Pharmacological and Structure-Activity Relationship Evaluation of 4-aryl-1-Diphenylacetyl(thio)semicarbazides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

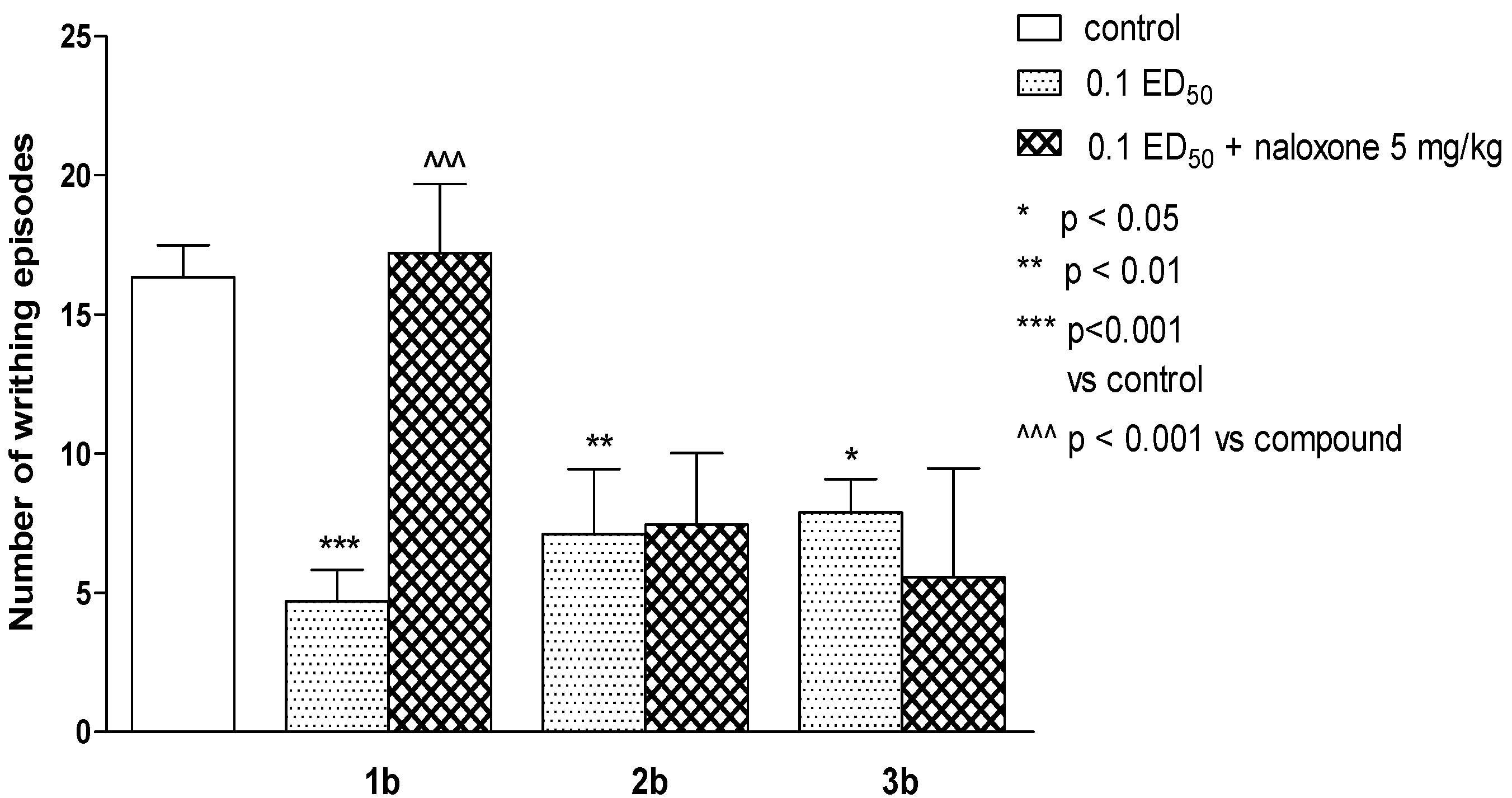
2.2. Pharmacological Activity
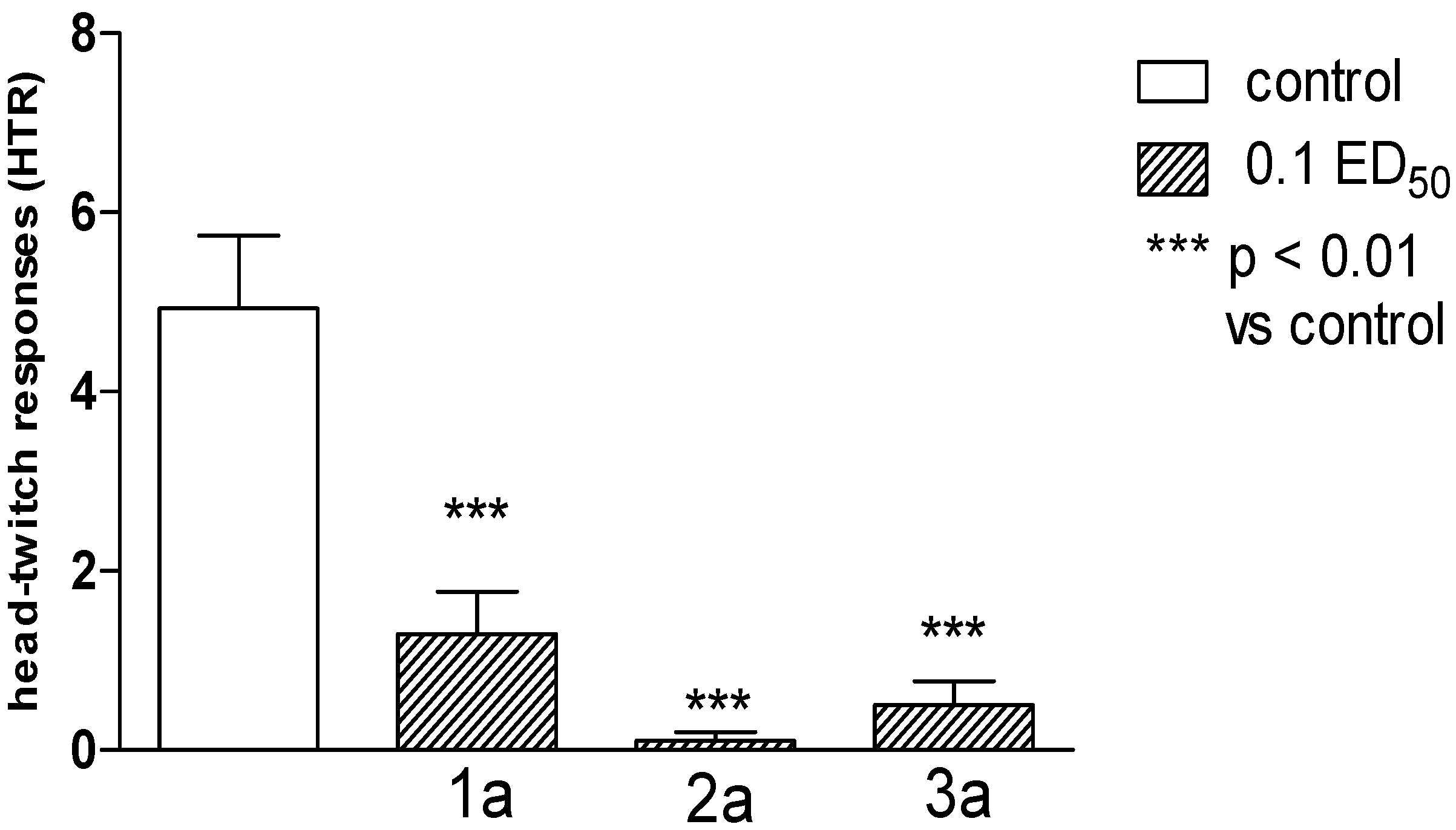

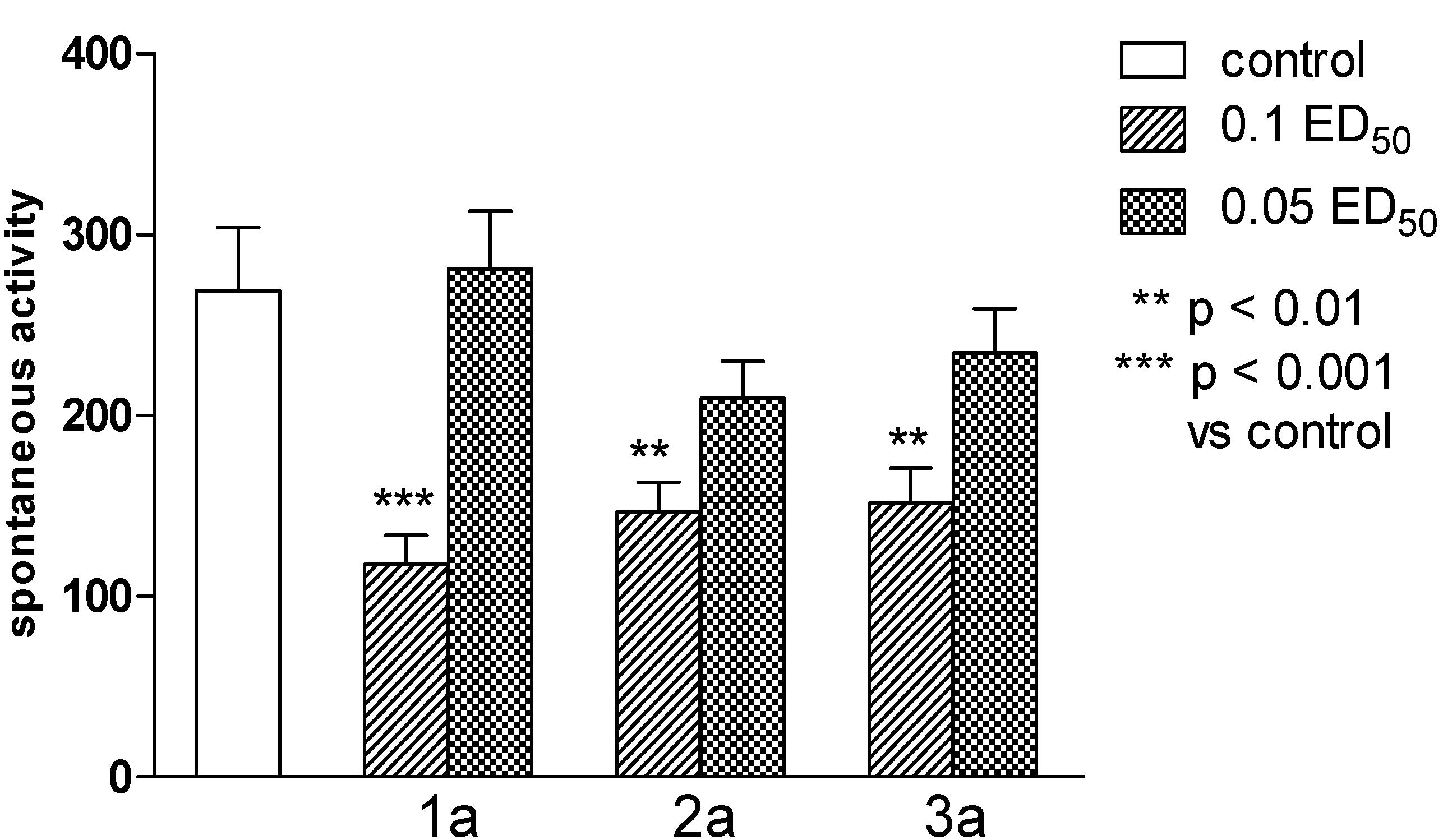
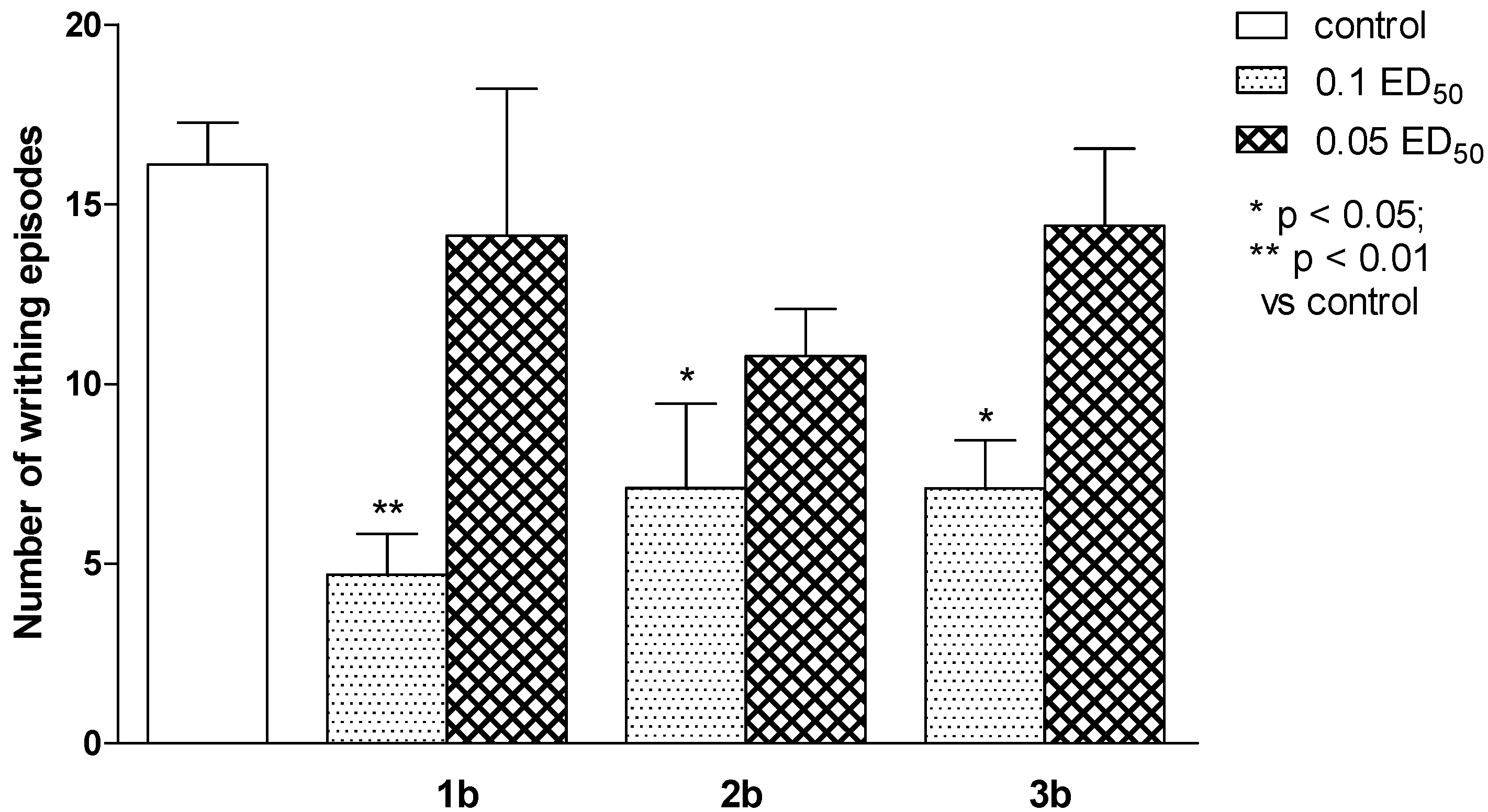
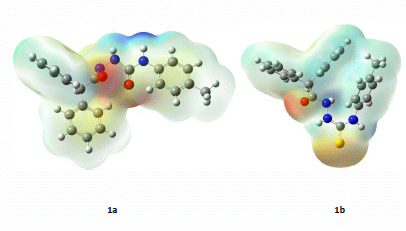
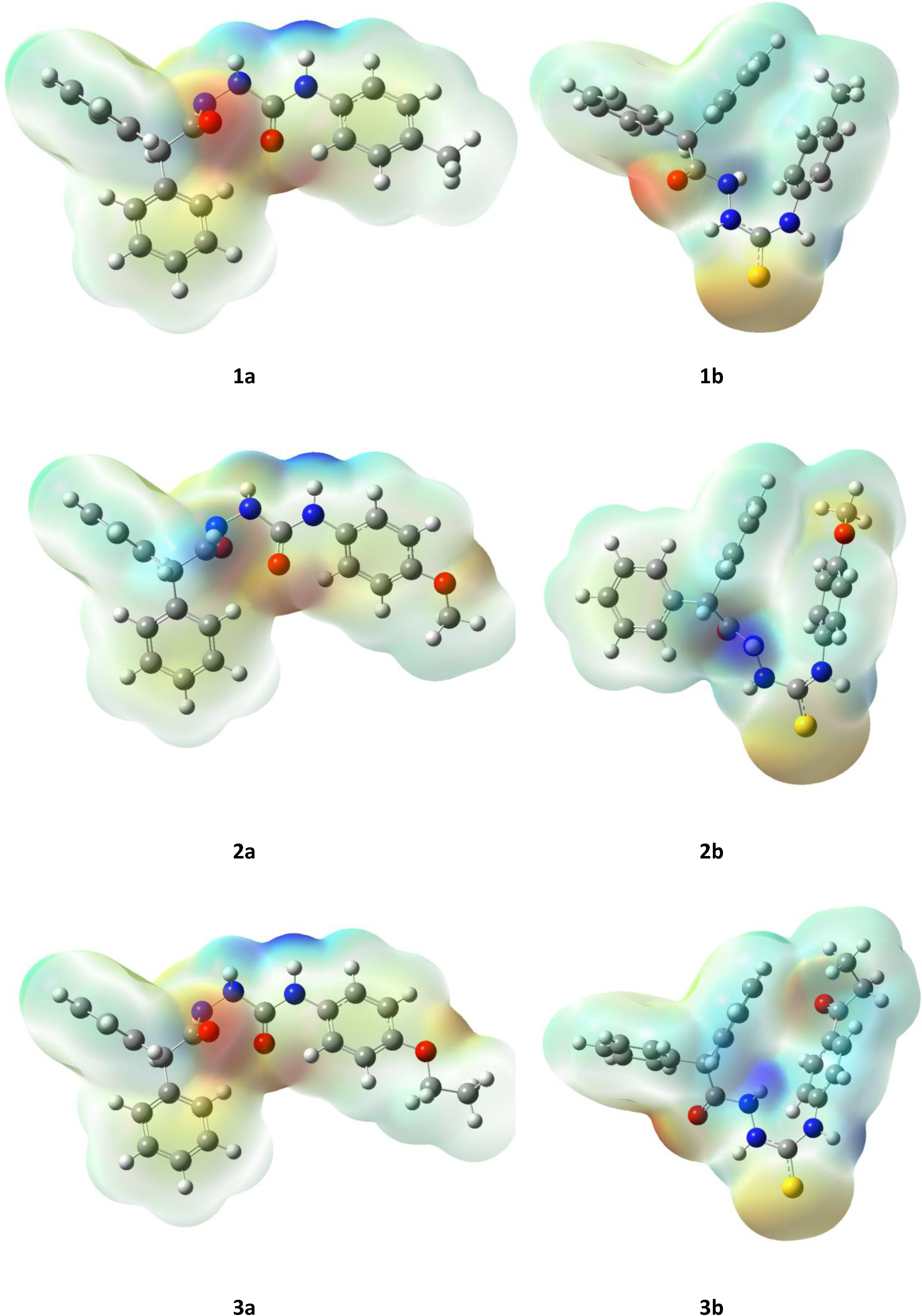
2.3. Computational Part

3. Experimental
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of 4-aryl-1-Diphenylacetylsemicarbazides 1a and 2a
3.2.2. Procedure for the Synthesis of 4-(4-Ethoxyphenyl)-1-(diphenylacetyl)semicarbazide 3a and 4-Aryl-1-(diphenylacetyl)thiosemicarbazides 1b–3b
3.3. Pharmacology
- (a)
- spontaneous activity
- (b)
- amphetamine-induced hyperactivity: mice received s.c. 5 mg/kg of amphetamine 30 min before the test;
3.4. Statistics
3.5. Computational Part
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Guzel, O.; Karalı, N.; Salman, A. Synthesis and antituberculosis activity of 5-methyl/trifluoromethoxy-1H-indole-2,3-dione 3-thiosemicarbazone derivatives. Bioorg. Med. Chem. 2008, 16, 8976–8987. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V.; Pellerano, M.L.; De Logu, A. Synthesis and antimycobacterial activity of new s-alkylisothiosemicarbazone derivatives. Bioorg. Med. Chem. 2002, 10, 501–506. [Google Scholar] [CrossRef]
- Al-Soud, Y.A.; Al-Dweri, M.N.; Al-Masoudi, N.A. Synthesis, antitumor and antiviral properties of some 1,2,4-triazole derivatives. Il Farmaco 2004, 59, 775–783. [Google Scholar] [CrossRef]
- Iyidogan, A.K.; Tasdemir, D.; Oruç-Emre, E.E.; Balzarini, J. Novel platinum(II) and palladium(II) complexes of thiosemicarbazones derived from 5-substitutedthiophene-2-carboxaldehydes and their antiviral and cytotoxic activities. Eur. J. Med. Chem. 2011, 46, 5616–5624. [Google Scholar]
- Qu, J.Q.; Sun, G.C.; Wang, L.F.; Qu, L. Synthesis, characterization, and biological activities of some transition metal(II) complexes with the thiosemicarbazone derived from 4-[1-(4-methylphenylsulfonyl)-1-indol-3-yl]but-3-en-2-one. Chem. Pap. 2006, 60, 214–219. [Google Scholar]
- Aly, M.M.; Mohamed, Y.A.; El-Bayouki, K.A.M.; Basyouni, W.M.; Abbas, S.Y. Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities. Eur. J. Med. Chem. 2010, 45, 3365–3373. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D.; Mehta, S.; Nigam, D.; Kumar, M.M.; Murugesan, S.; Stables, J.P. Anticonvulsant and neurotoxicity evaluation of some 6-substituted benzothiazolyl-2-thiosemicarbazones. Il Farmaco 2005, 60, 1–5. [Google Scholar] [CrossRef]
- Jain, J.; Kumar, Y.; Stables, J.; Sinha, R. Menthone Semicarbazides and thiosemicarbazides as anticonvulsant agents. Med. Chem. 2010, 6, 44–50. [Google Scholar] [CrossRef]
- Siwek, A.; Stączek, P.; Wujec, M.; Stefańska, J.; Kosikowska, U.; Malm, A.; Jankowski, S.; Paneth, P. Biological and docking studies of topoisomerase IV inhibition by thiosemicarbazides. J. Mol. Model. 2011, 17, 2297–2303. [Google Scholar] [CrossRef]
- Siwek, A.; Stączek, P.; Stefańska, J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur. J. Med. Chem. 2011, 46, 5717–5726. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Siwek, A.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(49-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Umamatheswari, S.; Balaji, B.; Ramanathan, M.; Kabilan, S. Synthesis, stereochemistry, antimicrobial evaluation and QSAR studies of 2,6-diaryltetrahydropyran-4-one thiosemicarbazones. Eur. J. Med. Chem. 2011, 46, 1415–1424. [Google Scholar] [CrossRef]
- Zhang, H.J.; Qian, Y.; Zhu, D.D.; Yang, X.G.; Zhu, H.L. Synthesis, molecular modeling and biological evaluation of chalcone thiosemicarbazide derivatives as novel anticancer agents. Eur. J. Med. Chem. 2011, 46, 4702–4708. [Google Scholar] [CrossRef]
- Kritsanida, M.; Mouroutsou, A.; Marakos, P.; Pouli, N.; Papakonstantinou-Garoufalias, S.; Pannecougue, C.; Witvouw, M.; de Clerq, E. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b]thiadiazoles. Il Farmaco 2002, 57, 253–257. [Google Scholar] [CrossRef]
- Walczak, K.; Gondela, A.; Suwiński, J. Synthesis and anti-tuberculosis activity of N-aryl-C-nitroazoles. Eur. J. Med. Chem. 2004, 39, 849–853. [Google Scholar] [CrossRef]
- Karlsson, M.; Fellström, C.; Gunnarsson, A.; Landén, A.; Franklin, A. Antimicrobial susceptibility testing of porcine Brachyspira (Serpulina) species isolates. J. Clin. Microbiol. 2003, 41, 2596–2604. [Google Scholar] [CrossRef]
- Amir, M.; Shikha, K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives. Eur. J. Med. Chem. 2004, 39, 535–545. [Google Scholar] [CrossRef]
- Pitucha, M.; Chodkowska, A.; Maciejewski, M.; Jagiełło-Wójtowicz, E.; Pachuta-Stec, A. Synthesis and antinociceptive activity of 4,4′-bis(1-substituted-semicarbazidyl)phenylmethane and 4,4′-bis(5-substituted-2,4-dihydro-3-oxo-3H-1,2,4-triazol-4-yl)diphenylmethane derivatives. Monatsh. Chem. 2010, 141, 199–203. [Google Scholar] [CrossRef]
- Andurkar, S.V.; Béguin, C.; Stables, J.P.; Kohn, H. Synthesis and structural studies of aza analogues of functionalized amino acids: new anticonvulsant agents. J. Med. Chem. 2001, 44, 1475–1478. [Google Scholar] [CrossRef]
- Pitucha, M.; Polak, B.; Świeboda, R.; Kosikowska, U.; Malm, A. Determination of the lipophilicity of some new derivatives of semicarbazide and 1,2,4-triazol-5-one with potential antibacterial activity. Z. Naturforsch. B 2009, 64, 570–576. [Google Scholar]
- Listos, J.; Talarek, S.; Orzelska, J.; Fidecka, S.; Wujec, M.; Plech, T. The antinociceptive effect of 4-substituted derivatives of 5-(4-chlorophenyl)-2-(morpholin-4-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 367–375. [Google Scholar] [CrossRef]
- Kusmierz, E.; Siwek, A.; Kosikowska, U.; Malm, A.; Stefańska, J.; Dzitko, K.; Wujec, M. Antibacterial activity and structure-activity relationship studies of 4-substituted-5-(diphenylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Lett. Drug Design Discov. 2013, 10, 95–101. [Google Scholar]
- Wujec, M.; Pitucha, M.; Dobosz, M. New derivatives of 3-[(4-phenyl-5-oxo-1,2,4-triazolin-1-yl)methyl]-4-substituted-1,2,4-triazolin-5-one. Heterocycles 2006, 68, 779–785. [Google Scholar] [CrossRef]
- Corne, S.J.; Pickering, R.W.; Werner, B.T. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br. J. Pharmacol. 1963, 20, 106–120. [Google Scholar]
- Corne, S.J.; Pickering, R.W. A possible correlation between druginduced hallucinations in man and a behavioural response in mice. Psychopharmacology 1967, 11, 65–68. [Google Scholar] [CrossRef]
- Peroutka, S.J.; Lebovitz, R.M.; Snyder, S.H. Two distinct central serotonin receptors with different physiological functions. Science 1981, 212, 827–829. [Google Scholar]
- Goodwin, G.M; Green, A.R. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br. J. Pharmacol. 1985, 84, 743–753. [Google Scholar] [CrossRef]
- Darmani, N.A.; Martin, B.R.; Glennon, R.A. Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J. Pharmacol. Exp. Ther. 1992, 262, 692–698. [Google Scholar]
- Darmani, N.A.; Martin, B.R.; Glennon, R.A. Withdrawal from chronic treatment with (+/−)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 1990, 186, 115–118. [Google Scholar] [CrossRef]
- Darmani, N.A.; Martin, B.R.; Pandy, U.; Glennon, R.A. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol. Biochem. Behav. 1990, 36, 901–906. [Google Scholar] [CrossRef]
- Fantegrossi, W.E.; Kiessel, C.L.; Leach, P.T.; Van Martin, C.; Karabenick, R.L.; Chen, X.; Ohizumi, Y.; Ullrich, T.; Rice, K.C.; Woods, J.H. Nantenine: An antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology 2004, 173, 270–277. [Google Scholar] [CrossRef]
- Lucki, I.; Nobler, M.S.; Frazer, A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J. Pharmacol. Exp. Ther. 1984, 228, 133–139. [Google Scholar]
- Handley, S.L.; Singh, L. The modulation of head-twitch behaviour by drugs acting on beta-adrenoceptors: Evidence for the involvement of both beta 1- and beta 2-adrenoceptors. Pharmacology 1986, 7, 320–324. [Google Scholar]
- Ortmann, R.; Biscoff, S.; Radeke, E.; Bueche, O.; Delini-Stula, A. Correlation between different measures of antiserotonin activity of drugs. Naunyn Schmiedebergs Arch. Pharmacol. 1982, 321, 265–270. [Google Scholar] [CrossRef]
- Cox, B.; Lomax, P. Pharmacological studies on the central regulation of body temperature. Annu. Rev. Pharmacol. Toxicol. 1997, 17, 341–353. [Google Scholar]
- Ulugol, A.; Karadag, H.C.; Dokmeci, D.; Al.-Khatib, I.; Dokmeci, I. The protective effect of moclobemide against hypoxia-induced lethality in mice is not due to a decrease in body temperature. Pharmacol. Biochem. Behav. 1995, 51, 245–247. [Google Scholar] [CrossRef]
- Gao, B.; Ducan, W.C., Jr; Wehr, T.A. Clorgyline-induced reduction in body temperature and its relationship to vigilance states in Syrian hamsters. Neuropsychopharmacology 1991, 4, 187–1897. [Google Scholar]
- Adell, A.; Bigg, T.A.; Myers, R.D. Action of harman (1-methyl-β-carboline) on the brain: Body temperature and in vivo efflux of 5-HT from hippocampus of the rat. Neuropharmacology 1996, 35, 1101–1107. [Google Scholar] [CrossRef]
- Vogel, G.H.; Vogel, W.H. Drug Discovery and Evaluation. Pharmacological Assays; Springer-Verlag: Berlin, Germany, 1997. [Google Scholar]
- Gutstein, H. B.; Akil, H. Opioid analgesics. In Goodman and Gilman’s. The Pharmacological Basis of Therapeutics, XIth ed.; MacGrow Hill: New York, NY, USA, 2006; pp. 547–590. [Google Scholar]
- Siwek, A.; Świderek, K.; Jankowski, S. Problems with molecular mechanics implementations on the example of 4-benzoyl-1-(4-methyl-imidazol-5-yl)-carbonylthiosemicarbazide. J. Mol. Model. 2012, 18, 843–849. [Google Scholar] [CrossRef]
- Litchfield, L.T.; Wilcoxon, F. Simplified method of evaluating dose effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Koster, R.; Anderson, M.; DeBeer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–416. [Google Scholar]
- Gross, F.; Tripod, J.; Meir, R. Zur pharmakologischen Charakterisierung des schlafmittelsdoriden. Schweiz. Med. Wochschr. 1955, 85, 305–309. [Google Scholar]
- Boissier, J.R.; Tardy, J.; Diverres, J.C. Une nouvelle méthode simple pour explorer l'actiontranquilisante: le test de la cheminée. Med. Exp. (Basel) 1960, 3, 81–84. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Hyperchem 8.0.3, HyperCube Inc.: Gainsville, FL, USA, 2007.
- Gaussian 09, revision A.02; Gaussian Inc.: Wallingford, SA, USA, 2009.
- Hariharan, P.C.; Pople. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wujec, M.; Kędzierska, E.; Kuśmierz, E.; Plech, T.; Wróbel, A.; Paneth, A.; Orzelska, J.; Fidecka, S.; Paneth, P. Pharmacological and Structure-Activity Relationship Evaluation of 4-aryl-1-Diphenylacetyl(thio)semicarbazides. Molecules 2014, 19, 4745-4759. https://doi.org/10.3390/molecules19044745
Wujec M, Kędzierska E, Kuśmierz E, Plech T, Wróbel A, Paneth A, Orzelska J, Fidecka S, Paneth P. Pharmacological and Structure-Activity Relationship Evaluation of 4-aryl-1-Diphenylacetyl(thio)semicarbazides. Molecules. 2014; 19(4):4745-4759. https://doi.org/10.3390/molecules19044745
Chicago/Turabian StyleWujec, Monika, Ewa Kędzierska, Edyta Kuśmierz, Tomasz Plech, Andrzej Wróbel, Agata Paneth, Jolanta Orzelska, Sylwia Fidecka, and Piotr Paneth. 2014. "Pharmacological and Structure-Activity Relationship Evaluation of 4-aryl-1-Diphenylacetyl(thio)semicarbazides" Molecules 19, no. 4: 4745-4759. https://doi.org/10.3390/molecules19044745
APA StyleWujec, M., Kędzierska, E., Kuśmierz, E., Plech, T., Wróbel, A., Paneth, A., Orzelska, J., Fidecka, S., & Paneth, P. (2014). Pharmacological and Structure-Activity Relationship Evaluation of 4-aryl-1-Diphenylacetyl(thio)semicarbazides. Molecules, 19(4), 4745-4759. https://doi.org/10.3390/molecules19044745