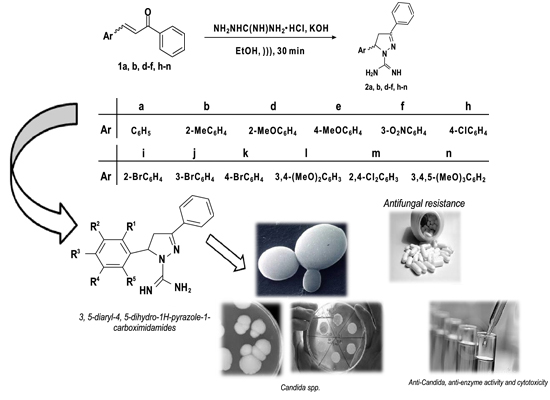
Anti-Candida, Anti-Enzyme Activity and Cytotoxicity of 3,5-Diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides
Abstract
:1. Introduction
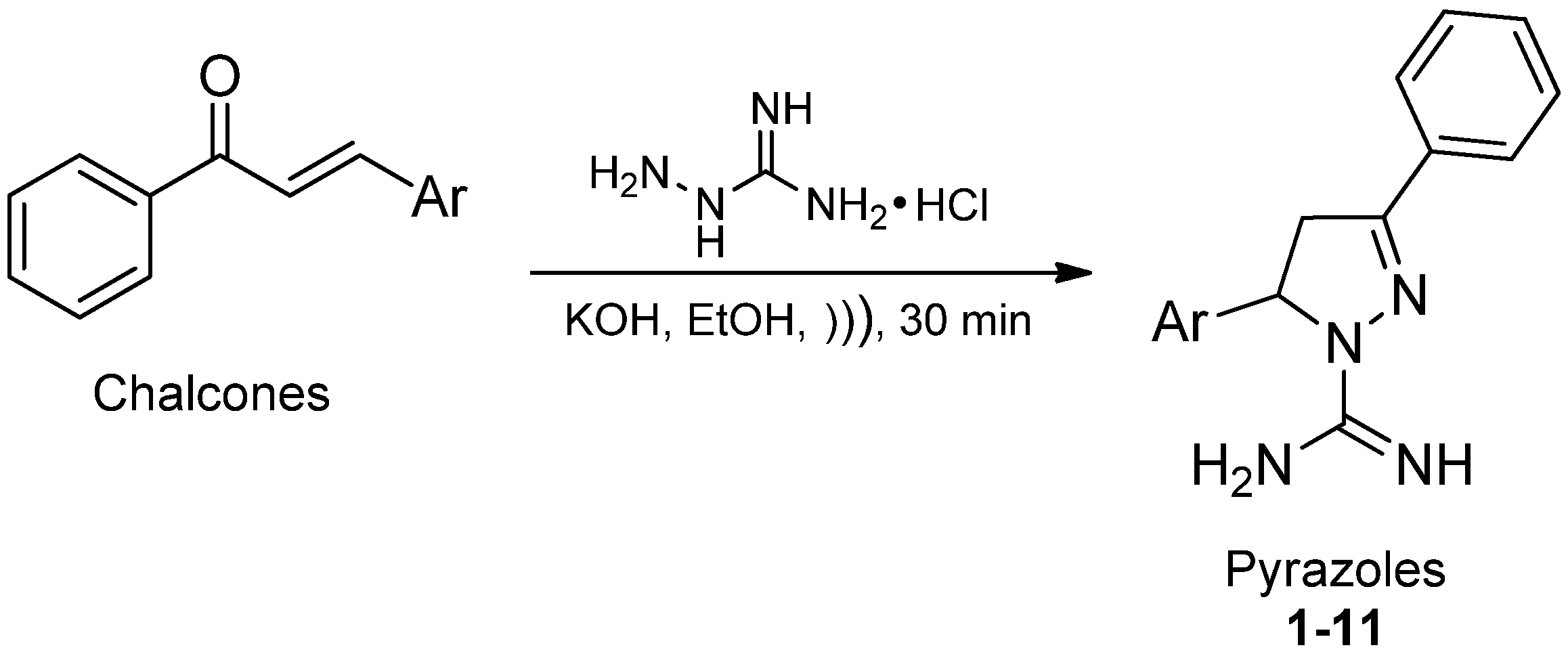
2. Results and Discussion
2.1. Chemistry

| Pyrazole a | Molecular Formula | HRMS m/z [MH]+ | |
|---|---|---|---|
| Calculated | Found | ||
| 1 | C17H19N4O | 295.1559 | 295.1557 |
| 2 | C16H17N4 | 265.1453 | 265.1452 |
| 3 | C16H16ClN4 | 299.1063 | 299.1047 |
| 4 | C16H16BrN4 | 343.0558 | 343.0547 |
| 5 | C17H19N4O | 295.1559 | 295.1550 |
| 6 | C16H15Cl2N4 | 333.0674 | 333.0685 |
| 7 | C17H19N4 | 279.1610 | 279.1610 |
| 8 | C18H21N4O2 | 325.1664 | 325.1661 |
| 9 | C16H16N5O2 | 310.1304 | 310.1318 |
| 10 | C16H16BrN4 | 343.0558 | 343.0546 |
| 11 | C17H19N4 | 279.1610 | 279.1602 |
2.2. Biological Activity
| Compound | Structural Formula | Chemical Formula | C. albicans (n = 33) Median (range) | R. mucillaginosa (n = 2) | C. parapsilosis (n = 2) | C. famata (n = 2) | C. glabrata (n = 2) | C. lypolytica (n = 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSO | 70% ethanol | DMSO | 70% ethanol | DMSO | 70% ethanol | DMSO | 70% ethanol | DMSO | 70% ethanol | DMSO | 70% ethanol | |||
| MIC µg/mL | MFC µg/mL | MIC µg/mL | MFC µg/mL | MIC µg/mL | MFC µg/mL | MIC µg/mL | MFC µg/mL | MIC µg/mL | MFC µg/mL | MIC µg/mL | MFC µg/mL | |||
| 1 | 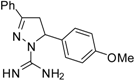 | C17H18N4O | 250 | >250 | 250 | 250 | >62.5 | >62.5 | >62.5 | >62.5 | 125 | 125 | 15.6 | 15.6 |
| 2 | 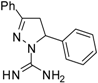 | C16H16N4 | 250 | 250 | 125 | 125 | 125 | 125 | >62.5 | >62.5 | 62.5 | 62.5 | 31.2 | 31.2 |
| 3 |  | C16H15ClN4 | 125 | 125 | 250 | 250 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 31.2 | 31.2 |
| 4 | 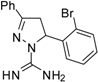 | C16H15BrN4 | 250 | 250 | 250 | 250 | 125 | 125 | >62.5 | >62.5 | 62.5 | 62.5 | >31.2 | >31.3 |
| 5 | 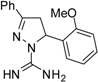 | C17H18N4O | 15.6 | 15.6 | 62.5 | 62.5 | >62.5 | >62.5 | 62.5 | 62.5 | 125 | 125 | 15.6 | 15.6 |
| 6 | 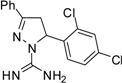 | C16H14Cl2N4 | 250 | 250 | 250 | 250 | 125 | 125 | 125 | 125 | 125 | 125 | >15.6 | >15.6 |
| 7 | 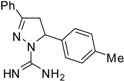 | C17H18N4 | 250 | >250 | 250 | 250 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 62.5 |
| 8 | 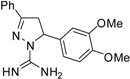 | C18H20N4O2 | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | 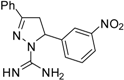 | C16H15N5O2 | - | - | - | - | - | - | - | - | - | - | - | - |
| 10 | 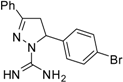 | C16H15BrN4 | 125 | 125 | 250 | 250 | 125 | 125 | 125 | 125 | >125 | >125 | <62.5 | <62.5 |
| 11 | 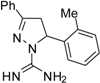 | C17H18N4 | - | - | - | - | - | - | - | - | - | - | - | - |
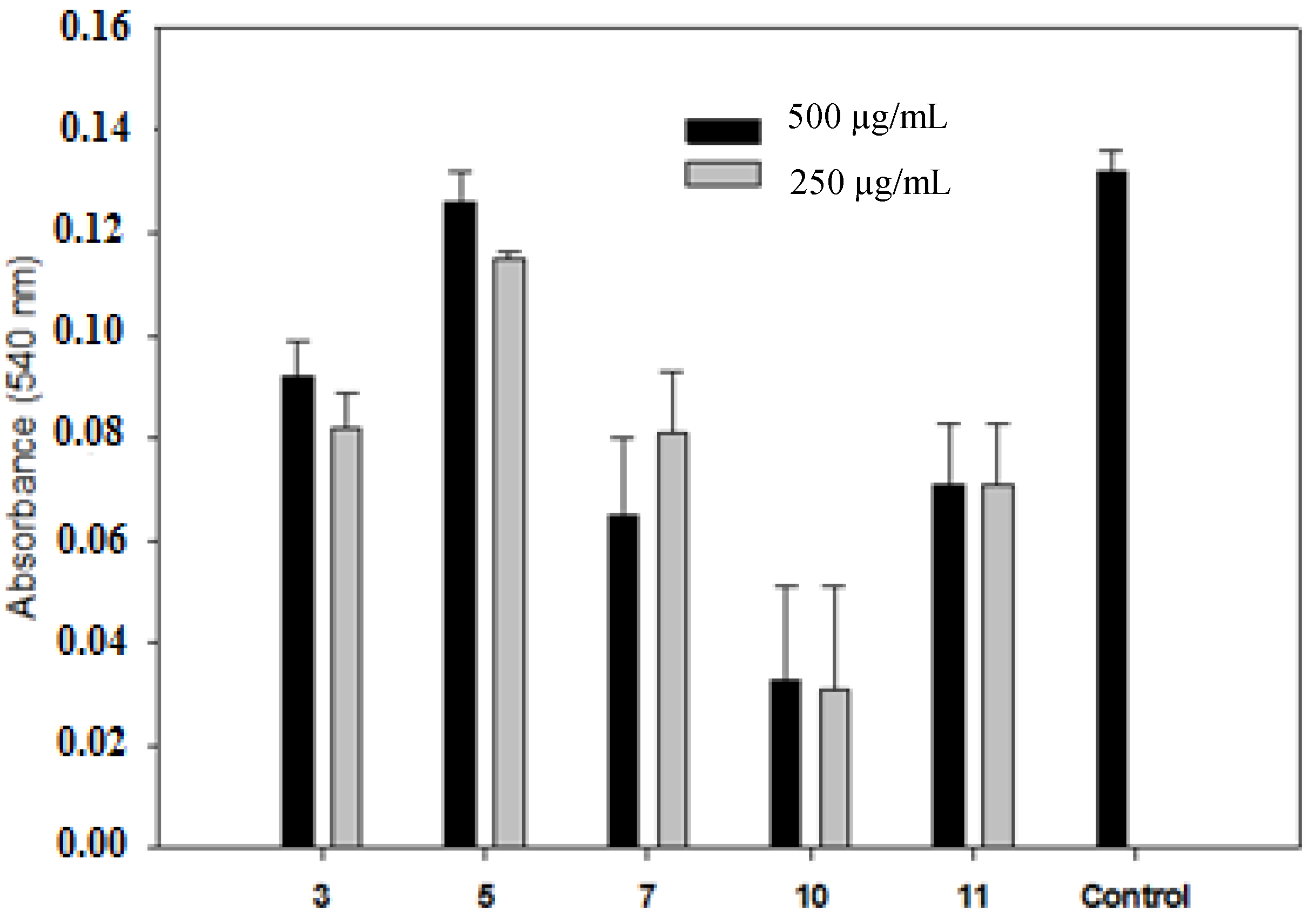
3. Experimental
3.1. General Information
3.2. Strains
3.3. In Vitro Antifungal Activity
3.3.1. Inoculum
3.3.2. Determination of the MIC and MFC
3.4. In Vitro Anti-Enzyme Activity
3.4.1. Phospholipase
3.4.2. Proteinase
3.5. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marchini, L.; Tamashiro, E.; Nascimento, D.F.; Cunha, V.P. Self reported denture hygiene of a sample of edentulous attendees at a University dental clinic and the relationship to the condition of the oral tissues. Gerodontology 2004, 21, 226–228. [Google Scholar]
- Fontenelle, R.O.S.; Morais, S.M.; Brito, E.H.S.; Brilhante, R.S.N.; Cordeiro, R.A.; Nascimento, N.R.F.; Kerntopf, M.R.; Sidrim, J.J.C.; Rocha, M.F.G. Antifungal activity of essential oils of Croton species from the Brazilian Caatinga biome. J. Appl. Microbiol. 2008, 104, 1383–1390. [Google Scholar] [CrossRef]
- El-Azizi, M.A.; Starks, S.E.; Khardori, N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J. Appl. Microbiol. 2004, 96, 1067–1073. [Google Scholar] [CrossRef]
- Jha, B.K.; Dey, S.; Tamang, M.D.; Joshy, M.E.; Shivananda, P.G.; Brahmadatan, K.N. Characterization of candida species isolated from cases of lower respiratory tract infection. Kathmandu Univ. Med. J. 2006, 4, 290–294. [Google Scholar]
- Moran, G.; Sanglard, D.; Donnelly, S.; Shanley, D.; Sullivan, D.; Coleman, D. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob. Agents Chemother. 1998, 42, 1819–1830. [Google Scholar]
- Harris, R. Progress with superficial mycoses using essential oils. Int. J. Aromath. 2002, 12, 83–91. [Google Scholar] [CrossRef]
- Cross, L.J.; Bagg, J.; Wray, D.; Aitchison, T. A comparison of fluconazole and itraconazole in the management of denture stomatitis: A pilot study. J. Dent. 1998, 26, 657–664. [Google Scholar] [CrossRef]
- Sun, L.M.; Cheng, A.X.; Wu, X.Z.; Zhang, H.J.; Lou, H.X. Synergistic mechanisms of retigeric acid B and azoles against Candida albicans. J. Appl. Microbiol. 2010, 108, 341–348. [Google Scholar] [CrossRef]
- Gökhan-Kelekçi, N.; Yabanoglu, S.; Küpeli, E.; Salgin, U.; Ösgen, O.; Uçar, G.; Yesilada, E.; Kendi, E.; Yesilada, A.; Bilgin, A.A. A new therapeutic approach in Alzheimer disease: Some novel pyrazole derivatives as dual MAO-B inhibitors and anti-inflammatory analgesics. Bioorg. Med. Chem. 2007, 15, 5775–5786. [Google Scholar] [CrossRef]
- Özdemir, Z.; Kandilci, H.B.; Gümüsel, B.; Calıs, U.; Bilgin, A. A synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 373–379. [Google Scholar] [CrossRef]
- Choy, A.L.; Prasad, J.V.N.V.; Boyer, F.E.; Huband, M.D.; Dermyer, M.R. Synthesis and SAR of novel conformationally restricted oxazolidinones possessing gram-positive and fastidious gram-negative antibacterial activity. Part 2: Amino substitutions on heterocyclic D-ring system. Bioorg. Med. Chem. Lett. 2007, 17, 4699–4702. [Google Scholar] [CrossRef]
- Turan-Zitouni, G.; Chevallet, P.; Kiliç, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Badawey, E.S.A.M.; El-Ashmawey, I.M. Nonsteroidal antiinflammatory agents—part 2 antiinflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin-5-ones and 1,2,4,5,6,7–3H-hexahydroindazol-3-ones. Eur. J. Med. Chem. 2006, 41, 155–165. [Google Scholar] [CrossRef]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Villar, R.; Vicente, E.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Monge, A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues. Bioorg. Med. Chem. Lett. 2007, 17, 6439–6443. [Google Scholar] [CrossRef]
- Dräger, G.; Solodenko, W.; Messinger, J.; Schön, U.; Kirschning, A. A new reagent and its polymer-supported variant for the amidation of amines. Tetrahedron Lett. 2002, 43, 1401–1403. [Google Scholar]
- Silva, F.A.N.; Galluzzi, M.P.; Albuquerque, B.; Pizzuti, L.; Gressler, V.; Rivelli, D.P.; Barros, S.B.M.; Pereira, C.M.P. Ultrasound irradiation promoted large-scale preparation in aqueous media and antioxidant activity of azoles. Lett. Drug Des. Discov. 2009, 6, 323–326. [Google Scholar]
- Silva, F.A.N.; Pizzuti, L.; Quina, F.H.; Souza, S.P.; Rosales, P.F.; Siqueira, G.M.; Pereira, C.M.P.; Barros, S.B.M.; Rivelli, D.P. Antioxidant capacity of 2-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazoles. Lett. Drug Des. Discov. 2010, 7, 657–660. [Google Scholar] [CrossRef]
- Pizzuti, L.; Martins, P.L.; Ribeiro, B.A.; Quina, F.H.; Pinto, E.; Flores, A.F.C.; Venzke, D.; Pereira, C.M.P. Efficient sonochemical synthesis of novel 3,5-diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Ultrason. Sonochem. 2010, 17, 34–37. [Google Scholar] [CrossRef]
- Flores, D.C.; Fiss, G.F.; Wbatuba, L.S.; Martins, M.A.P.; Burrow, R.A.; Flores, A.F.C. Synthesis of new 2-(5-aryl-3-styryl-4,5-dihydro-1H-pyrazol-1-yl)-4-(trifluoromethyl)-pyrimidines. Synthesis 2006, 14, 2349–2356. [Google Scholar]
- Pizzuti, L.; Franco, M.S.F.; Flores, A.F.C.; Quina, F.H.; Pereira, C.M.P. Recent advances in the ultrasound-assisted synthesis of azoles. In Green Chemistry—Environmentally Benign Approaches; Kidwai, M., Ed.; InTech: Rijeka, Croatia, 2010. [Google Scholar]
- Arthington-Skaggs, B.A.; Lee-Yang, W.; Ciblak, M.A.; Frade, J.P.; Brandt, M.E.; Hajjeh, R.A.; Harrison, L.H.; Sofair, A.N.; Warnock, D.W. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 2002, 46, 2477–2481. [Google Scholar] [CrossRef]
- Ostrosky, E.A.; Mizumoto, M.K.; Lima, M.E.L.; Kaneko, T.M.; Nishikawa, S.O.; Freitas, B.R. Methods for evaluation of the antimicrobial activity and determination of minimum inhibitory concentration (MIC) of plant extracts. Braz. J. Pharm. Sci. 2008, 18, 301–307. [Google Scholar]
- Eggimann, P.; Garbino, J.; Pittet, D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003, 3, 685–702. [Google Scholar] [CrossRef]
- Ruhnke, M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr. Drug Targets 2006, 7, 495–504. [Google Scholar] [CrossRef]
- Dolan, K.; Montgomery, S.; Buchheit, B.; DiDone, L.; Wellington, M.; Krysan, D.J. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob. Agents Chemoter. 2009, 53, 3337–3346. [Google Scholar] [CrossRef]
- Marsh, P.D.; Martin, M.V. Oral Microbiology, 5th ed.; Churchill Livingstone: Edinburgh, UK, 2009. [Google Scholar]
- Elewki, B.E.; Ohio, C. Mechanism of action of systemic antifungal agents. J. Am. Acad. Dermatol. 1993, 28, 28–34. [Google Scholar] [CrossRef]
- Farah, C.S.; Lynch, N.; McCullough, M.J. Oral fungal infections: An update for the general practitioner. Aust. Dent. J. 2010, 55, 48–54. [Google Scholar] [CrossRef]
- Frohner, I.E.; Bourgeois, C.; Yatsyk, K.; Majer, O.; Kuchler, K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 2009, 71, 240–252. [Google Scholar] [CrossRef]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef]
- Barros, L.M.; Boriollo, M.F.G.; Alves, A.C.B.A.; Klein, M.I.; Gonçalves, R.B.; Höfling, J.F. Genetic diversity and exoenzyme activities of Candida albicans and Candida dubliniensis isolated from the oral cavity of Brazilian periodontal patients. Arch. Oral Biol. 2008, 53, 1172–1178. [Google Scholar] [CrossRef]
- Kadir, T.; Gümrü, B.; Uygun-Can, B. Phospholipase activity of Candida albicans isolates form patients with denture stomatitis: The influence of chlorhexidine gluconate on phospholipase production. Arch. Oral Biol. 2007, 52, 691–696. [Google Scholar] [CrossRef]
- Price, M.F.; Wilkinson, I.D.; Gentr, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 15, 179–185. [Google Scholar]
- Neville, B.W. Oral and Maxillofacial Pathology, 2nd ed.; Guanabara Koogan: Rio de Janeiro, RJ, Brazil, 2004. [Google Scholar]
- Giordani, R.; Trebaux, J.; Masi, M.; Regli, P. Enhanced antifungal activity of ketoconazole by Euphorbia characias latex against Candida albicans. J. Ethnopharmacol. 2001, 78, 1–5. [Google Scholar] [CrossRef]
- Nobre, M.O.; Nascente, P.S.; Meireles, M.C.; Ferreiro, L. Antifungal drugsfor small and large animals. Cienc. Rural 2002, 32, 175–184. [Google Scholar]
- Elliott, T.S.J.; Foweraker, J.; Gould, F.K.; Perry, J.D.; Sandoe, J.A.T. Guidelines for the antibiotic treatment of endocarditis in adults: Report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2004, 54, 971–981. [Google Scholar] [CrossRef]
- Johnson, E.M. Issues in antifungal susceptibility testing. J. Antimicrob. Chemother. 2008, 61, i13–i18. [Google Scholar] [CrossRef]
- Wingeter, M.A.; Guilhermetti, E.; Shinobu, C.S.; Takaki, I.; Svidzinski, T.I.E. Microbiological identification and in vitro sensitivity of Candida isolates from the oral cavity of HIV-positive individuals. Rev. Soc. Bras. Med. Trop. 2007, 40, 272–276. [Google Scholar] [CrossRef]
- Lund, R.G.; Nascente, P.S.; Etges, A.; Ribeiro, G.A.; Rosalen, P.L.; del Pino, F.A.B. Occurrence, isolation and differentiation of Candida spp. and prevalence of variables associated to chronic atrophic candidiasis. Mycoses 2010, 53, 232–238. [Google Scholar] [CrossRef]
- Nedel, F.; Begnini, K.R.; Carvalho, P.H.; Lund, R.G.; Beira, F.T.; del Pino, F.A.B. Antiproliferative activity of flower hexane extract obtained from Mentha spicata associated with against the MCF7, KB, and NIH/3T3 cell lines. J. Med. Food 2012, 15, 955–958. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds tested are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oliveira, S.; Pizzuti, L.; Quina, F.; Flores, A.; Lund, R.; Lencina, C.; Pacheco, B.S.; De Pereira, C.M.P.; Piva, E. Anti-Candida, Anti-Enzyme Activity and Cytotoxicity of 3,5-Diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Molecules 2014, 19, 5806-5820. https://doi.org/10.3390/molecules19055806
Oliveira S, Pizzuti L, Quina F, Flores A, Lund R, Lencina C, Pacheco BS, De Pereira CMP, Piva E. Anti-Candida, Anti-Enzyme Activity and Cytotoxicity of 3,5-Diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Molecules. 2014; 19(5):5806-5820. https://doi.org/10.3390/molecules19055806
Chicago/Turabian StyleOliveira, Simone, Lucas Pizzuti, Frank Quina, Alex Flores, Rafael Lund, Claiton Lencina, Bruna S. Pacheco, Claudio M. P. De Pereira, and Evandro Piva. 2014. "Anti-Candida, Anti-Enzyme Activity and Cytotoxicity of 3,5-Diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides" Molecules 19, no. 5: 5806-5820. https://doi.org/10.3390/molecules19055806
APA StyleOliveira, S., Pizzuti, L., Quina, F., Flores, A., Lund, R., Lencina, C., Pacheco, B. S., De Pereira, C. M. P., & Piva, E. (2014). Anti-Candida, Anti-Enzyme Activity and Cytotoxicity of 3,5-Diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Molecules, 19(5), 5806-5820. https://doi.org/10.3390/molecules19055806