Cornu Cervi Pantotrichum Supplementation Improves Exercise Performance and Protects against Physical Fatigue in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight and Other Metabolism-Related Organ Weights
| Characteristic | Vehicle | CCP-1X | CCP-2X | Trend analysis |
|---|---|---|---|---|
| Initial BW (g) | 30.1 ± 0.3 | 29.4 ± 0.4 | 30.5 ± 0.3 | 0.5798 |
| Final BW (g) | 33.8 ± 0.8 | 34.6 ± 0.6 | 34.7 ± 0.7 | 0.5583 |
| Food intake (g/day) | 6.91 ± 0.28 | 7.23 ± 0.22 | 7.29 ± 0.24 | 0.3765 |
| Water intake (mL/day) | 7.93 ± 0.14 | 7.51 ± 0.22 | 8.27 ± 0.25 | 0.4492 |
| Liver (g) | 1.86 ± 0.06 | 1.87 ± 0.02 | 1.81 ± 0.04 | 0.2206 |
| Muscle (g) | 0.34 ± 0.01 | 0.32 ± 0.01 | 0.35 ± 0.01 | 0.7708 |
| Heart (g) | 0.21 ± 0.00 | 0.21 ± 0.01 | 0.22 ± 0.00 | 0.5426 |
| Lung (g) | 0.32 ± 0.04 | 0.32 ± 0.04 | 0.35 ± 0.04 | 0.4720 |
| Kidney (g) | 0.57 ± 0.02 | 0.55 ± 0.01 | 0.58 ± 0.02 | 0.8445 |
| Testis (g) | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.00 | 0.2130 |
| EFP (g) | 0.65 ± 0.04 | 0.56 ± 0.05 | 0.58 ± 0.04 | 0.4589 |
| BAT (g) | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.0882 |
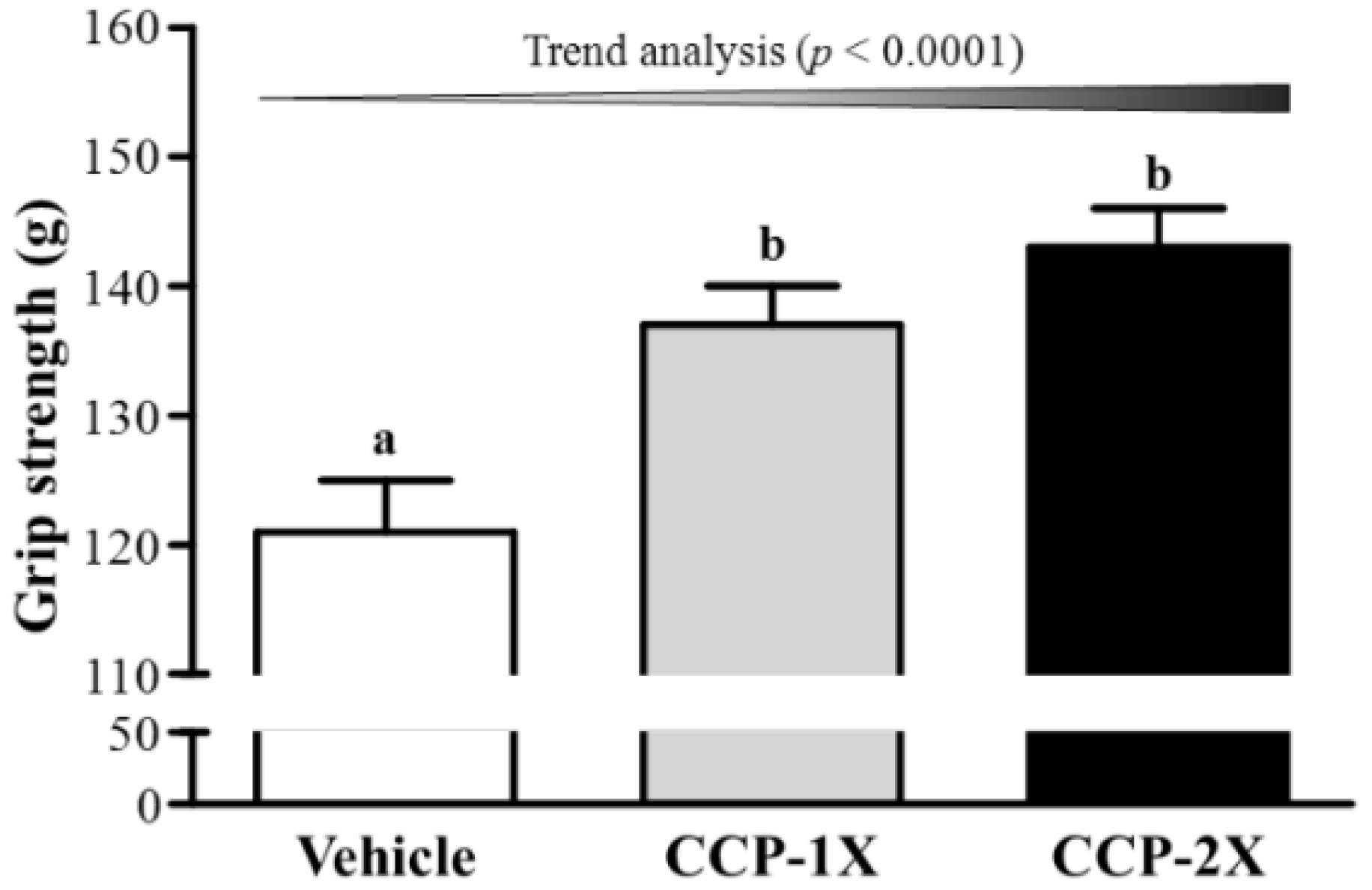
2.2. Effect of CCP Supplementation on Forelimb Grip Strength and Exercise Performance in a Weight-Loaded Swimming Test
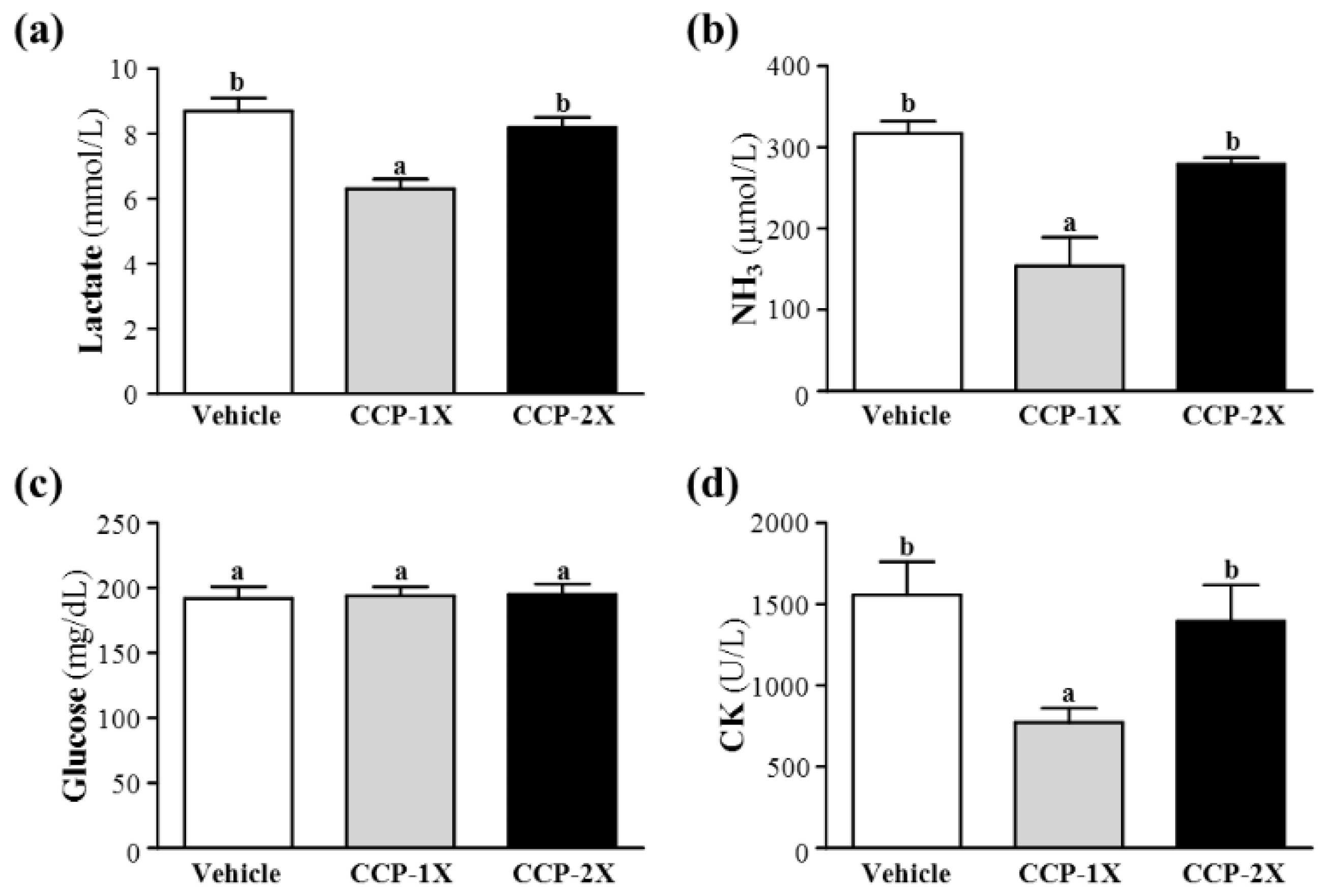
2.3. Effect of CCP Supplementation on Serum Lactate, Ammonia, Glucose, and Creatine Kinase Levels after Acute Exercise Challenge
2.4. Effect of CCP Supplementation on Biochemical Analyses at the End of the Experiment
| Parameter | Vehicle | CCP-1X | CCP-2X | Trend analysis |
|---|---|---|---|---|
| AST (U/L) | 78 ± 6 | 78 ± 10 | 72 ± 6 | 0.4773 |
| ALT (U/L) | 53 ± 4 | 44 ± 4 a | 46 ± 2 | 0.1381 |
| ALP (U/L) | 444 ± 29 b | 382 ± 35 ab | 332 ± 15 a | <0.0001 (↓) |
| LDH (U/L) | 396 ± 26 | 451 ± 37 | 428 ± 21 | 0.3274 |
| CK (U/L) | 245 ± 33 | 282 ± 90 | 187 ± 25 | 0.3077 |
| Albumin (g/dL) | 3.3 ± 0.1 | 3.4 ± 0.0 | 3.3 ± 0.1 | 1.0000 |
| TP (g/dL) | 5.6 ± 0.1 | 5.8 ± 0.1 | 5.6 ± 0.1 | 0.9163 |
| BUN (mg/dL) | 25.2 ± 0.9 | 25.2 ± 1.1 | 22.4 ± 0.9 | 0.0840 |
| Creatinine (mg/dL) | 0.30 ± 0.0 | 0.31 ± 0.02 | 0.33 ± 0.01 | 0.0600 |
| UA (mg/dL) | 1.16 ± 0.12 | 1.06 ± 0.13 | 0.89 ± 0.05 | 0.0111 (↓) |
| TC (mg/dL) | 160 ± 4 b | 168 ± 5 b | 144 ± 7 a | 0.0884 |
| TG (mg/dL) | 162 ± 13 b | 137 ± 10 b | 84 ± 8 a | <0.0001 (↓) |
| Glucose (mg/dL) | 201 ± 5 b | 186 ± 6 ab | 176 ± 7 a | 0.0055 (↓) |
2.5. Effect of CCP Supplementation on Histological Examinations at the End of the Experiment

3. Experimental
3.1. Materials, Animals, and Experiment Design

3.2. Forelimb Grip Strength
3.3. Swimming Exercise Performance Test
3.4. Determination of Fatigue-Associated Biochemical Indices
3.5. Blood Biochemical Assessments and Histological Staining of Tissues
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Wu, F.; Li, H.; Jin, L.; Li, X.; Ma, Y.; You, J.; Li, S.; Xu, Y. Deer antler base as a traditional Chinese medicine: A review of its traditional uses, chemistry and pharmacology. J. Ethnopharmacol. 2013, 145, 403–415. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.J.; Sun, S.Q.; Noda, I. Study on similar traditional Chinese medicines cornu Cervi pantotrichum, cornu Cervi and cornu Cervi degelatinatum by FT-IR and 2D-IR correlation spectroscopy. J. Pharm. Biomed. Anal. 2010, 52, 631–635. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, Y.C.; Wang, C.T.; Ji, B.P. Antioxidant activity of protein hydrolysates from aqueous extract of velvet antler (Cervus elaphus) as influenced by molecular weight and enzymes. Nat. Prod. Commun. 2011, 6, 1683–1688. [Google Scholar]
- Tseng, S.H.; Sung, H.C.; Chen, L.G.; Lai, Y.J.; Wang, K.T.; Sung, C.H.; Wang, C.C. Effects of Velvet Antler with Blood on Bone in Ovariectomized Rats. Molecules 2012, 17, 10574–10585. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, Y.H.; Kim, K.H.; Lee, Y.C.; Kim, C.H.; Moon, S.H.; Kang, S.G.; Park, Y.G. Protective and anti-arthritic effects of deer antler aqua-acupuncture (DAA), inhibiting dihydroorotate dehydrogenase, on phosphate ions-mediated chondrocyte apoptosis and rat collagen-induced arthritis. Int. Immunopharmacol. 2004, 4, 963–973. [Google Scholar] [CrossRef]
- Dragoni, F.; Minotti, C.; Palumbo, G.; Faillace, F.; Redi, R.; Bongarzoni, V.; Avvisati, G. As compared to kaolin clotting time, silica clotting time is a specific and sensitive automated method for detecting lupus anticoagulant. Thromb. Res. 2001, 101, 45–51. [Google Scholar] [CrossRef]
- Luo, J.; Yan, D.; Zhang, D.; Feng, X.; Yan, Y.; Dong, X.; Xiao, X. Substitutes for endangered medicinal animal horns and shells exposed by antithrombotic and anticoagulation effects. J. Ethnopharmacol. 2011, 136, 210–216. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wang, L.Z.; Gao, X.M. Immunopotentiating effect of a ‘Yang’-promoting formula of traditional Chinese medicine on aged female BALB/c mice. Phytother. Res. 2004, 18, 857–861. [Google Scholar] [CrossRef]
- Mehta, R.K.; Agnew, M.J. Influence of mental workload on muscle endurance, fatigue, and recovery during intermittent static work. Eur. J. Appl. Physiol. 2012, 112, 2891–2902. [Google Scholar] [CrossRef]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar]
- Coombes, J.S.; Rowell, B.; Dodd, S.L.; Demirel, H.A.; Naito, H.; Shanely, R.A.; Powers, S.K. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 2002, 87, 272–277. [Google Scholar] [CrossRef]
- Nybo, L. CNS fatigue and prolonged exercise: Effect of glucose supplementation. Med. Sci. Sports Exerc. 2003, 35, 589–594. [Google Scholar]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 364741. [Google Scholar]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Hou, C.C.; Chuang, H.L.; Huang, C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014. [Google Scholar] [CrossRef]
- Syrotuik, D.G.; MacFadyen, K.L.; Harber, V.J.; Bell, G.J. Effect of elk velvet antler supplementation on the hormonal response to acute and chronic exercise in male and female rowers. Int. J. Sport Nutr. Exerc. MeTable 2005, 15, 366–385. [Google Scholar]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. MeTable 2007, 32, 1186–1190. [Google Scholar] [CrossRef]
- Suh, S.H.; Paik, I.Y.; Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar]
- Fujii, N.; Jessen, N.; Goodyear, L.J. AMP-activated protein kinase and the regulation of glucose transport. Am. J. Physiol. Endocrinol. MeTable 2006, 291, E867–E877. [Google Scholar] [CrossRef]
- Zhang, H.; Wanwimolruk, S.; Coville, P.F.; Schofield, J.C.; Williams, G.; Haines, S.R.; Suttie, J.M. Toxicological evaluation of New Zealand deer velvet powder. Part I: acute and subchronic oral toxicity studies in rats. Food Chem. Toxicol. 2000, 38, 985–990. [Google Scholar]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, C.-C.; Chen, Y.-M.; Kan, N.-W.; Chao, H.-L.; Ho, C.-S.; Hsu, M.-C. Cornu Cervi Pantotrichum Supplementation Improves Exercise Performance and Protects against Physical Fatigue in Mice. Molecules 2014, 19, 4669-4680. https://doi.org/10.3390/molecules19044669
Huang C-C, Chen Y-M, Kan N-W, Chao H-L, Ho C-S, Hsu M-C. Cornu Cervi Pantotrichum Supplementation Improves Exercise Performance and Protects against Physical Fatigue in Mice. Molecules. 2014; 19(4):4669-4680. https://doi.org/10.3390/molecules19044669
Chicago/Turabian StyleHuang, Chi-Chang, Yi-Ming Chen, Nai-Wen Kan, Hui-Ling Chao, Chin-Shan Ho, and Mei-Chich Hsu. 2014. "Cornu Cervi Pantotrichum Supplementation Improves Exercise Performance and Protects against Physical Fatigue in Mice" Molecules 19, no. 4: 4669-4680. https://doi.org/10.3390/molecules19044669
APA StyleHuang, C.-C., Chen, Y.-M., Kan, N.-W., Chao, H.-L., Ho, C.-S., & Hsu, M.-C. (2014). Cornu Cervi Pantotrichum Supplementation Improves Exercise Performance and Protects against Physical Fatigue in Mice. Molecules, 19(4), 4669-4680. https://doi.org/10.3390/molecules19044669