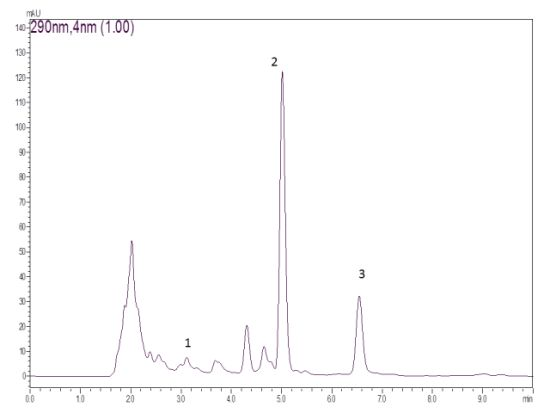
Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Content
| Plant part | Solvent | Phenolics (mg GAE g−1 of extract) | Capsaicin (mg g−1 extract ± SD) | Dihydrocapsaicin (mg g−1 extract ± SD) | Chrysoeriol (mg g−1 extract ± SD) |
|---|---|---|---|---|---|
| Seeds | Hexane | 9.4 ± 0.55 | 90.0 ± 8.60 | 42.0 ± 4.21 | 1.8 ± 0.69 |
| Acetonitrile | 61.3 ± 0.64 | 130.4 ± 4.98 | 53.3 ± 1.59 | 11.4 ± 0.38 | |
| Peel | Hexane | 3.2 ± 0.22 | 45.8 ± 0.1 | 23.3 ± 0.23 | 0.5 ± 0.27 |
| Acetonitrile | 14.0 ± 0.14 | 164.3 ± 10.84 | 73.6 ± 6.43 | 5.1 ± 1.31 | |
| Whole Fruits | Hexane | 4.9 ± 0.44 | 31.1 ± 2.04 | 15.0 ± 0.65 | 0.4 ± 0.03 |
| Acetonitrile | 110.6 ± 1.03 | 109.8 ± 13.66 | 42.0 ± 4.64 | 5.5 ± 0.49 |

| Species/part | Extraction solvent | Capsaicin | Dihydrocapsaicin | [Reference] |
|---|---|---|---|---|
| C. frutescens | Ethanol | 0.7 mg g−1 fresh weight | 0.4 mg g−1 fresh weight | Zhuang et al., 2012 [21] |
| C. annuum | Methanol | 2.3 mg g−1 extract | 0.8 mg g−1 extract | Alvarez-Parrilla et al., 2011 [5] |
| C. annuum | Ethanol | 0.3 mg g−1 extract | 0.2 mg g−1 extract | Othman et al., 2011 [9] |
| C. chinense Seeds | Acetonitrile | 14.0 mg g−1 dried fruits | 5.2 mg g−1 dried fruits | Chinn et al., 2011[23] |
| C. chinense Shells | 5.0 mg g−1 dried fruits | 1.3 mg g−1 dried fruits | ||
| C. chinense Whole fruits | 7.0 mg g−1 dried fruits | 2.0 mg g−1 dried fruits | ||
| C. frutescens Seeds | Methanol | 1.1 mg g−1 fresh weight | 0.5 mg g−1 fresh weight | Wahyuni et al., 2011 [26] |
| C. frutescens Pericarp | 0.6 mg g−1 fresh weight | 0.1 mg g−1 fresh weight | ||
| C. chinense | Ethanol | 4.3 mg g−1 fresh weight | 2.4 mg g−1 fresh weight | Menichini et al., 2009 [1] |
| C. frutescens | Acetonitrile | 3.7 mg g−1 dried weight | 2.4 mg g−1 dried weight | Garcés-Claver et al., 2006 [3] |
2.2. Radical Scavenging and Antioxidant Activity
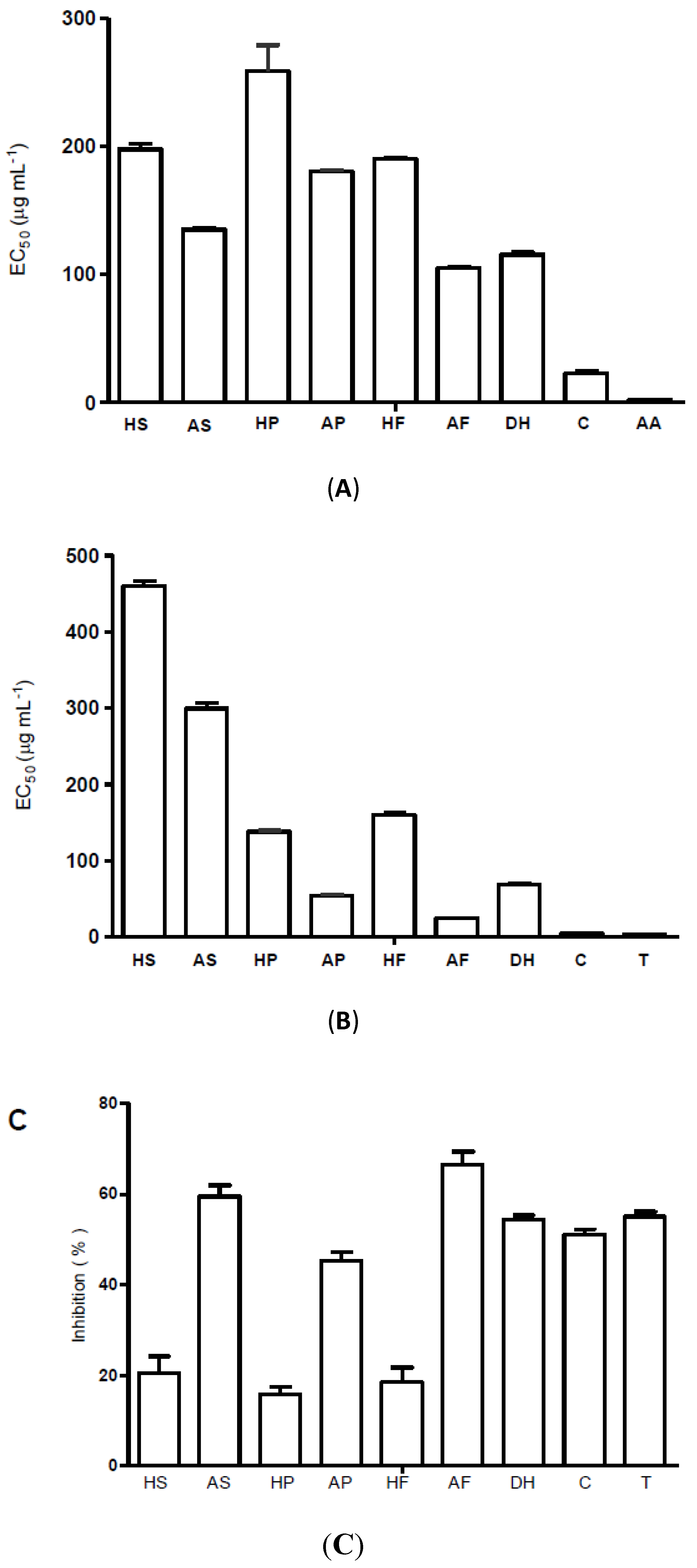
2.3. Antimicrobial Activity
| Microorganisms | MIC (µg mL−1) | ||
|---|---|---|---|
| Capsaicin | Dihydrocapsaicin | Chrysoeriol | |
| Gram positive bacteria | |||
| Enterococcus faecalis | 25 | 0.6 | 1 |
| Bacillus subtillis | 25 | 1.2 | 1 |
| Staphylococcus aureus | 1.2 | 5 | 0.25 |
| Gram negative bacteria | |||
| Pseudomonas aeruginosa | 10 | 2.5 | 0.12 |
| Klebsiella pneumoniae | 0.6 | 2.5 | 0.25 |
| Escherichia coli | 5 | 5 | 0.06 |
| Yeast | |||
| Candida albicans | 25 | 10 | ND * |
3. Experimental
3.1. Chemicals and Solvents
3.2. Preparation of Extracts from Capsicum frutescens
3.3. Isolation of Compounds from Pimenta malagueta
3.4. Quantification of Capsaicin, Dihydrocapsaicin and Chrysoeriol Content
3.5. Determination of Total Phenolic Content
3.6. DPPH Free Radical Scavenging Assay
3.7. ABTS Radical Cation Assay
3.9. Antimicrobial Assay
3.9.1. Microbial Strains
3.9.2. Determination of Minimal Inhibitory Concentration (MIC)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; de Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical contente and biological activity of Capsicum Chinese Jacq. Cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Barboza, G.E.; Bianchetti, L.B. Three new species of Capsicum (Solanaceae) and a key to the wild species from Brazil. Syst. Bot. 2005, 30, 863–871. [Google Scholar] [CrossRef]
- Garcés-Claver, A.; Arnedo-Andrés, M.S.; Abadia, J.; Gil-Ortega, R.; Álvarez-Fernández, A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruits by liquid chromatography-electrospray/time-of-flight mass spectrometry. J. Agric. Food Chem. 2006, 54, 9303–9311. [Google Scholar] [CrossRef]
- Cisneros-Pineda, O.; Torres-Tapia, L.W.; Gutiérrez-Pacheco, L.C.; Contreras-Martín, F.; González-Estrada, T.; Peraza-Sánchez, S.R. Capsaicinoids quantification in chili peppers cultivated in the state of Yucatan, Mexico. Food Chem. 2007, 104, 1755–1760. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant activity of fresh and processed jalapeño and Serrano peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Taxonomic classification helps identify flavonoid-containing foods on a semiquantitative food frequency questionnaire. J. Am. Diet. Assoc. 1998, 98, 677–685. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Jifon, J.; Patil, B.S. Extraction efficiency and validation of an HPLC method for flavonoid analysis. Food Chem. 2012, 130, 751–758. [Google Scholar] [CrossRef]
- Othman, Z.A.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef]
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol. 2000, 57, 125–128. [Google Scholar] [CrossRef]
- Sokmen, A.; Gulluce, M.; Akpulat, A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control. 2004, 15, 627–634. [Google Scholar]
- Zimmer, A.R.; Leonardi, B.; Miron, D.; Schapoval, E.; Oliveira, J.R.; Gosmann, G. Antioxidant and anti-inflammatory proprerties of Capsicum baccatum: From traditional use to scientific approach. J. Ethnopharmacol. 2012, 139, 228–233. [Google Scholar] [CrossRef]
- Al-Fartosy, A.J.M.; Zearah, S.A. Antioxidant, antibacterial and cytotoxicity activities of flavonoid extract from Capsicum annum L. seeds. Iraqi Natl. J. Chem. 2013, 49, 100–112. [Google Scholar]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Kozukue, N.; Han, J.S.; Kozukue, E.; Lee, S.J.; Kim, J.A.; Lee, K.R.; Levin, C.E.; Friedman, M. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2005, 53, 9172–9181. [Google Scholar] [CrossRef]
- Higashiguchi, F.; Nakamura, H.; Hayashi, H.; Kometani, T. Purification and structure determination of glucosides of capsaicin and dihydrocapsaicin from various Capsicum fruits. J. Agric. Food Chem. 2006, 54, 5948–5953. [Google Scholar]
- Cichewicz, R.H.; Thorpe, P.A. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J. Ethnopharmacol. 1996, 52, 61–70. [Google Scholar] [CrossRef]
- Jin, J.R.; Huiming, P.; Beibei, X.; Xia, Z.X. Separation and quantitative analysis of capsaicinoids in chili peppers by reversed-phase argentation LC. Chromatographia 2009, 70, 1011–1013. [Google Scholar] [CrossRef]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Câmara, C.A.; Silva, T.M.S.; Moreira, K.A.; Porto, A.L.F. Antimicrobial and antioxidant activities of pimenta malagueta (Capsicum frutescens). Afr. J. Microbiol. Res. 2013, 7, 3526–3533. [Google Scholar]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive characteristics and antioxidant activities of nine peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunruku, O.O. Cyclophosphamide-induced oxidative stress in brain: Protective effect of hot short pepper (Capsicum frutescens L. var. abbreviatum). Exp. Toxicol. Pathol. 2010, 62, 227–233. [Google Scholar] [CrossRef]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Tapia, J.C.; Garcia, R.; Escamilla, E.M.; Calva, C.; Rocha, J.A. Capsaicin recovery from a cell culture broth. Ind. Eng. Chem. Res. 1993, 32, 2242–2246. [Google Scholar] [CrossRef]
- Collins, M.D.; Wasmund, L.M.; Bosland, P.W. Improved Method for Quantifying Capsaicinoids in Capsicum Using High-performance Liquid Chromatography. Hortscience 1995, 30, 137–139. [Google Scholar]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Kirschbaum-Titze, P.; Hiepler, C.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicum annuum). 1. Decrease of capsaicinoid content following cellular disruption. J. Agric. Food Chem. 2002, 50, 1260–1263. [Google Scholar] [CrossRef]
- Marín, A.; Ferreres, F.; Tomás-Barberán, F.A.; Gil, M.I. Characterization and Quantitation of Antioxidant Constituents of Sweet Pepper (Capsicum annuum L.). J. Agric. Food Chem. 2004, 52, 3861–3869. [Google Scholar] [CrossRef]
- Bamoniri, A.; Ebrahimabadi, A.H.; Mazoochi, A.; Behpour, M.; Kashi, F.J.; Batooli, H. Antioxidant and antimicrobial activity evaluation and essential oil analysis of Semenovia. tragioides Boiss. from Iran. Food Chem. 2010, 122, 553–558. [Google Scholar] [CrossRef]
- Flagan, S.F.; Leadbetter, J.R. Utilization of capsaicin and vanillylamine as growth substrates by Capsicum (hot pepper)—associated bacteria. Environ. Microbiol. 2006, 8, 560–565. [Google Scholar] [CrossRef]
- Molina-Torres, J.; García-Chávez, A.; Ramírez-Chávez, E. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J. Ethnopharmacol. 1999, 64, 241–248. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar]
- Silva, T.M.S.; Câmara, C.A.; Lins, A.C.S.; Barbosa, J.M.; Silva, E.M.S.; Freitas, B.M.; Santos, F.A.R. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona. subnitida Ducke. J. Food Compos. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 11231–1237. [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, In Approved Standand NCCLS Document M7-A6, 6th ed.; NCCLS: Wayne, NJ, USA, 2003; Volume 23, pp. 1–50. [Google Scholar]
- Clinical and Laboratory Standarts Institute (CLSI). Reference Method for broth dilution antifungal susceptibility testing of yeasts, In Approved Standard M27-A3, 3rd ed.; CLSI: Wayne, NJ, USA, 2008; pp. 1–25. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434-5447. https://doi.org/10.3390/molecules19045434
Nascimento PLA, Nascimento TCES, Ramos NSM, Silva GR, Gomes JEG, Falcão REA, Moreira KA, Porto ALF, Silva TMS. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules. 2014; 19(4):5434-5447. https://doi.org/10.3390/molecules19045434
Chicago/Turabian StyleNascimento, Patrícia L. A., Talita C. E. S. Nascimento, Natália S. M. Ramos, Girliane R. Silva, José Erick Galindo Gomes, Rosângela E. A. Falcão, Keila A. Moreira, Ana L. F. Porto, and Tania M. S. Silva. 2014. "Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta)" Molecules 19, no. 4: 5434-5447. https://doi.org/10.3390/molecules19045434
APA StyleNascimento, P. L. A., Nascimento, T. C. E. S., Ramos, N. S. M., Silva, G. R., Gomes, J. E. G., Falcão, R. E. A., Moreira, K. A., Porto, A. L. F., & Silva, T. M. S. (2014). Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules, 19(4), 5434-5447. https://doi.org/10.3390/molecules19045434