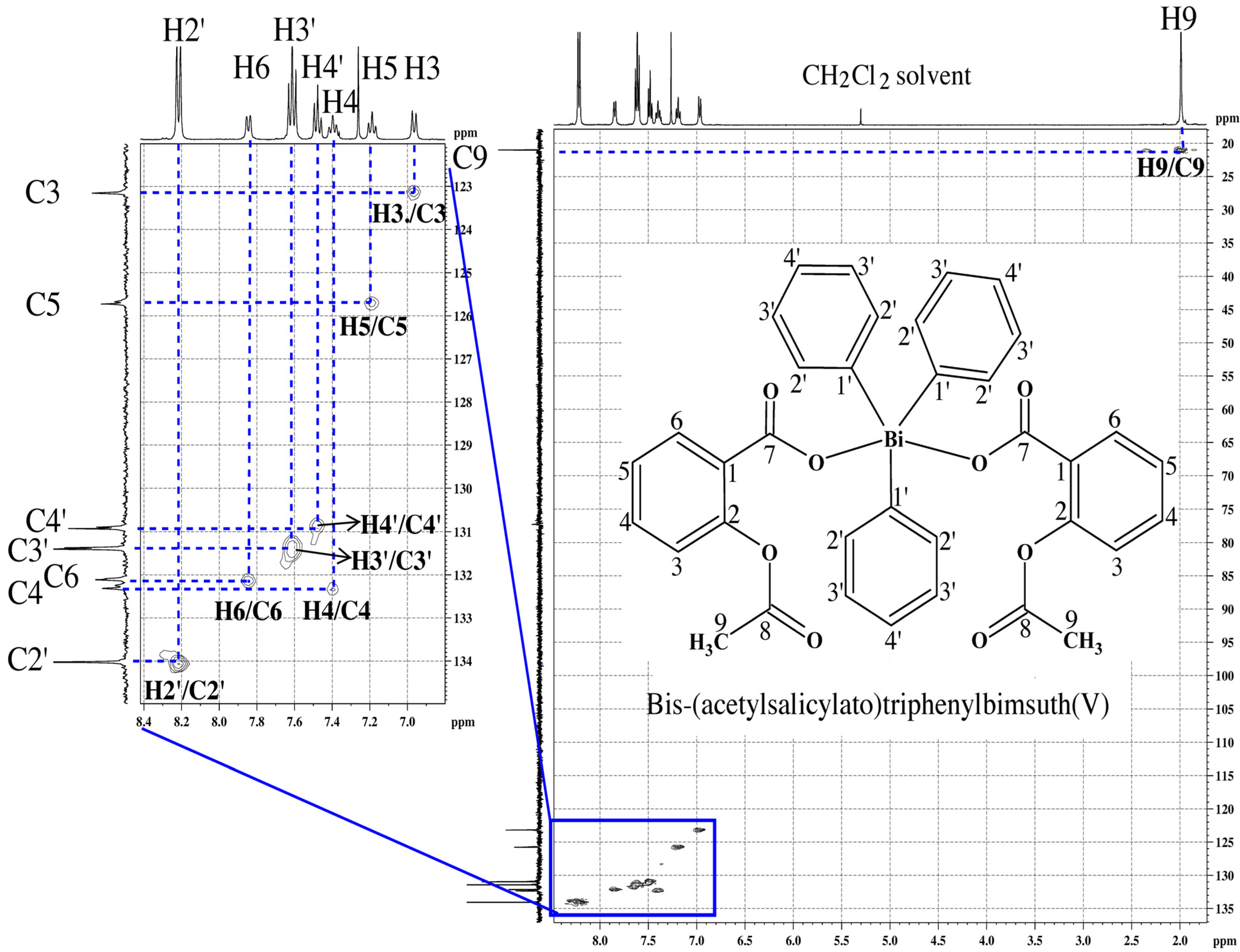
2.1.2. NMR Spectra
2.1.3. Crystal Structure of Complexes 1 and 2
| Complex 1 | Complex 2 | |
|---|---|---|
| CCDC deposition number | 981904 | 981905 |
| Empirical formula | C36H29O8Sb | C37H30Cl3O8Sb |
| Molecular mass (g mol−1) | 711.34 | 830.71 |
| Temperature (K) | 293(2) | 150(2) |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | Pī |
| Unit cell dimensions | ||
| a (Å) | 16.5940(5) | 10.6597(3) |
| b (Å) | 9.9184(3) | 11.1818(3) |
| c (Å) | 19.2259(6) | 16.2321(4) |
| α (°) | 90° | 70.446(3)° |
| β (°) | 96.3222(3)° | 88.098(2)° |
| γ (°) | 90° | 7.643(2)° |
| Volume (Å3)Z | 3145.07(17)4 | 71779.40(8)2 |
| Dcalc (mg/m3) | 1.502 | 1.55 |
| Crystal dimensions (mm3) | 0.38 × 0.29 × 0.05 | 0.45 × 0.12 × 0.04 |
| μ = Absorption coefficient (mm–1) | 0.930 | 1.05 |
| F(000) | 1440 | 836 |
| θ Range (°) | θmax = 29.6°, θmin = 2.1° | θmax = 29.6°, θmin = 2.0° |
| Index ranges | –22 ≤ h ≤ 22, | –14 ≤ h ≤ 14, |
| –13 ≤ k ≤ 13, | –15 ≤ k ≤ 15, | |
| –26 ≤ l ≤ 26 | –22 ≤ l ≤ 21 | |
| Reflections collected | 25501 | 34656 |
| Independent reflections | 4119 | 8931 |
| Rint | 0.0431 | 0.041 |
| Completeness to theta full | 93.94% (θ = 26.32°) | 99.96% (θ = 26.32°) |
| Data/restraints/parameters | 4119/0/206 | 8931/0/444 |
| Goodness-of-fit on F2 | 1.07 | 1.08 |
| Final R indices [ I > 2σ (I)] | R1 = 0.0277, wR2 = 0.0680 | R1 = 0.0272, wR2 = 0.0641 |
| R indices (all data) | R1 = 0.0325, wR2 = 0.0713 | R1 = 0.0319, wR2 = 0.0669 |
| Largest difference in peak/hole (e Å–3) | 0.55 and −0.49 | 0.75 and −0.83 |
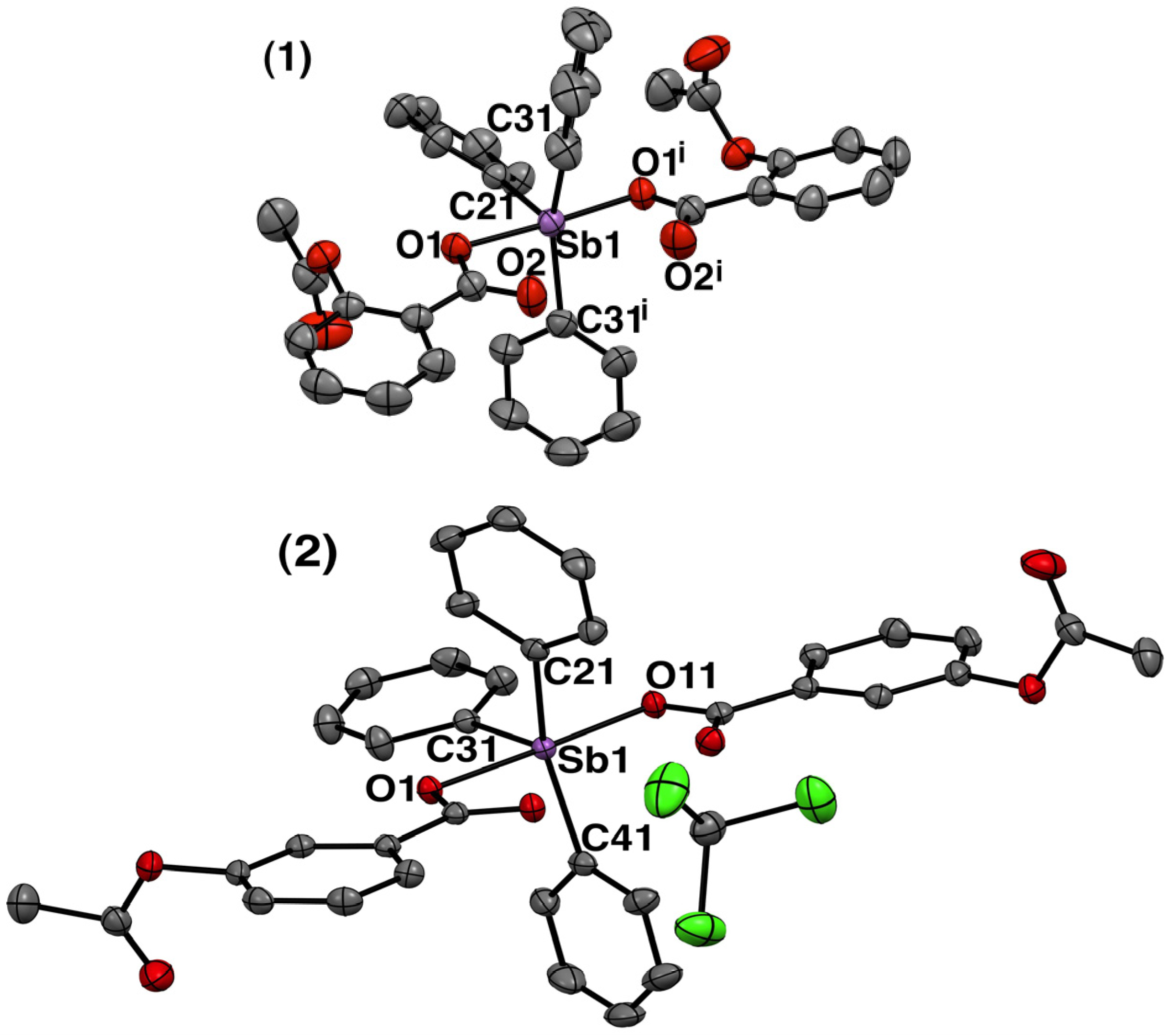
| 1 | 2 | ||
|---|---|---|---|
| Bond angles | (°) | Bond angles | (°) |
| Sb(1)–O(1) | 2.1524(14) | Sb(1)–O(11) | 2.1228 (13) |
| Sb(1)–O(1i) | 2.1524(14) | Sb(1)–O(1) | 2.1410 (12) |
| Sb(1)–C(21) | 2.122(3) | Sb(1)–C(41) | 2.1124 (19) |
| Sb(1)–(C(31) | 2.127(2) | Sb(1)–C(31) | 2.1196 (18) |
| Sb(1)– C(31i) | 2.127(2) | Sb(1)–C(21) | 2.1219 (19) |
| Bond lengths | (Å) | Bond lengths | (Å) |
| C(21)–Sb(1)–C(31) | 103.48(6) | C(41)–Sb(1)–C(31) | 105.29 (7) |
| C(31)–Sb(1)–C(31i) | 153.04(12) | C(41)–Sb(1)–C(21) | 149.52 (7) |
| C(21)–Sb(1)–C(31i) | 103.48(6) | C(31)–Sb(1)–C(21) | 104.76 (7) |
| C(21)–Sb(1)–O(1i) | 87.82(3) | C(41)–Sb(1)–O(11) | 92.81 (6) |
| C(31)–Sb(1)–O(1i) | 89.65(7) | C(31)–Sb(1)–O(11) | 88.90 (6) |
| C(21)–Sb(1)–O(1) | 87.82(3) | C(21)–Sb(1)–O(11) | 92.71 (6) |
| C(31)–Sb(1)–O(1) | 91.37(7) | C(41)–Sb(1)–O(1) | 88.35 (6) |
| C(31i)–Sb(1)–O(1) | 89.65(7) | C(31)–Sb(1)–O(1) | 89.15 (6) |
| O(1i)–Sb(1)–O(1) | 175.64(7) | C(21)–Sb(1)–O(1) | 87.13 (6) |
| C(1)–O(1)–Sb(1) | 104.71(12) | O(11)–Sb(1)–O(1) | 177.94 (5) |
2.2. Biological Activity
2.2.1. Anti-Leishmanial Activity against Promastigotes
| L. infantum strain | L. amazonensis strain | ||
|---|---|---|---|
| Compounds | IC50 (μM) ± SEM | ||
| Acetylsalicylic acid (HL1) | 8.99 × 103 ± 1.89 × 103 | 2.18 × 106 ± 0.12 × 106 | |
| Ph3Sb(L1)2 (1) | 13.3 ± 0.74 | 30.7 ± 3.43 | |
| Ph3Sb(L2)2∙CHCl3 (2) | 12.2 ± 0.91 | 8.9 ± 0.36 | |
| Ph3SbCl2 (M1) | 13.2 ± 2.03 | 9.3 ± 0.26 | |
| 3-acetoxybenzoic acid (HL2) | 3.19 × 107 ± 0.55 × 107 | 1.40 × 107 ± 0.12 × 106 | |
| Ph3Bi(L1)2 (3) | 8.6 ± 1.36 | 8.5 ± 0.56 | |
| Ph3Bi(L2)2 (4) | 4.0 ± 0.39 | 2.9 ± 0.17 | |
| Ph3BiCO3 (M2) | 1.1 ± 0.37 | 2.7 ± 0.34 | |
| TA | 100 ± 3 | 83 ± 1 [16] | |
2.2.2. Antibacterial Activity
| MIC in μM | ||
|---|---|---|
| Compounds | S. aureus | P. aeruginosa |
| Acetylsalicylic acid (HL1) | >555 | >555 |
| Ph3Sb(L1)2 (1) | 140.6 | 140.6 |
| Ph3Sb(L2)2·CHCl3 (2) | 140.6 | 140.6 |
| Ph3SbCl2 (M1) | 235.8 | 235.8 |
| 3-acetoxybenzoic acid (HL2) | >555 | >555 |
| Ph3Bi(L1)2 (3) | 4.1 | 8.2 |
| Ph3Bi(L2)2 (4) | 4.1 | 16.5 |
| Ph3BiCO3 (M2) | 24.5 | 48.9 |
| Tetracycline | 3.5 | >96.7 [48] |
2.2.3. Cytotoxicity against Mammalian Cells
| Complex 1Ph3Sb(L1)2 | Complex 2Ph3Sb(L2)2 | Complex 3Ph3Bi(L1)2 | Complex 4Ph3Bi(L2)2 | M1Ph3SbCl2 | M2 Ph3BiCO3 | HL1 | HL2 | |
|---|---|---|---|---|---|---|---|---|
| * CC50 (μM) | 60.5 ± 3.4 | 30.0 ± 2.3 | 6.4 ± 0.5 | 2.0 ± 0.1 | 22.7 ± 0.93 | 12.1 ± 0.45 | 580.1 ± 1.07 | 2768 ± 9 |
| a SI | 4.51 | 2.45 | 0.74 | <1 | 1.7 | 10.6 | <1 | <1 |
| b SI | 2.0 | 3.40 | 0.75 | <1 | 2.4 | 4.6 | <1 | <1 |
| c SI | <1 | <1 | 1.6 | 1.6 | <1 | <1 | <1 | <1 |
| d SI | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
3. Experimental
3.1. Material and Methods
3.2. Syntheses of Complexes 1–4
3.2.1. Syntheses of Triphenylantimony(V) Complexes 1 and 2
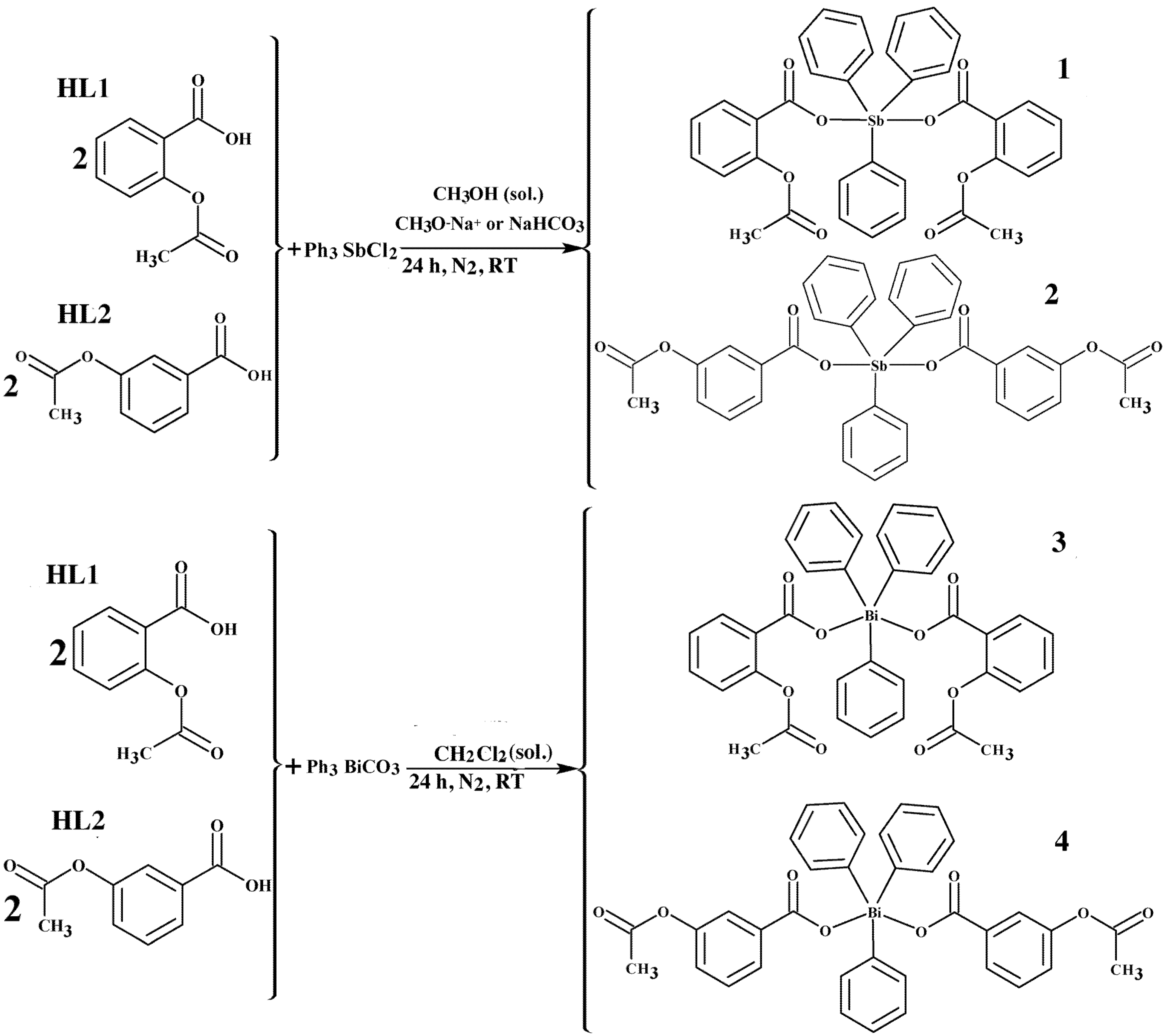
3.2.2. Syntheses of Triphenylbimsuth(V) Complexes 3 and 4
3.3. Biological Activity
3.3.1. Evaluation of Activity against Leishmania promastigotes
3.3.1.1. Parasite Culture
3.3.1.2. Antileishmanial Activity Assay
3.3.2. Evaluation of Activity against Gram-Positive and Gram-Negative Strain of Bacteria
3.3.3. Cytotoxicity Assay against Mouse Peritoneal Macrophages
3.3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Briand, G.G.; Burford, N. Bismuth compounds and preparations with biological or medicinal relevance. Chem. Rev. 1999, 99, 2601–2658. [Google Scholar] [CrossRef]
- Estes, J.W. The Medical Skills of Ancient Egypt, 1st ed.; Science History Publications: Canton, MA, USA, 1989; p. 155. [Google Scholar]
- Lambert, J.R.; Midolo, P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1997, 11, 27–33. [Google Scholar]
- Mishra, J.; Saxena, A.; Singh, S. Chemotherapy of leishmaniasis: Past, present and future. Curr. Med. Chem. 2007, 14, 1153–1169. [Google Scholar] [CrossRef]
- Vianna, G. Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. In Proceedings of the Anais do 7 Congresso Brasileiro de Medicina e Cirurgia, Belo Horizonte, Minas Gerais, Brasil, 21 April 1912; Volume 4, pp. 426–428.
- Berman, J.D. Chemotherapy for leishmaniasis: Biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988, 10, 560–586. [Google Scholar] [CrossRef]
- Mohan, R.S. In Your Element: Green Bismuth. Nat. Chem. 2010, 2, 336–336. [Google Scholar] [CrossRef]
- Yamey, G. The world’s most neglected diseases: Ignored by the pharmaceutical industry and by public-private partnerships. BMJ 2002, 325, 176–177. [Google Scholar] [CrossRef]
- Gradoni, L.; Gramiccia, M.; Scalone, A. Visceral leishmaniasis treatment, Italy. Emerg. Infect. Dis. 2003, 9, 1617–1620. [Google Scholar] [CrossRef]
- Berman, J. Miltefosine to treat leishmaniasis. Expert Opin. Pharmacother. 2005, 6, 1381–1388. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Sinha, P.K.; Sundar, S.; Thakur, C.P.; Jha, T.K.; Pandey, K.; Das, V.R.; Kumar, N.; Lal, C.; Verma, N. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J. Infect. Dis. 2007, 196, 591–598. [Google Scholar] [CrossRef]
- Jha, T.K.; Sundar, S.; Thakur, C.P.; Felton, J.M.; Sabin, A.J.; Riera, C.; Fisa, R.; Ribera, E.; Carria, J.; Falca, V. A phase II dose-ranging study of sitamaquine for the treatment of visceral leishmaniasis in India. Am. J. Trop. Med. Hyg. 2005, 73, 1005–1011. [Google Scholar]
- Wasunna, M.K.; Rashid, J.R.; Mbui, J.; Kirigi, G.; Kinoti, D.; Lodenyo, H.; Felton, J.M.; Sabin, A.J.; Horton, J. A phase II dose-increasing study of sitamaquine for the treatment of visceral leishmaniasis in Kenya. Am. J. Trop. Med. Hyg. 2005, 73, 871–876. [Google Scholar]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.K.; Murray, H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef]
- Andrews, P.C.; Frank, R.; Junk, P.C.; Kedzierski, L.; Kumar, I.; MacLellan, J.G. Anti-Leishmanial activity of homo-and heteroleptic bismuth (III) carboxylates. J. Inorg. Biochem. 2011, 105, 454–461. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.H.; Monte-Neto, R.L.; Reis, P.G.; Fernandes, N.G.; Speziali, N.L.; Melo, M.N.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622–12635. [Google Scholar] [CrossRef]
- Rocha, M.N.; Nogueira, P.M.; Demicheli, C.; de Oliveira, L.G.; da Silva, M.M.; Frézard, F.; Melo, M.N.; Soares, R.P. Cytotoxicity and in vitro antileishmanial activity of antimony (V), Bismuth (V), and Tin (IV) complexes of lapachol. Bioinorg. Chem. Appl. 2013, 2013, 961783:1–961783:7. [Google Scholar]
- Andrews, P.C.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Kedzierski, L.; Peiris, R.M. Bismuth (III) β-thioxoketonates as antibiotics against Helicobacter pylori and as anti-leishmanial agents. Dalton Trans. 2014, 43, 1279–1291. [Google Scholar]
- Ma, Y.-Q; Yu, L.; Li, J.-S. Synthesis and biological activity of some triarylantimony dipyrazolecarboxylates. Heteroato. Chem. 2002, 13, 299–301. [Google Scholar] [CrossRef]
- De Oliveira, L.G.; Silva, M.M.; Paula, F.; Pereira-Maia, E.C.; Donnici, C.L.; de Simone, C.A.; Frézard, F.; Júnior, E.N.; Demicheli, C. Antimony (V) and bismuth (V) complexes of lapachol: Synthesis, crystal structure and cytotoxic activity. Molecules 2011, 16, 10314–10323. [Google Scholar] [CrossRef]
- Ali, M.I.; Rauf, M.K.; Badshah, A.; Kumar, I.; Forsyth, C.M.; Junk, P.C.; Kedzierski, L.; Andrews, P.C. Anti-leishmanial activity of heteroleptic organometallic Sb (V) compounds. Dalton Trans. 2013, 42, 16733–16741. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.H.; Reis, P.G.; Bezerra, F.M.; Rodrigues, B.L.; Monte-Neto, R.L.; Melo, M.N.; Frézard, F.; Demicheli, C. Complexes of different nitrogen donor heterocyclic ligands with SbCl3 and PhSbCl2 as potential antileishmanial agents against SbIII-sensitive and-resistant parasites. J. Inorg. Biochem. 2014, 132, 30–36. [Google Scholar] [CrossRef]
- Sushma, R.A.N.I. Synthesis and characterization of some tetraorganobismuth(V) aryloxyacetate for their biological screening. World Res. J. Appl. Med. Chem. 2011, 1, 24–27. [Google Scholar]
- O’Neill, A.J. New antibacterial agents for treating infections caused by multi-drug resistant Gram-negative bacteria. Expert Opin. Invest. Drugs 2008, 17, 297–302. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Infrared Spectrometry. In In Spectrometric Identification of Organic Compounds, 7th ed.; Brennan, D., Yee, J., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2005; pp. 95–96. [Google Scholar]
- Minzanova, S.T.; Mironov, V.F.; Vyshtakalyuk, A.B.; Tsepaeva, O.V.; Mironova, L.G.; Ryzhkina, I.S.; Murtazina, L.I.; Gubaidullin, A.T. Complexes of pectin polysaccharide with acetylsalicylic acid. Doklady Chem. 2013, 452, 230–233. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Kumar, H.V. The reaction of aspirin with base. Tetrahedron Lett. 2011, 52, 3561–3564. [Google Scholar] [CrossRef]
- Helen Pricilla Bai, E.; Vairam, S. Hydrazine Complexes of Lanthanides with 3-Acetoxy-and 4-Acetoxybenzoic Acids: Spectroscopic, Thermal, and XRD Studies. J. Chem. 2012, 2013, 717618:1–717618:10. [Google Scholar]
- Fregona, D.; Giovagnini, L.; Ronconi, L.; Marzano, C.; Trevisan, A.; Sitran, S.; Biondi, B.; Bordin, F. Pt (II) and Pd (II) derivatives of ter-butylsarcosinedithiocarbamate: Synthesis, chemical and biological characterization and in vitro nephrotoxicity. J. Inorg. Biochem. 2003, 93, 181–189. [Google Scholar] [CrossRef]
- Wiles, D.M.; Gingras, B.A.; Suprunchuk, T. The C=S stretching vibration in the infrared spectra of some thiosemicarbazones. Can. J. Chem. 1967, 45, 469–473. [Google Scholar] [CrossRef]
- Cesaro, S.N. FTIR study of a silver–thiourea complex generated in argon and nitrogen cryogenic matrices. Vib. Spectrosc. 1998, 16, 55–59. [Google Scholar] [CrossRef]
- Garje, S.S.; Jain, V.K. Triorganoantimony (V) bis (dialkylthiophosphates): Synthesis and characterization. Main Group Met. Chem. 1995, 18, 387–390. [Google Scholar]
- Li, J.S.; Ma, Y.Q.; Cui, J.R.; Wang, R.Q. Synthesis and in vitro antitumor activity of some tetraphenylantimony derivatives of exo-7-oxa-bicyclo[2,2,1] heptane (ene)-3-arylamide-2-acid. Appl. Organomet. Chem. 2001, 15, 639–645. [Google Scholar] [CrossRef]
- Lawal, A.; Obaleye, J.A. Synthesis, characterization and antibacterial activity of aspirin and paracetamolmetal complexes. Biokemistri 2007, 19, 9–15. [Google Scholar]
- Claridge, T.D.W. High Resolution NMR Techniques in Organic Chemistry, 2nd ed.; Elsevier: Oxford, UK, 2009; pp. 11–34. [Google Scholar]
- Sharutin, V.V.; Egorova, I.V.; Sharutina, O.K.; Ivaneko, T.K.; Pushilin, M.A.; Gerasimenko, A.V. Structural characteristics of triarylbimsuth dicarboxylates Ar3Bi[OC(O)r]2. Chem. Comput. Simul. Butlerov Commun. 2002, 9, 59–64. [Google Scholar]
- Ferguson, G.; Glidewell, C.; Kaitner, B.; Lloyd, D.; Metcalfe, S. Second determination of the structure of dimeric triphenylstibine oxide. Acta Crystallogr. Sect. C: Cryst. Struct. Commun. 1987, 43, 824–826. [Google Scholar] [CrossRef]
- Yin, H.-D.; Wen, L.-Y.; Cui, J.-C.; Li, W.-K. Synthesis, characterizations and crystal structures of new organoantimony (V) complexes with various isomers of fluoromethylbenzoate ligands. Polyhedron 2009, 28, 2919–2926. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Pakusina, A.P.; Platonova, T.P.; Smirnova, S.V.; Pushilin, M.A.; Gerasimenko, A.V. Structural Features of Triorganylantimony Dicarboxylates R3Sb[OC(O)R')]2. Russ. J. Coord. Chem. 2003, 29, 780–789. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2'-yl)-2, 6-dithiaheptane] copper (II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar]
- Shaw, J.J. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the aetiological agent of American visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 2006, 101, 577–579. [Google Scholar] [CrossRef]
- Marzochi, M.C.D.A.; Marzochi, K.B.F. Tegumentary and visceral leishmaniases in Brazil: Emerging anthropozoonosis and possibilities for their control. Cad. Saúde Pública 1994, 10, S359–S375. [Google Scholar] [CrossRef]
- Sinagra, Á.; Luna, C.; Abraham, D.; Iannella, M.D.C.; Riarte, A.; Krolewiecki, A.J. The activity of azithromycin against Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the golden hamster model. Rev. Soc. Bras. Med. Trop. 2007, 40, 627–630. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Centers for Disease Control and Prevention. A Public Health Action Plan to Combat Antimicrobial Resistance. Part I: Domestic Issues; The Centers: Atlanta, GA, USA, 2001. [Google Scholar]
- McGowan, J.E. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev. Infect. Dis. 1983, 5, 1033–1048. [Google Scholar] [CrossRef]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- De Lima, G.M.; Menezes, D.C.; Cavalcanti, C.A.; Dos Santos, J.A.F.; Ferreira, I.P.; Paniago, E.B.; Wardell, J.L.; Wardell, S.M.S.V.; Krambrock, K.; Mendes, I.C.; et al. Synthesis, characterisation and biological aspects of copper(II) dithiocarbamate complexes, [Cu{S2CNR(CH2CH2OH)}2], (R = Me, Et, Pr and CH2CH2OH). J. Mol. Struct. 2011, 988, 1–8. [Google Scholar] [CrossRef]
- CrysAlisPro, v 1.171.35.15; Agilent Technologies: Yarnton, UK, 2011.
- Sheldrick, G.M.S. SHELXL-97—A Program for Crystal Structure Refinement; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M.S. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Rubens, L.; Coelho, A.C.; Raymond, F.; Légaré, D.; Corbeil, J.; Melo, M.N.; Frézard, F.; Ouellette, M. Gene expression profiling and molecular characterization of antimony resistance in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2011, 5, e1167. [Google Scholar] [CrossRef]
- Ma, G.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Li, Z.; Pasco, D.S.; Walker, L.A.; Khan, I.A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004, 48, 4450–4452. [Google Scholar] [CrossRef]
- Habtemariam, S. In vitro antileishmanial effects of antibacterial diterpenes from two Ethiopian Premma species: P. chimperi and P. oligotricha. BMC Pharmacol. 2003, 3, 1–6. [Google Scholar]
- Ouellette, M.; Fase-Fowler, F.; Borst, P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990, 9, 1027–1033. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antimicrobial Susceptibility Testing for Bacteria that Grow Aerobically, In Approved Standard- Sixth Edition; NCCLS Document M7-A6; NCCLS: Wayne, PA, USA, 2003; ISBN 1-56238-486-4. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 2, 3 and 4 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Islam, A.; Da Silva, J.G.; Berbet, F.M.; Da Silva, S.M.; Rodrigues, B.L.; Beraldo, H.; Melo, M.N.; Frézard, F.; Demicheli, C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules 2014, 19, 6009-6030. https://doi.org/10.3390/molecules19056009
Islam A, Da Silva JG, Berbet FM, Da Silva SM, Rodrigues BL, Beraldo H, Melo MN, Frézard F, Demicheli C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules. 2014; 19(5):6009-6030. https://doi.org/10.3390/molecules19056009
Chicago/Turabian StyleIslam, Arshad, Jeferson Gomes Da Silva, Filipe Moan Berbet, Sydnei Magno Da Silva, Bernardo Lages Rodrigues, Heloisa Beraldo, Maria Norma Melo, Frédéric Frézard, and Cynthia Demicheli. 2014. "Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages" Molecules 19, no. 5: 6009-6030. https://doi.org/10.3390/molecules19056009
APA StyleIslam, A., Da Silva, J. G., Berbet, F. M., Da Silva, S. M., Rodrigues, B. L., Beraldo, H., Melo, M. N., Frézard, F., & Demicheli, C. (2014). Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules, 19(5), 6009-6030. https://doi.org/10.3390/molecules19056009





