Three New Ring-A Modified Ursane Triterpenes from Davidia involucrata
Abstract
:1. Introduction
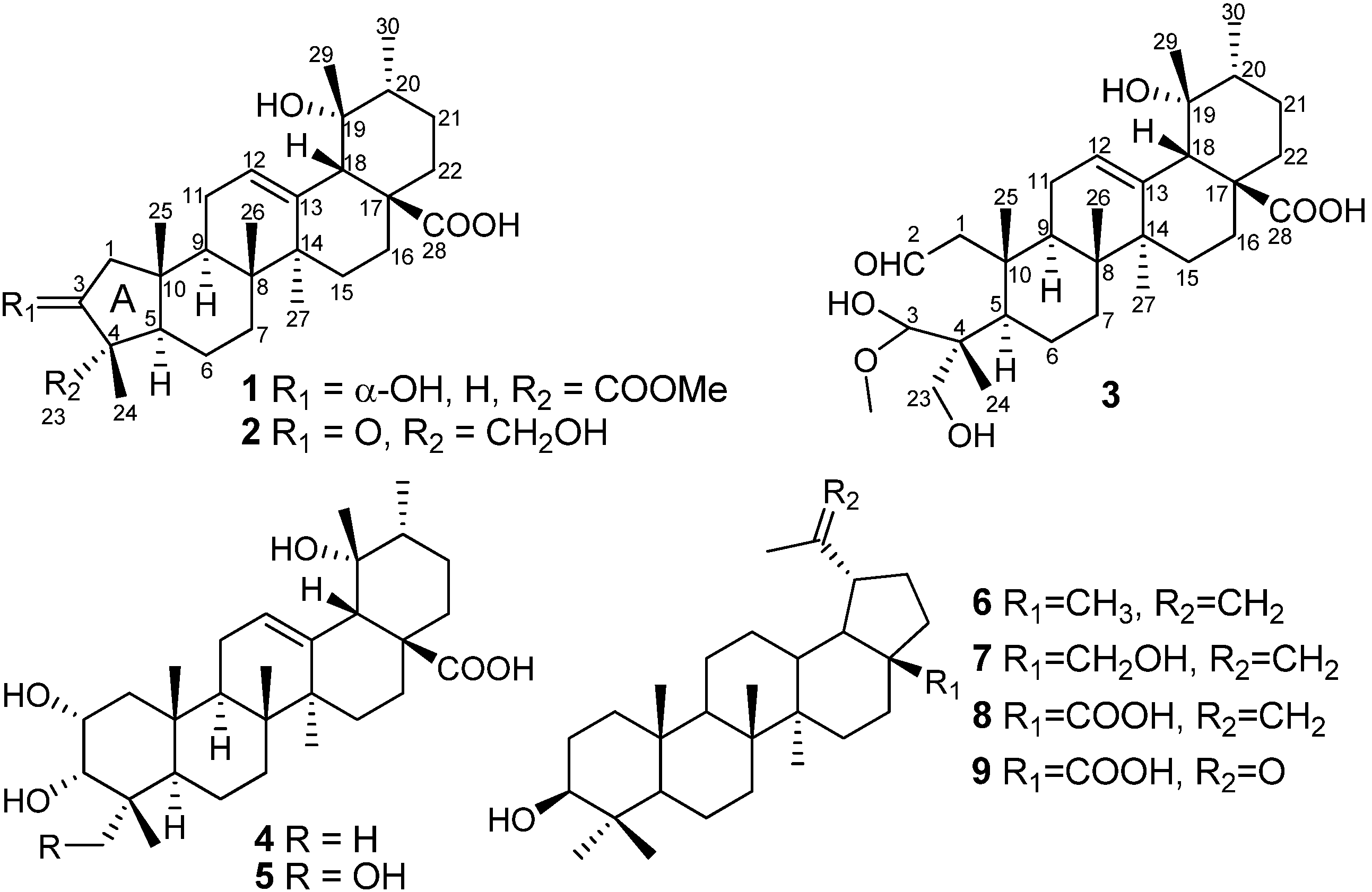
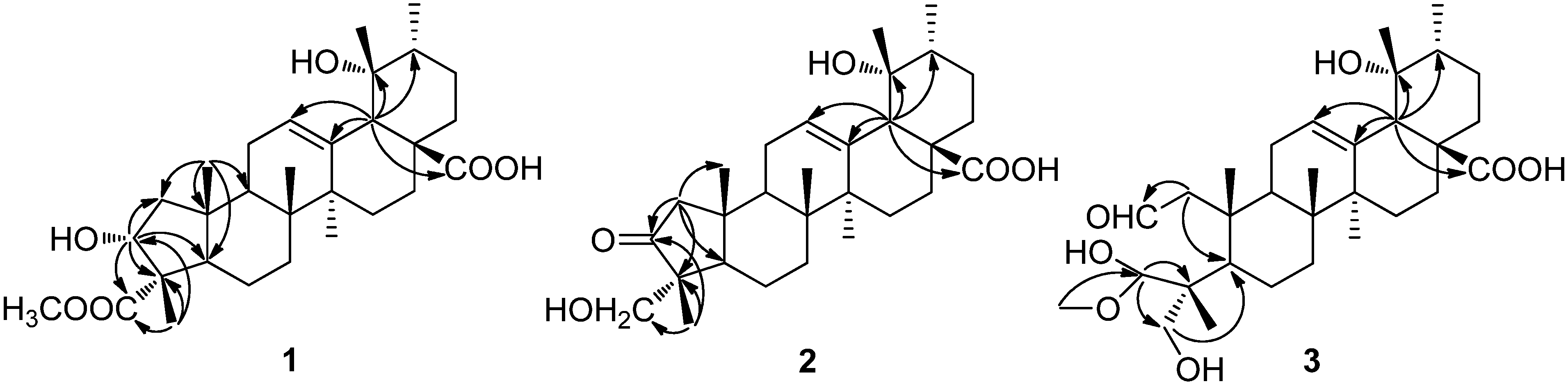
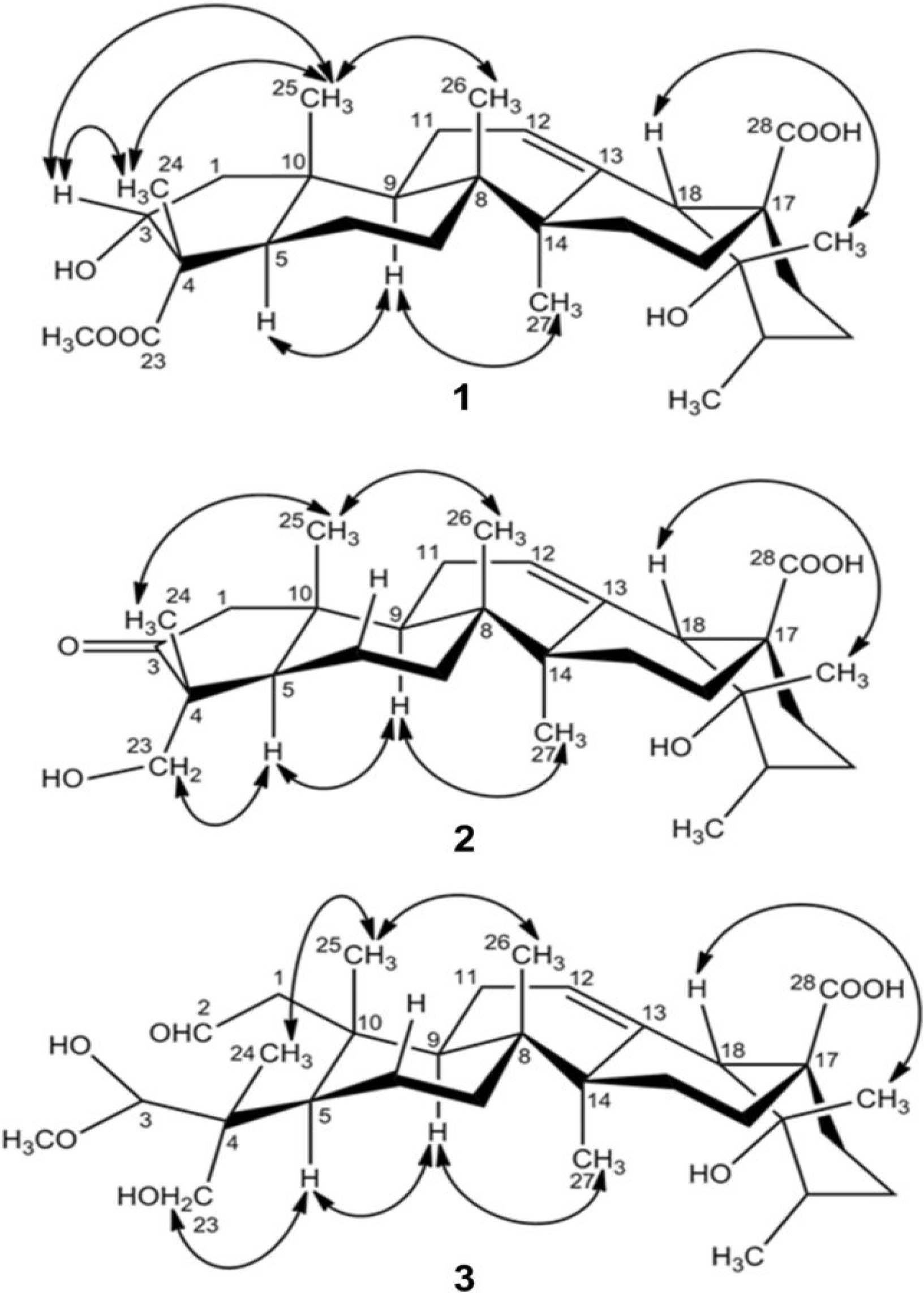
2. Results and Discussion
| Compounds | Cell lines | ||
|---|---|---|---|
| SGC-7901 | MCF-7 | BEL-7404 | |
| 1 | – a | – | – |
| 2 | Nt | Nt | Nt |
| 3 | 36.3 ± 2.00 | 37.4 ± 1.57 | 47.41 ± 20.4 |
| 4 | – | – | – |
| 5 | – | – | – |
| 6 | 42.35 ± 1.79 | 45.8 ± 2.15 | 38.24 ± 1.57 |
| 7 | 39.25 ± 1.83 | 21.24 ± 1.15 | 24.59 ± 1.51 |
| 8 | 7.26 ± 0.08 | 12.37 ± 0.18 | 8.15 ± 0.13 |
| 9 | 10.26 ± 0.52 | 15.21 ± 0.28 | 18.14 ± 0.30 |
| Doxorubicin | 0.19 ± 0.032 | 0.08 ± 0.007 | 0.12 ± 0.011 |
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
| No. | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δC (DEPT) | δH, mult. (J in Hz) | δC (DEPT) | δH, mult. (J in Hz) | δC (DEPT) | δH, mult. (J in Hz) | |
| 1 | 50.3 (CH2) | 0.97 d (11.5), 2.09 dd (11.5, 7.5) | 56.5 (CH2) | 2.07 s | 43.9 (CH2) | 1.30 m, 2.03 m |
3.4. Spectral Data
 = +87.5 (c 0.12, CHCl3). IR (KBr) νmax (cm−1): 3430, 2950, 1750, 1728. HR-TOF-MS m/z = 525.3187 [M + Na]+ (calcd. for C30H46O6Na+ 525.3192). 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (125 MHz, CDCl3 and CD3OD) data (see Table 2).
= +87.5 (c 0.12, CHCl3). IR (KBr) νmax (cm−1): 3430, 2950, 1750, 1728. HR-TOF-MS m/z = 525.3187 [M + Na]+ (calcd. for C30H46O6Na+ 525.3192). 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (125 MHz, CDCl3 and CD3OD) data (see Table 2). = +109.8 (c 0.22, CHCl3). IR (KBr) νmax (cm−1): 3428, 2920, 1740, 1686. HR-TOF-MS m/z = 495.3080 [M + Na]+ (calcd. for C29H44O5Na 495.3086). 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (100 MHz, CDCl3 and CD3OD) data (see Table 2).
= +109.8 (c 0.22, CHCl3). IR (KBr) νmax (cm−1): 3428, 2920, 1740, 1686. HR-TOF-MS m/z = 495.3080 [M + Na]+ (calcd. for C29H44O5Na 495.3086). 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (100 MHz, CDCl3 and CD3OD) data (see Table 2). = +124.2 (c 0.15, CHCl3). IR (KBr) νmax (cm−1): 3437, 2930, 1741, 1720. HR-TOF-MS m/z 557.3448 [M + Na]+ (calcd. for C31H50O7Na 557.3454). 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (100 MHz, CDCl3 and CD3OD) data (see Table 2).
= +124.2 (c 0.15, CHCl3). IR (KBr) νmax (cm−1): 3437, 2930, 1741, 1720. HR-TOF-MS m/z 557.3448 [M + Na]+ (calcd. for C31H50O7Na 557.3454). 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (100 MHz, CDCl3 and CD3OD) data (see Table 2).3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.X.; Chen, L.; Lin, J.; Li, Y.F.; Chen, F. Suppression subtractive hybridization cloning of cDNAs of differentially expressed genes in dovetree (Davidia involucrata) bracts. Plant Mol. Biol. Rep. 2002, 20, 231–238. [Google Scholar] [CrossRef]
- He, Z.C.; Li, J.Q.; Wang, H.C. Karyomorphology of Davidia involucrata and Camptotheca acuminate, with special reference to their systematic positions. Bot. J. Linn. Soc. 2004, 2, 193–198. [Google Scholar]
- Sun, J.F.; Gong, Y.B.; Renner, S.S.; Huang, S.Q. Multifunctiona bracts in the dove tree Davidia involucrata (Nyssaceae: Cornales): Rain protection and pollinator attraction. Am. Nat. 2008, 171, 119–124. [Google Scholar] [CrossRef]
- Ouyang, M.A.; Zhou, J.N.; Wang, S.B. New caffeoyl derivatives from the leaves of Davidia involucrata. Nat. Prod. Res. 2008, 22, 471–476. [Google Scholar] [CrossRef]
- Wu, Z.J.; Ouyang, M.A.; Wang, S.B. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 2008, 22, 483–488. [Google Scholar] [CrossRef]
- Ouyang, M.A.; Huang, J.; Tan, Q.W. Neolignan and lignan glycosides from branch bark of Davidia involucrata. J. Asian Nat. Prod. Res. 2007, 9, 487–492. [Google Scholar] [CrossRef]
- Liang, G.Y.; Gray, A.I.; Waterman, P.G. Pentacyclic triterpenes from fruits of Rosa sterilis. J. Nat. Prod. 1989, 52, 162–166. [Google Scholar] [CrossRef]
- Wandji, J.; Tillequin, F.; Mulholland, D.A.; Shirri, J.C.; Tsabang, N.; Seguin, E.; Verite, P.; Libot, F.; Fomum, Z.T. Pentacyclic triterpenoid and saponins from Gambeya boukokoensis. Phytochemistry 2003, 64, 845–849. [Google Scholar] [CrossRef]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef]
- O’Connell, M.M.; Bentley, M.D.; Campbell, C.S.; Cole, B.J.W. Betulin and lupeol in bark from four white-barked birches. Phytochemistry 1988, 27, 2175–2176. [Google Scholar] [CrossRef]
- Bastos, D.Z.L.; Pimentel, I.C.; de Jesus, D.A.; de Oliveira, B.H. Biotransformation of betulinic and betulonic acids by fungi. Phytochemistry 2007, 68, 834–839. [Google Scholar] [CrossRef]
- Fujikoka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef]
- Aquino, R.; de Simone, F.; Vincieri, F.F.; Pizza, C.; Gacs-Baitz, E. New polyhydroxylated triterpenes from Uncaria tomentosa. J. Nat. Prod. 1990, 53, 559–564. [Google Scholar] [CrossRef]
- Jang, D.S.; Kim, J.M.; Kim, J.H.; Kim, J.S. 24-nor-Ursane type triterpenoids from the stems of Rumex japonicus. Chem. Pharm. Bull. 2005, 53, 1594–1596. [Google Scholar] [CrossRef]
- Taniguchi, S.; Imayoshi, Y.; Kobayashi, E.; Takamatsu, Y.; Ito, H.; Hatano, T.; Sakagami, H.; Tokuda, H.; Nishino, H.; Sugita, D.; Shimura, S.; Yoshida, T. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002, 59, 315–323. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, Y.H.; Lee, S.M.; Jung, H.J.; Lee, J.S.; Na, M.K.; Lee, C.O.; Lee, J.P.; Bae, K.H. Cytotoxic triterpenes from Crataegus pinnatifida. Arch. Pharm. Res. 2000, 23, 155–158. [Google Scholar] [CrossRef]
- He, X.F.; Wang, X.N.; Yin, S.; Dong, L.; Yue, J.M. Ring A-seco triterpenoids with antibacterial activity from Dysoxylum hainanense. Eur. J. Org. Chem. 2009, 28, 4818–4824. [Google Scholar]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. J. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Alkakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef]
- Kommera, H.; Kaluđerović, G.N.; Dittrich, S.; Kalbitz, J.; Dräger, B.; Mueller, T.; Paschke, R. Carbamate derivatives of betulinic acid and botulin with selective cytotoxic activity. Bioorg. Med. Chem. Lett. 2010, 20, 3409–3412. [Google Scholar] [CrossRef]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Paschke, R. Lupane Triterpenoids–Betulin and Betulinic acid derivatives induce apoptosis in tumor cells. Invest. New Drugs 2011, 29, 266–272. [Google Scholar] [CrossRef]
- Kessler, J.H.; Mullauer, F.B.; Roo, G.M.D.; Medema, J.P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic acid, a natural compound with potent anticancer effects. Anti-Cancer Drug. 2010, 21, 215–227. [Google Scholar] [CrossRef]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulin is a potent anti-tumor agent that is enhanced by cholesterol. PLoS One 2009, 4, e1. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds in the manuscripts are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, Q.-W.; Ouyang, M.-A.; Gao, B. Three New Ring-A Modified Ursane Triterpenes from Davidia involucrata. Molecules 2014, 19, 4897-4906. https://doi.org/10.3390/molecules19044897
Tan Q-W, Ouyang M-A, Gao B. Three New Ring-A Modified Ursane Triterpenes from Davidia involucrata. Molecules. 2014; 19(4):4897-4906. https://doi.org/10.3390/molecules19044897
Chicago/Turabian StyleTan, Qing-Wei, Ming-An Ouyang, and Bo Gao. 2014. "Three New Ring-A Modified Ursane Triterpenes from Davidia involucrata" Molecules 19, no. 4: 4897-4906. https://doi.org/10.3390/molecules19044897





