Bioactivity of a Family of Chiral Nonracemic Aminobenzylnaphthols towards Candida albicans
Abstract
:1. Introduction
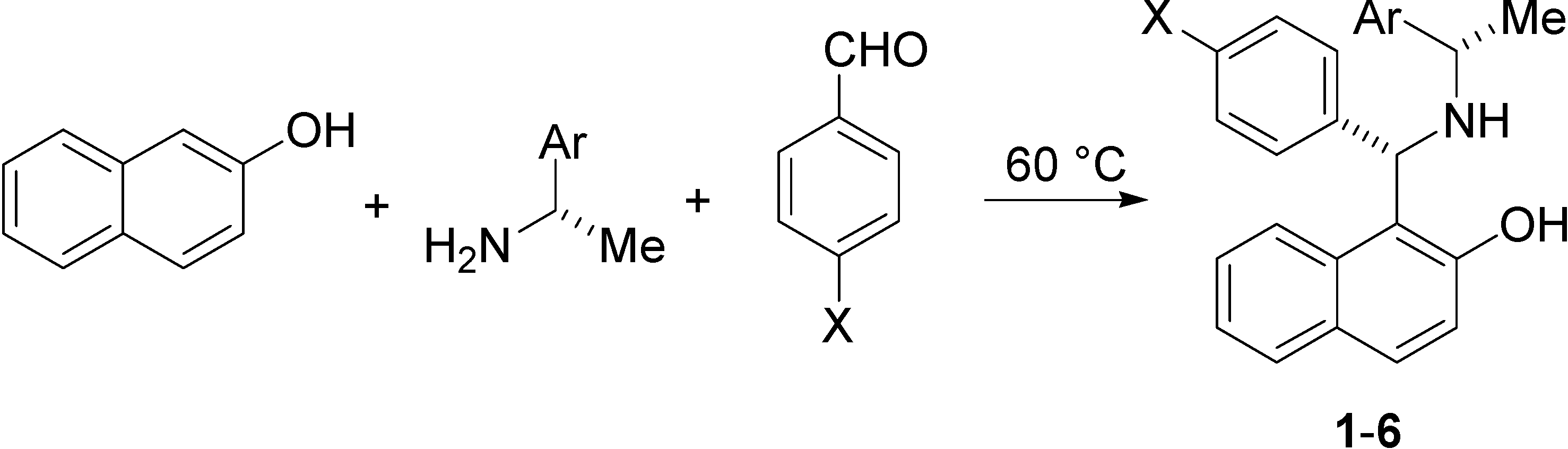
| Compound | X | Ar |
|---|---|---|
| (S, S)-1 | H | Ph |
| (S, S)-2 | F | Ph |
| (S, S)-3 | Cl | Ph |
| (S, S)-4 | H | 1-Np |
| (S, S)-5 | F | 1-Np |
| (S, S)-6 | Br | 1-Np |
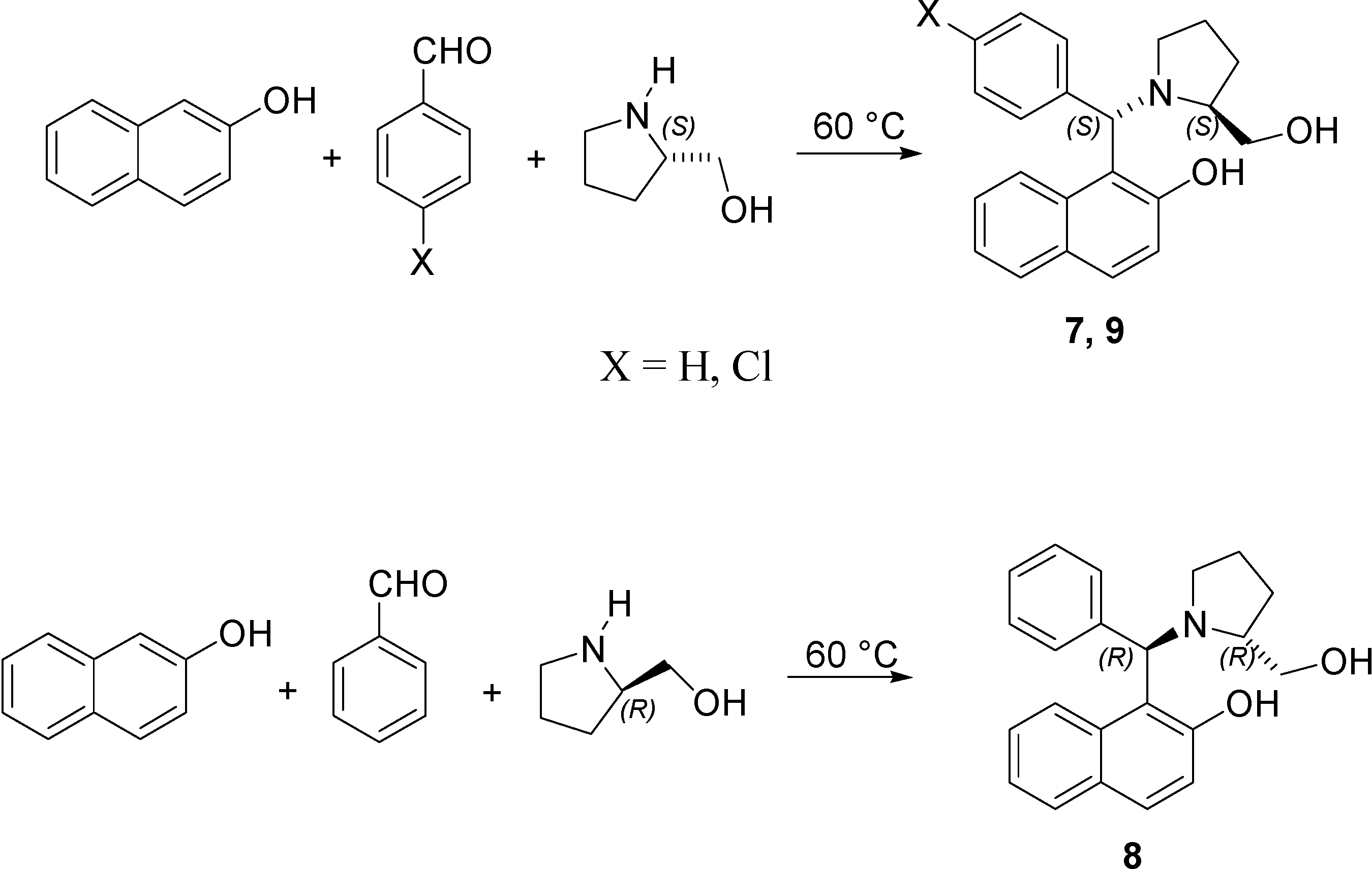
| Compound | X | Amine |
|---|---|---|
| (S, S)-7 | H | (S)-Prolinol |
| (R, R)-8 | H | (R)-Prolinol |
| (S, S)-9 | Cl | (S)-Prolinol |
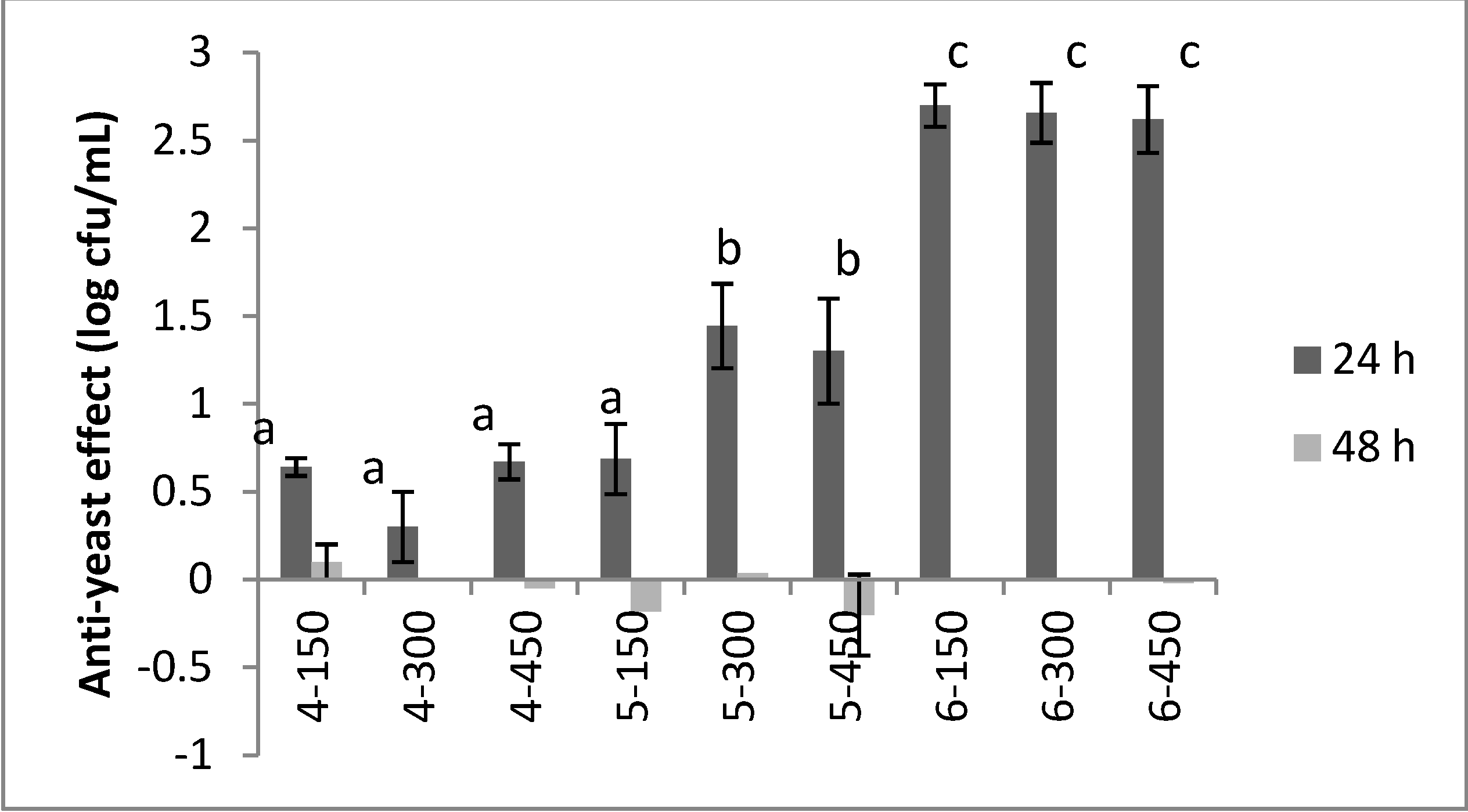
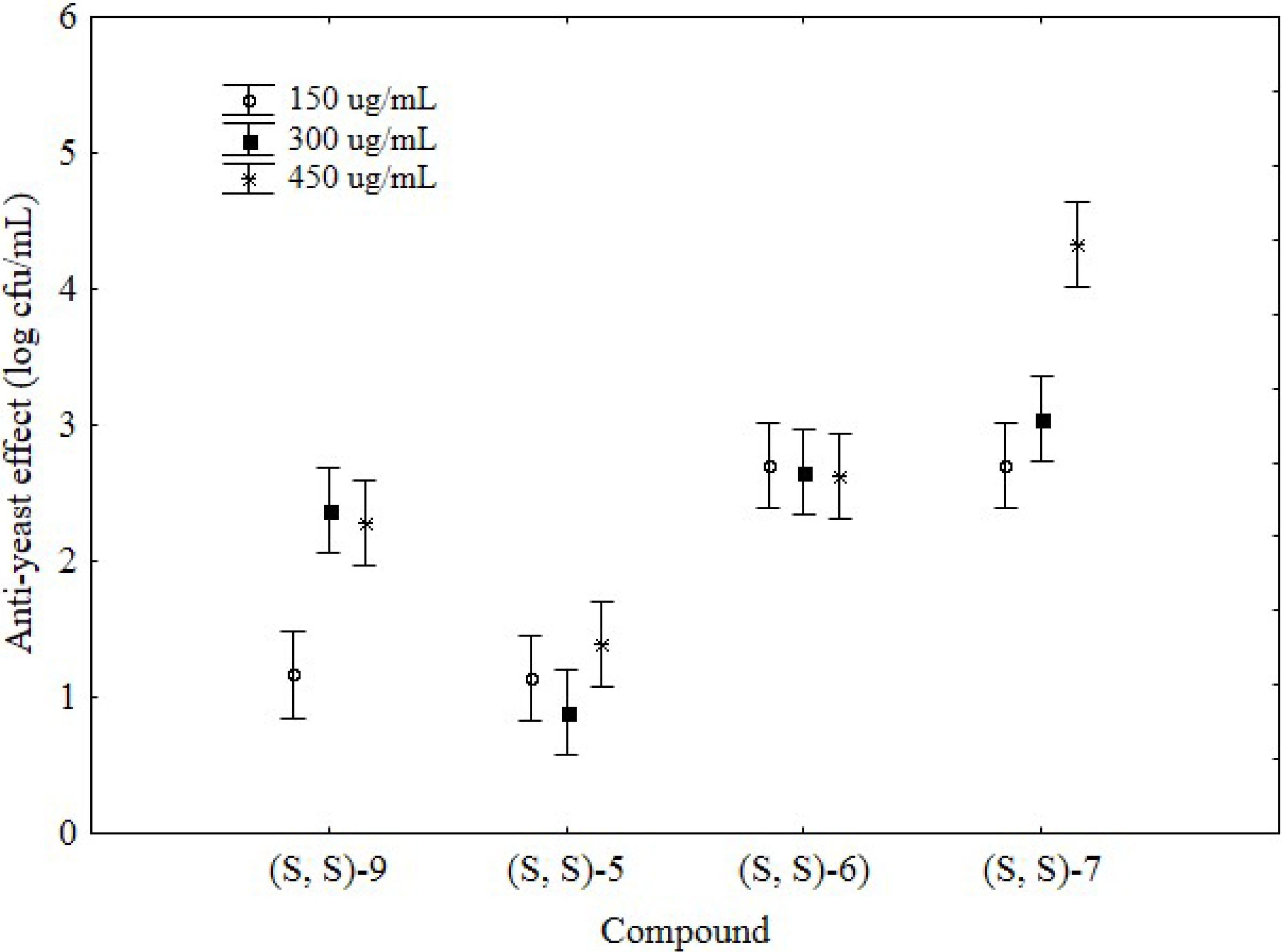
2. Results and Discussion
| Samples/ concentrations | Time (h) | |
|---|---|---|
| 6 | 24 | |
| (S,S)-1 | ||
| 150 μg/mL | / | / |
| 300 μg/mL | / | / |
| 450 μg/mL | / | / |
| (S,S)-2 | ||
| 150 μg/mL | 0.50 a | / |
| 300 μg/mL | 0.55 a | / |
| 450 μg/mL | / | / |
| (S,S)-3 | ||
| 150 μg/mL | 0.53 a | / |
| 300 μg/mL | 0.55 a | / |
| 450 μg/mL | / | / |

| Effects | SS | df | MS | F | p-value |
|---|---|---|---|---|---|
| Compound | 24.6148 | 3 | 8.20494 | 119.3 | 1.49E-14 |
| Amount | 3.17882 | 2 | 1.58941 | 23.1 | 2.55E-06 |
| Interaction | 4.30922 | 6 | 0.7182 | 10.44 | 1.03E-05 |
| Within | 1.65102 | 24 | 0.06879 | ||
| Total | 33.7539 | 35 |
3. Experimental Section
3.1. Strain
3.2. Tested Molecules
3.2.1. Synthesis of Aminobenzylnaphthols
3.2.2. 1-[(2-Hydroxymethyl-pyrrolidin-1-yl)-benzyl]-naphth-2-ols 7 and 8—Predominant Stereoisomer
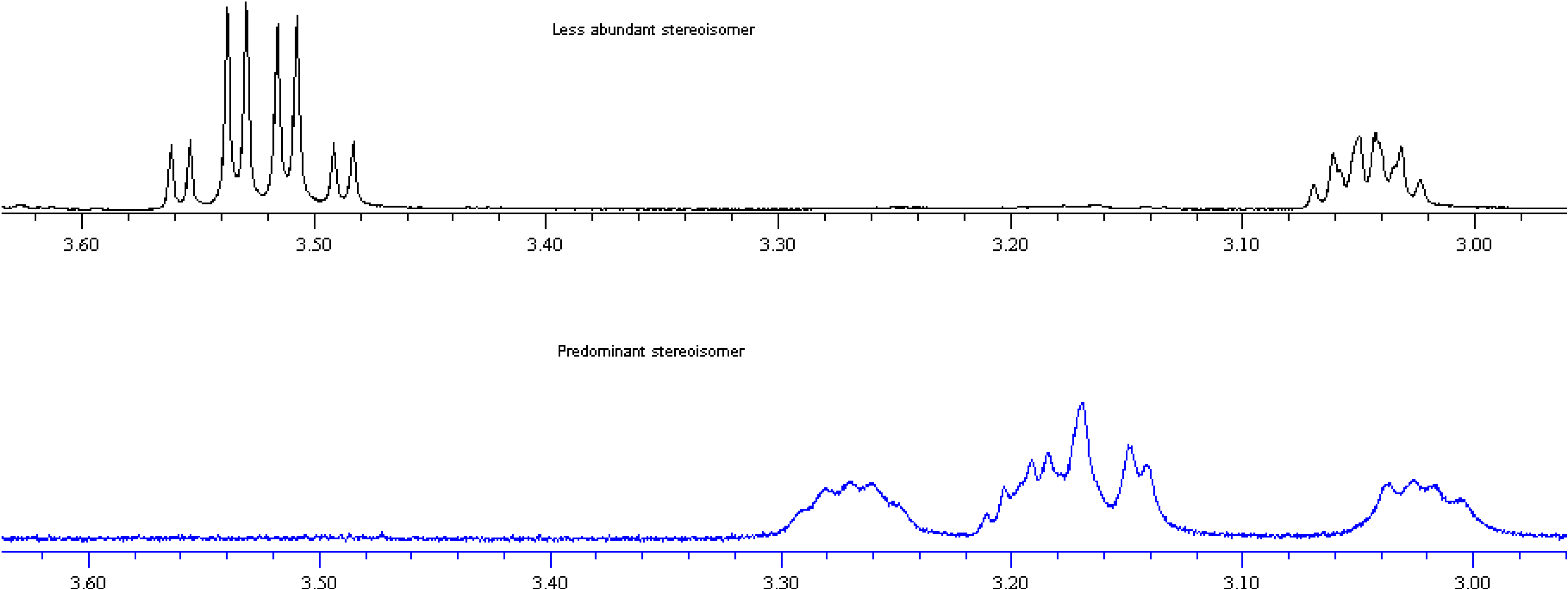
3.2.3. 1-[(4-Chlorophenyl)-(2-hydroxymethylpyrrolidin-1-yl)-methyl]-naphth-2-ol (9)
3.3. Evaluation of the Anti-Yeast Activity
| Samples | Stock solution (mL) | DMSO (mL) | Saline solution (mL) | Inoculum (mL) |
|---|---|---|---|---|
| 150 μg/mL | 0.2 | 0.4 | - | 0.1 |
| 300 μg/mL | 0.4 | 0.2 | - | 0.1 |
| 450 μg/mL | 0.6 | - | - | 0.1 |
| Control 1 (C1) | - | - | 0.6 | 0.1 |
| Control 2 (C2) | - | 0.6 | - | 0.1 |
4. Conclusions
- The structure of the compounds, since biological activity was detected for aminobenzyl-naphthols synthesized starting from 1-(1-naphthylethylamine) or from prolinol (groups 2 and 3), but not observed for molecules derived from 2-phenyethylamine.
- The presence on the tested molecules of the halogen atom in the para-position of the phenyl group deriving from the aryl aldehyde employed in the Betti reaction, since chlorine or bromine atom can affect the bioactivity.
- The stereochemistry of the amine used in the Betti reaction, since the aminobenzylnaphthols deriving from the natural (S)-prolinol showed a strong effect towards C. albicans, whilst the compound prepared by the unnatural (R)- prolinol does not.
Author Contributions
Conflicts of Interest
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef]
- Calderone, R.A.; Clancy, C.J. Candida and Candidiasis; ASM Press: Washington, DC, USA, 2012. [Google Scholar]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Mishra, N.N.; Prasad, T.; Sharma, N.; Payasi, A.; Gupta, D.K.; Singh, R. Pathogenicity and drug resistance in Candida albicans and other yeast species. Acta Microbiol. Immunol. Hung. 2007, 54, 201–235. [Google Scholar] [CrossRef]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficiency of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef]
- Prasad, R.; Kapoor, K. Multidrug resistance in yeast Candida. Int. Rev. Cytol. 2005, 242, 215–248. [Google Scholar] [CrossRef]
- Chipeva, V.A.; Petrova, D.C.; Geneva, M.E.; Dimitrova, M.A.; Monchdevaa, P.A.; Kapcina-Toteva, V.M. Antimicrobial activity of extracts from in vivo and in vitro propagated Lamium album L. plants. Afr. J. Trad. Complement. Altern. Med. 2013, 10, 559–562. [Google Scholar] [CrossRef]
- Naeini, A.; Jalayer Naderi, N.; Shokri, H. Analysis and in vitro anti-Candida antifungal activity of Cuminum cyminum and Salvadora persica herbs extracts against pathogenic Candida strains. J. Mycol. Med. 2014, 24, 13–18. [Google Scholar] [CrossRef]
- Pemmaraju, S.C.; Pruthi, P.A.; Prasad, R.; Pruthi, V. Candida albicans biofilm inhibition by synergistic action of terpenes and fluconazole. Indian J. Exp. Biol. 2013, 51, 1032–1037. [Google Scholar]
- Bhattacharyya, S.; Gupta, P.; Banerjee, G.; Jain, A.; Singh, M. In vitro inhibition of biofilm formation in Candida albicans and Candida tropicalis by heat stable compounds in culture filtrate of Aspergillus flavus. J. Clin. Diagn. Res. 2013, 7, 2167–2169. [Google Scholar]
- Chaaban, I.; Khawass, E.S.M.; Mahran, M.A.; El Salamouni, N.S.; Abdel Wahab, A.E. Synthesis and biological evaluation of novel benzoquinones as potential antimicrobial agents. Med. Chem. Res. 2013, 22, 841–851. [Google Scholar] [CrossRef]
- Jamel, A.A.; Padush, M.S.A. Synthesis, characterization and antimicrobial activities of some novel derivatives of phenol. Asian J. Chem. 2010, 22, 3422–3428. [Google Scholar]
- Cardellicchio, C.; Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella, J.F.; Capitelli, F. Investigation on the weak interactions assembling the crystal structure of Betti bases. CrystEngComm 2012, 14, 3972–3981. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Capozzi, M.A.M.; Naso, F. The Betti base: The awakening of a sleeping beauty. Tetrahedron: Asymmetr. 2010, 21, 507–517. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Ciccarella, G.; Naso, F.; Perna, F.; Tortorella, P. Use of readily available chiral compounds related to the Betti base in the enantioselective addition of diethylzinc to aryl aldehydes. Tetrahedron 1999, 55, 14685–14692. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Ciccarella, G.; Naso, F.; Schingaro, E.; Scordari, F. The Betti base: Absolute configuration and routes to a family of related chiral nonracemic bases. Tetrahedron: Asymmetr. 1998, 9, 3667–3675. [Google Scholar] [CrossRef]
- Szatmári, F.; Fülöp, F. Syntheses, transformations and applications of aminonaphthol derivatives prepared via modified Mannich reactions. Tetrahedron 2013, 69, 1255–1278. [Google Scholar] [CrossRef] [Green Version]
- Szatmári, I.; Martinek, T.A.; Lázár, L.; Fülöp, F. Synthesis of 2,4-Diaryl-3,4-dihydro-2H-naphth[2,1-e][1,3]oxazines and Study of the Effects of the Substituents on Their Ring-Chain Tautomerism. Curr. Org. Synth. 2004, 1, 155–165. [Google Scholar]
- De Graaff, C.; Ruijter, E.; Orru, R.V.A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 2012, 41, 3969–4009. [Google Scholar] [CrossRef]
- Hazen, K.C. Influence of DMSO on antifungal activity during susceptibility testing in vitro. Diagn. Micr. Infect. Dis. 2013, 75, 60–63. [Google Scholar] [CrossRef]
- Szatmari, I.; Sillanpaa, R.; Fulop, F. Microwave assisted, highly enantioselective addition of diethylzinc to aromatic aldehydes catalyzed by chiral aminonaphthols. Tetrahedron: Asymmetr. 2008, 19, 612–617. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds tested in this research are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Capozzi, M.A.M.; Cardellicchio, C.; Magaletti, A.; Bevilacqua, A.; Perricone, M.; Corbo, M.R. Bioactivity of a Family of Chiral Nonracemic Aminobenzylnaphthols towards Candida albicans. Molecules 2014, 19, 5219-5230. https://doi.org/10.3390/molecules19045219
Capozzi MAM, Cardellicchio C, Magaletti A, Bevilacqua A, Perricone M, Corbo MR. Bioactivity of a Family of Chiral Nonracemic Aminobenzylnaphthols towards Candida albicans. Molecules. 2014; 19(4):5219-5230. https://doi.org/10.3390/molecules19045219
Chicago/Turabian StyleCapozzi, Maria Annunziata M., Cosimo Cardellicchio, Angela Magaletti, Antonio Bevilacqua, Marianne Perricone, and Maria Rosaria Corbo. 2014. "Bioactivity of a Family of Chiral Nonracemic Aminobenzylnaphthols towards Candida albicans" Molecules 19, no. 4: 5219-5230. https://doi.org/10.3390/molecules19045219




