Comparative Study of Tributyltin Adsorption onto Mesoporous Silica Functionalized with Calix[4]arene, p-tert-Butylcalix[4]arene and p-Sulfonatocalix[4]arene
Abstract
:1. Introduction
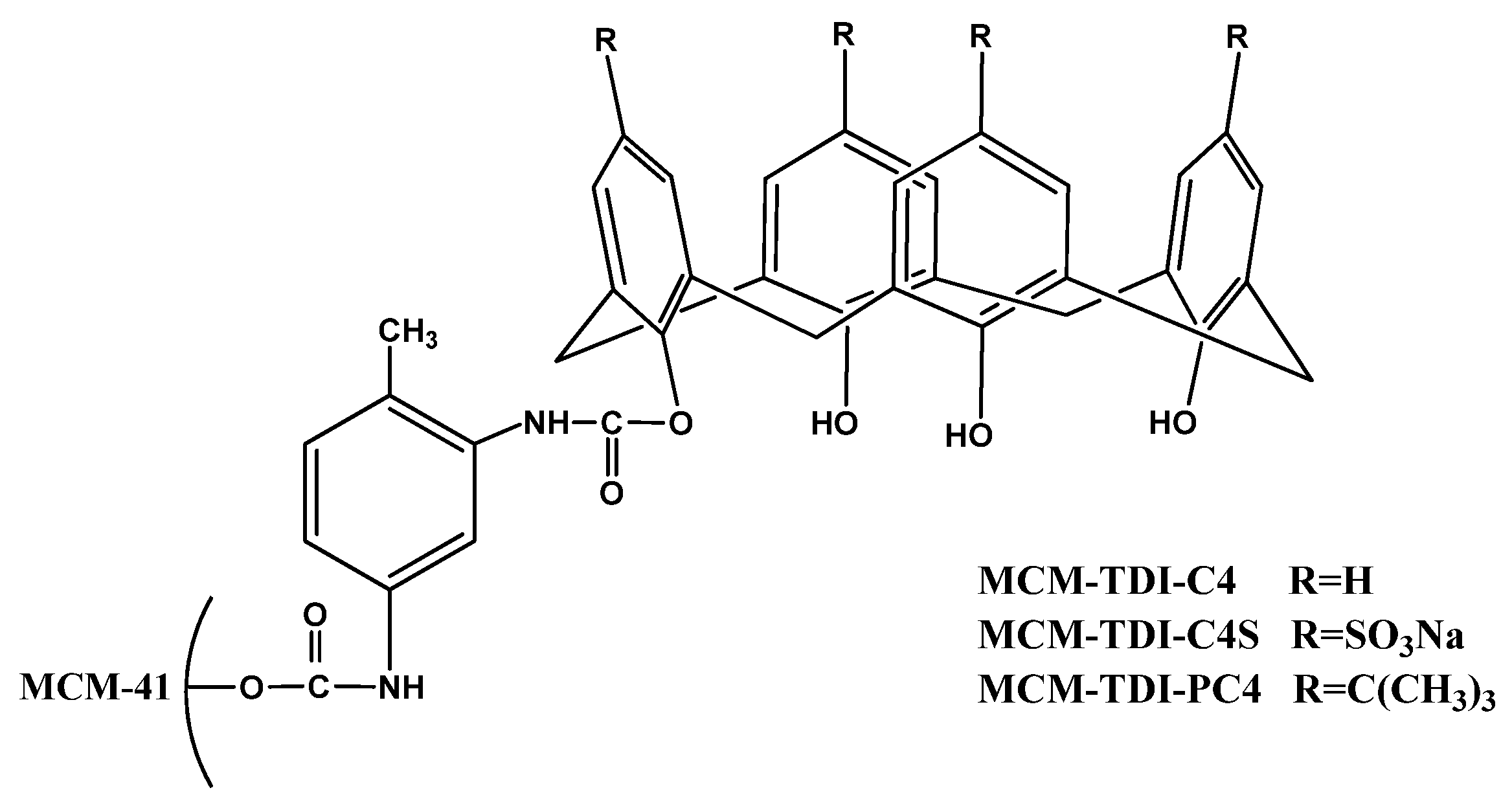
2. Results and Discussion
2.1. Characterization of Functionalized Mesoporous Silica with Calix[4]arene Derivatives
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR)

2.1.2. Nitrogen Adsorption-Desorption Measurements
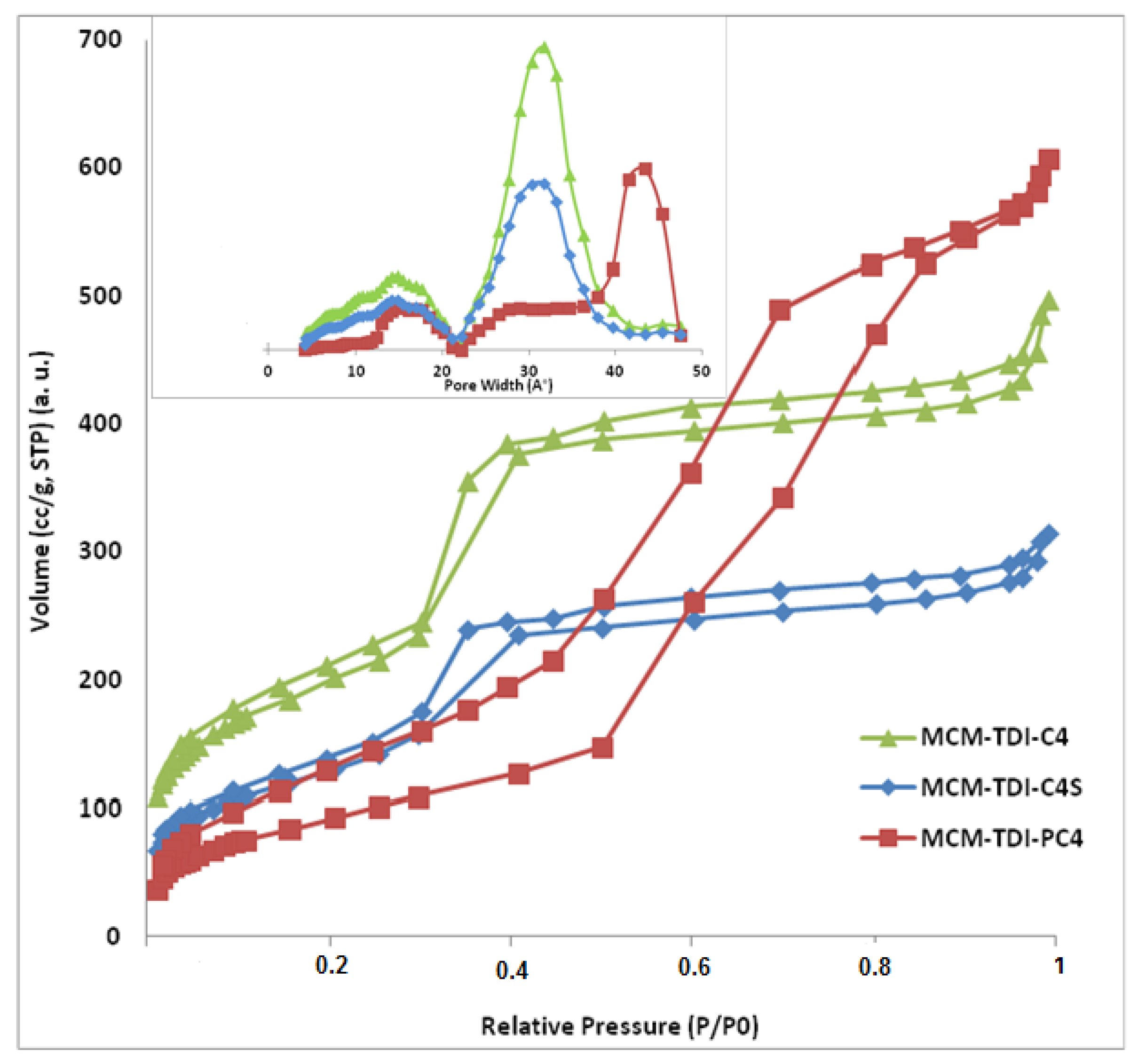
| Sample | SBET (m2/g) | V (cm3/g) | D (nm) |
|---|---|---|---|
| MCM-TDI-C4 | 733 | 0.67 | 3.6 |
| MCM-TDI-C4S | 452 | 0.43 | 3.8 |
| MCM-TDI-PC4 | 339 | 0.32 | 3.9 |
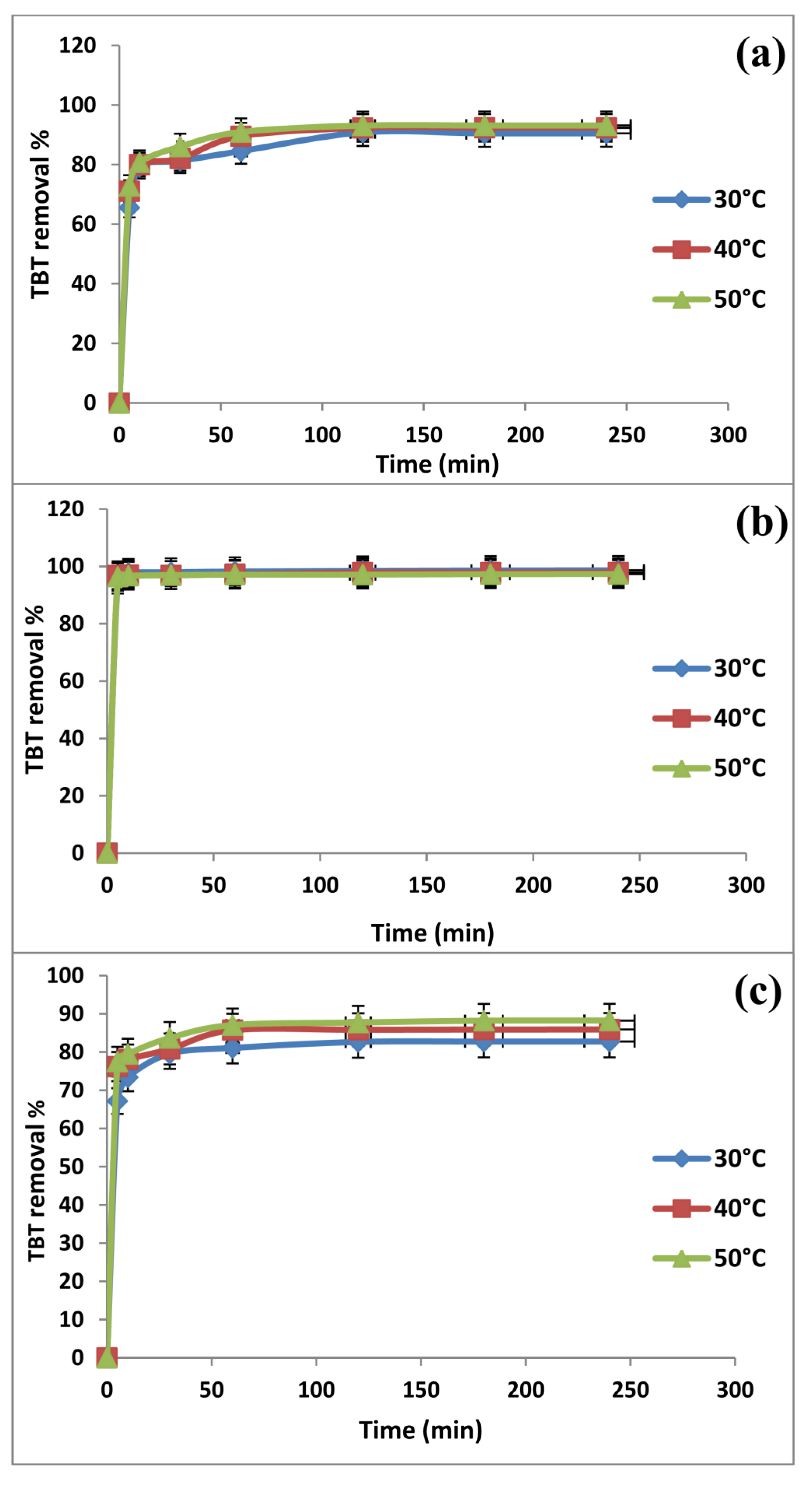
2.2. Effect of Contact Time
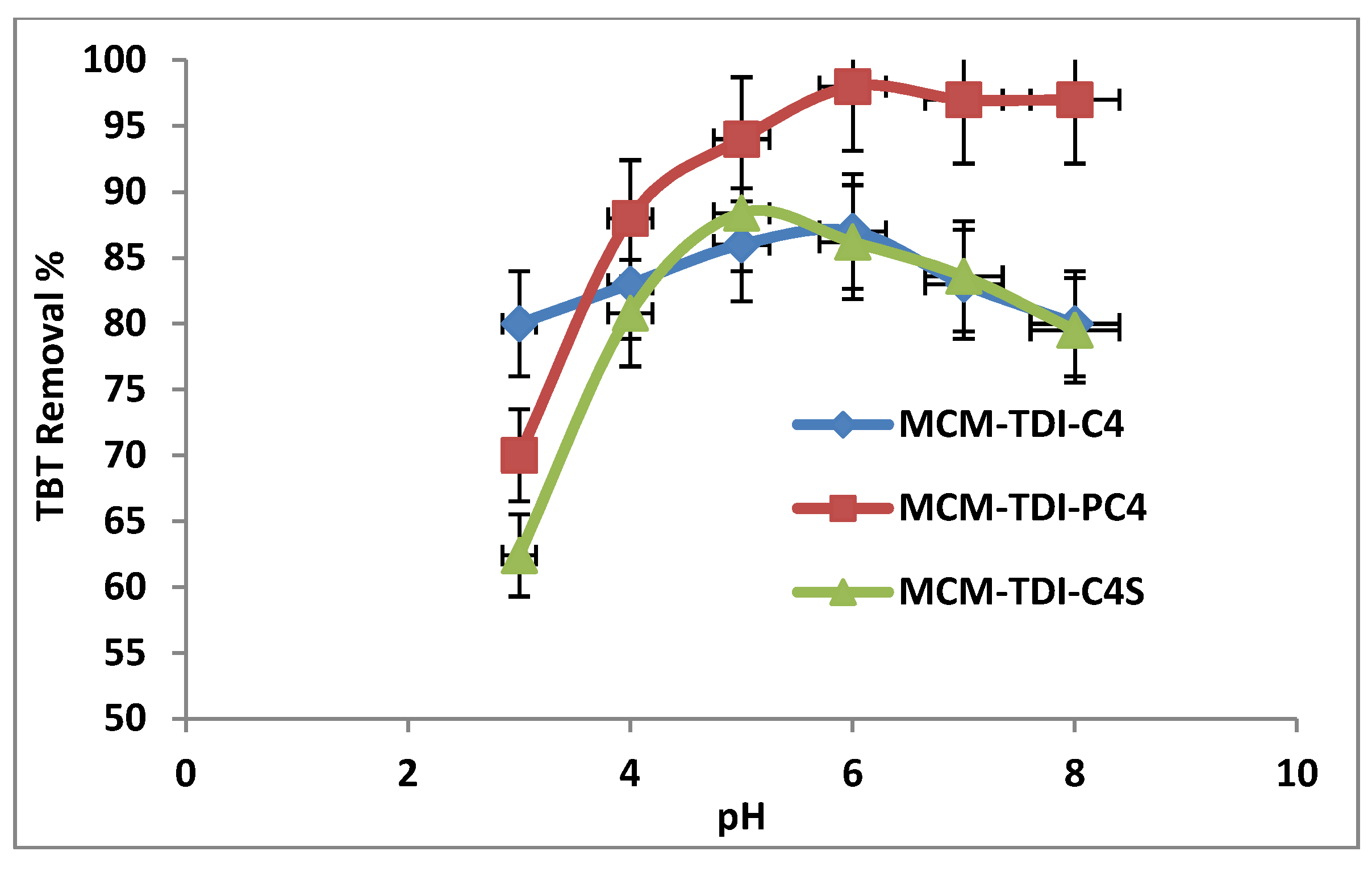
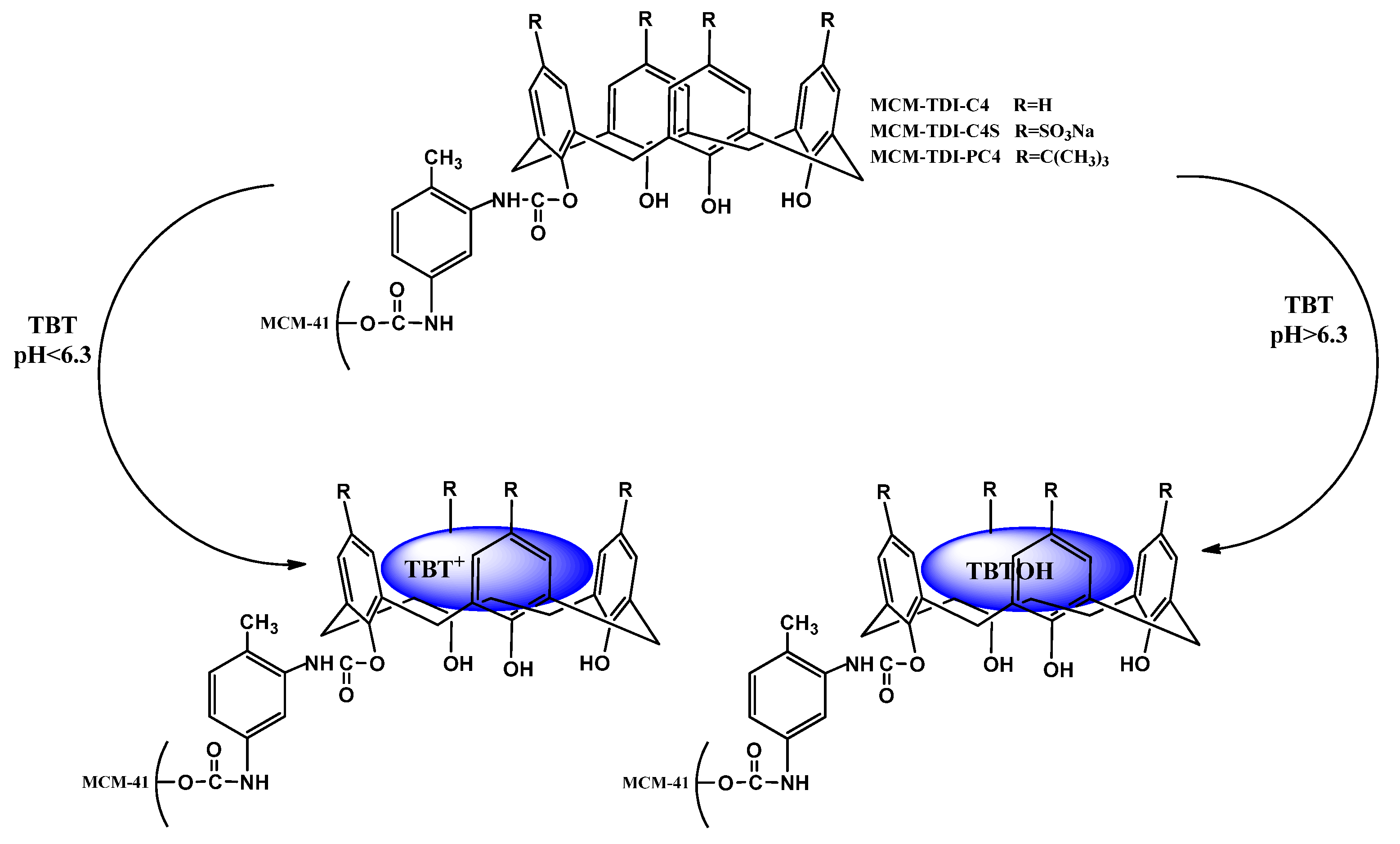
2.3. Influence of pH on Removal Efficiency of TBT
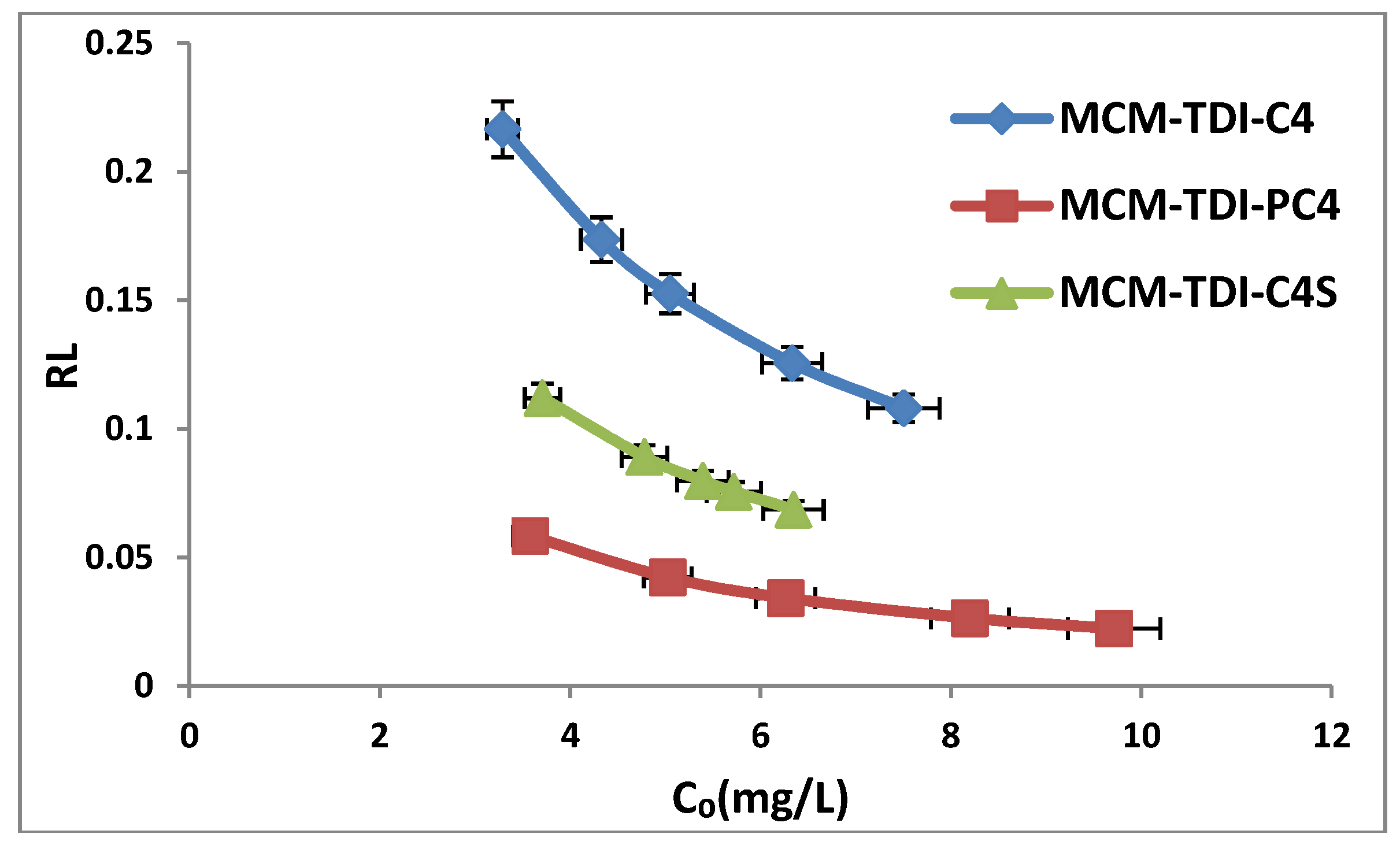
2.4. Effect of Initial TBT Concentration

2.5. Effect of Solution Temperature
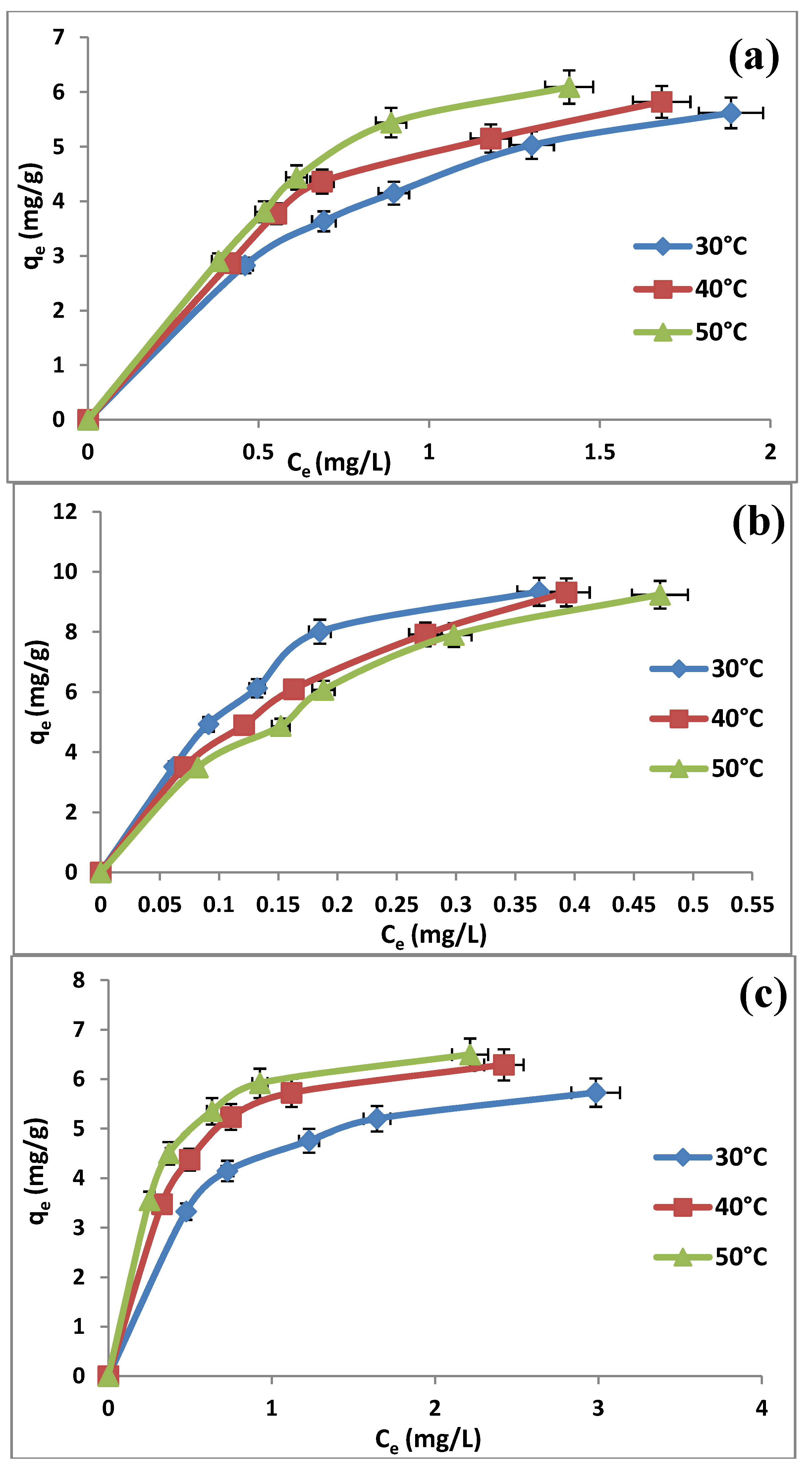
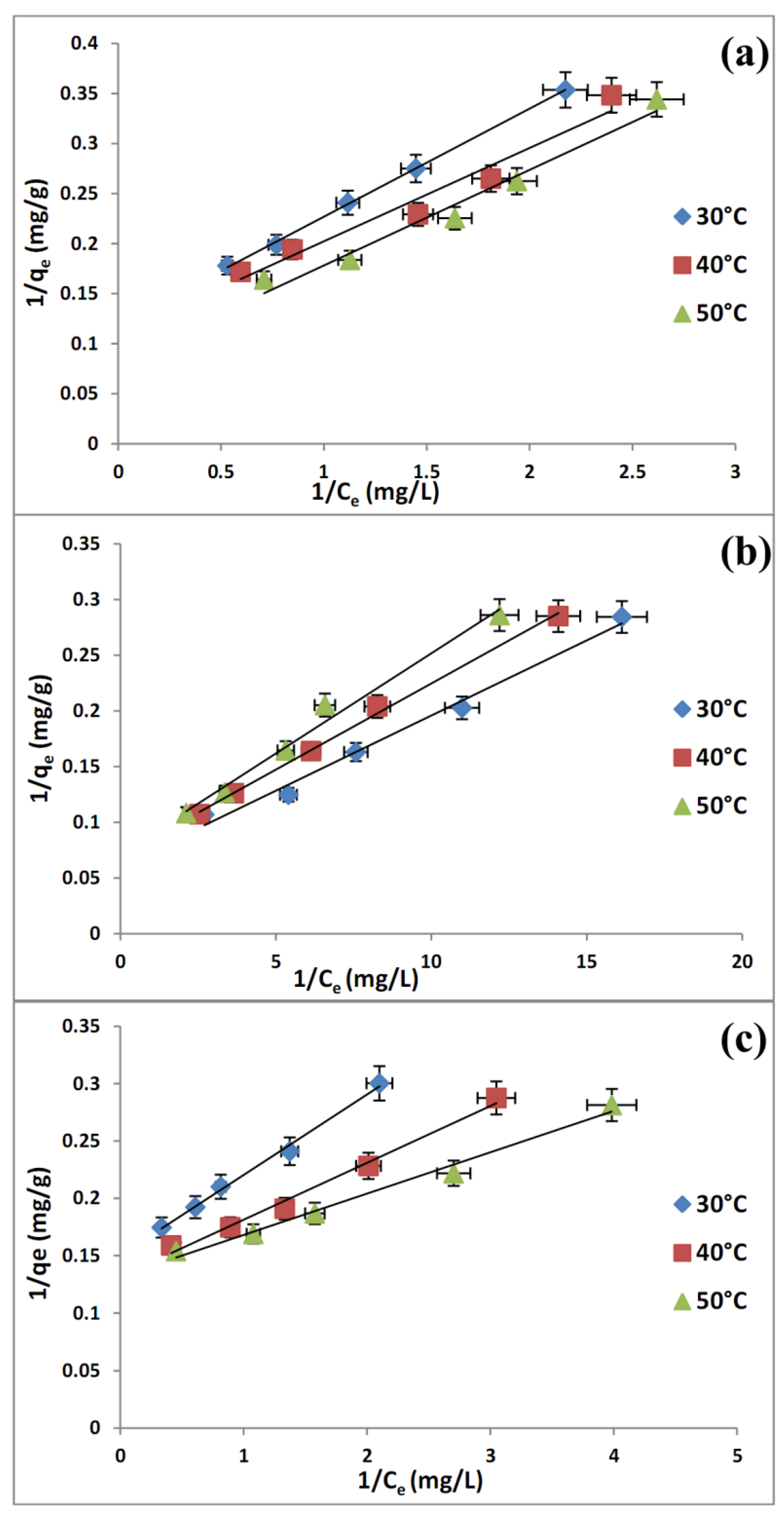
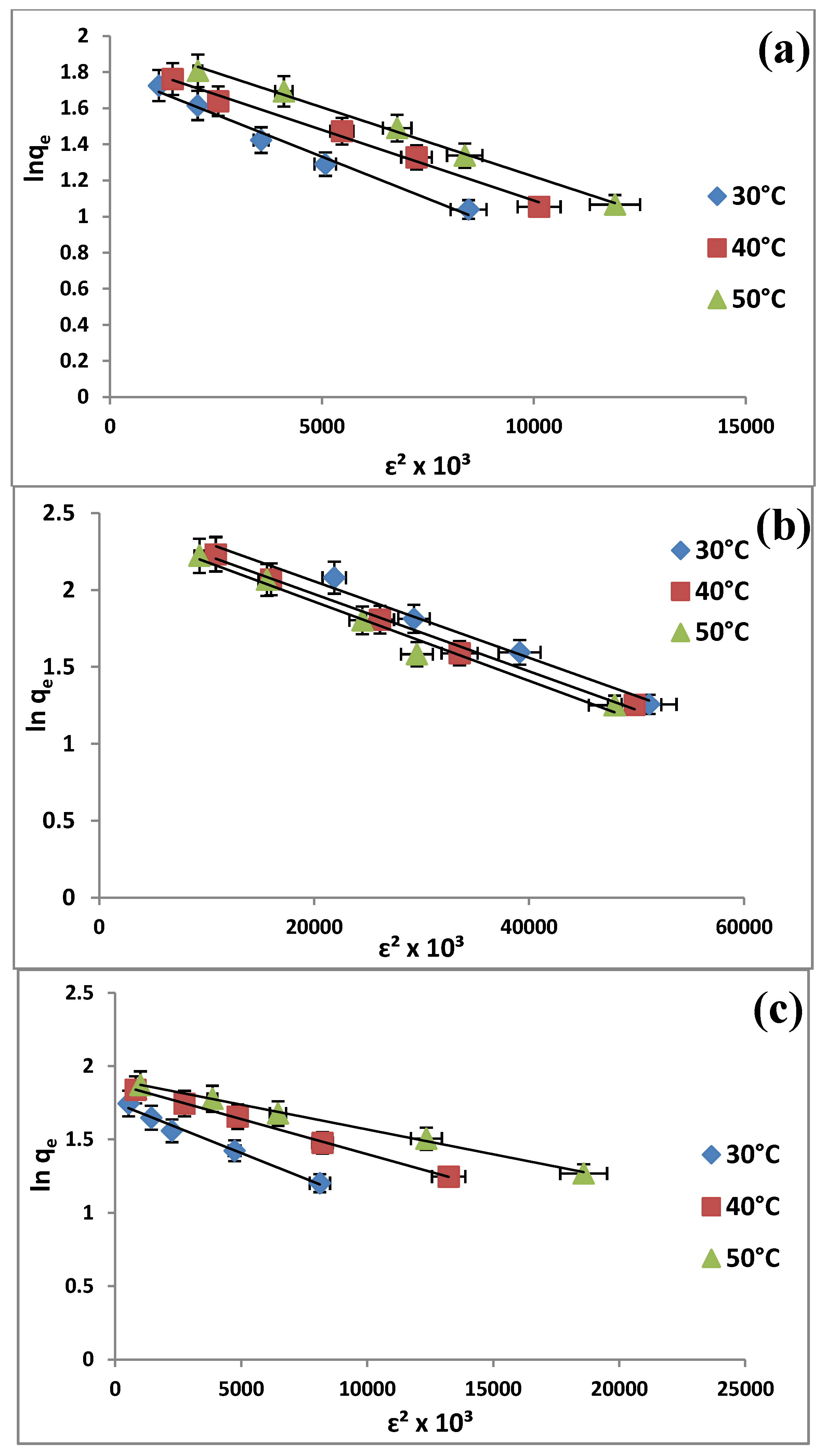
2.6. Adsorption Isotherms
| Adsorbent | Adsorption isotherm | Isotherm parameter | Temperature | ||
|---|---|---|---|---|---|
| 30 | 40 | 50 | |||
| (a) | Freundlich | KF (L/g) | 4.2815 | 4.7588 | 5.4225 |
| n | 2.0354 | 2.1322 | 1.7879 | ||
| R2 | 0.9845 | 0.9309 | 0.9307 | ||
| Langmuir | qm (mg/g) | 8.4104 | 9.1659 | 12.1212 | |
| KL (L/mg) | 1.1009 | 1.1693 | 0.8629 | ||
| R2 | 0.9992 | 0.9609 | 0.9719 | ||
| Temkin | AT | 2.0189 | 2.0038 | 2.4706 | |
| KT (L/mg) | 8.8305 | 11.3189 | 9.1593 | ||
| R2 | 0.997 | 0.9735 | 0.9704 | ||
| Dubinin-Radushkevitch | qd (mg/g) | 6.0376 | 6.4967 | 7.3075 | |
| β (mol2/kJ2) | 3.01 × 10−3 | 2.6 × 10−3 | 2.6× 10−3 | ||
| E (kJ/mol) | 12.8689 | 13.6495 | 13.6495 | ||
| R2 | 0.9823 | 0.9898 | 0.9957 | ||
| Redlich–Peterson | g | 0.9301 | 0.9689 | 0.8918 | |
| BR(L/mg) | 1.2792 | 1.3577 | 1.1522 | ||
| AR(L/g) | 10.0043 | 11.5734 | 11.9312 | ||
| R2 | 0.9986 | 0.9554 | 0.924 | ||
| Koble–Corrigan | p | 1.0176 | 2.1208 | 1.8879 | |
| AK | 9.4697 | 35.4609 | 30.8642 | ||
| BK | 1.1468 | 5.9397 | 4.4907 | ||
| R2 | 0.9992 | 0.9959 | 0.9986 | ||
| (b) | Freundlich | KF (L/g) | 17.8525 | 16.4626 | 15.0038 |
| n | 1.8205 | 1.7422 | 1.7382 | ||
| R2 | 0.9365 | 0.9928 | 0.9814 | ||
| Langmuir | qm (mg/g) | 16.4204 | 14.2653 | 13.9276 | |
| KL (L/mg) | 4.5111 | 4.5226 | 3.9888 | ||
| R2 | 0.9875 | 0.9975 | 0.9852 | ||
| Temkin | AT | 3.3637 | 3.4397 | 3.427 | |
| KT (L/mg) | 48.1922 | 36.8125 | 31.3889 | ||
| R2 | 0.9731 | 0.9945 | 0.9832 | ||
| Dubinin-Radushkevitch | qd(mg/g) | 12.8559 | 11.8829 | 11.4662 | |
| β (mol2/kJ2) | 6.7 × 10−4 | 1.01 × 10−3 | 1.01 × 10−3 | ||
| E (kJ/mol) | 27.2991 | 22.2896 | 22.2896 | ||
| R2 | 0.9861 | 0.9937 | 0.9788 | ||
| Redlich–Peterson | g | 0.998 | 0.9992 | 0.9883 | |
| BR (L/mg) | 4.8264 | 4.4406 | 3.8395 | ||
| AR (L/g) | 76.1627 | 64.2147 | 55.2684 | ||
| R2 | 0.9657 | 0.9943 | 0.971 | ||
| Koble–Corrigan | p | 1.383 | 0.849 | 0.742 | |
| AK | 238.0952 | 40.4858 | 25.4453 | ||
| BK | 21.0238 | 2.1053 | 0.9033 | ||
| R2 | 0.9958 | 0.9988 | 0.9897 | ||
| (c) | Freundlich | KF (L/g) | 4.3631 | 5.2396 | 5.6572 |
| n | 3.4507 | 3.4602 | 3.7439 | ||
| R2 | 0.9526 | 0.8943 | 0.8919 | ||
| Langmuir | qm (mg/g) | 6.6577 | 7.593 | 7.5757 | |
| KL (L/mg) | 2.1396 | 2.6552 | 3.6666 | ||
| R2 | 0.9952 | 0.9881 | 0.9875 | ||
| Temkin | AT | 1.2949 | 1.3988 | 1.3319 | |
| KT (L/mg) | 30.9003 | 45.1689 | 73.7734 | ||
| R2 | 0.9801 | 0.9367 | 0.9366 | ||
| Dubinin-Radushkevitch | qd (mg/g) | 5.7552 | 6.5463 | 6.7282 | |
| β (mol2/kJ2) | 2.3 × 10−3 | 1.6 × 10−3 | 1.0 × 10−3 | ||
| E (kJ/mol) | 14.5919 | 17.2655 | 22.2896 | ||
| R2 | 0.9866 | 0.9993 | 0.9979 | ||
| Redlich–Peterson | g | 0.9893 | 0.999 | 0.9937 | |
| BR (L/mg) | 2.2506 | 2.8092 | 3.9357 | ||
| AR (L/g) | 14.7273 | 20.9121 | 29.1639 | ||
| R2 | 0.9989 | 0.9968 | 0.9972 | ||
| Koble–Corrigan | p | 1.1583 | 1.4241 | 1.4278 | |
| AK | 16.8919 | 35.5872 | 54.6448 | ||
| BK | 2.6892 | 5.3594 | 8.1366 | ||
| R2 | 0.9964 | 0.9993 | 0.9984 |
| T (°C) | Thermodynamic parameters | |||
|---|---|---|---|---|
| ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) | ||
| 30 | −4.5779 | 8.669 | 43.7192 | |
| MCM-TDI-C4 | 40 | −5.0151 | ||
| 50 | −5.4523 | |||
| 30 | −10.0418 | −21.3952 | −37.4704 | |
| MCM-TDI-PC4 | 40 | −9.6671 | ||
| 50 | −9.2924 | |||
| 30 | −36.5595 | 30.8066 | 120.7609 | |
| MCM-TDI-C4S | 40 | −37.7671 | ||
| 50 | −38.9747 | |||
2.7. Adsorption Thermodynamic

3. Experimental
3.1. Materials and Instrumentation
3.2. Preparation of Functionalized Mesoporous Silica
3.3. Adsorption Isotherms

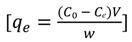
3.4. Mathematical Modelling
3.4.1. Isotherm Models
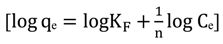
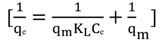
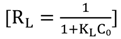
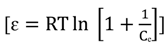
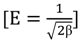
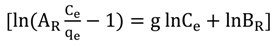
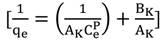
3.4.2. Adsorption Thermodynamic
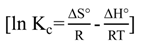
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoch, M. Organotin compounds in the enviroment — an overreview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Alahmadi, S. Organotin speciation analysis based on liquid or gas chromatography. Asian J. Chem. 2011, 23, 3787–3791. [Google Scholar]
- Goldberg, E.D. Tbt: An environmental dilemma. Environment: Science and Policy for Sustainable Development 1986, 28, 17–44. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (tbt)-contaminated marine environment. A review. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef]
- Bryan, G.W.; Gibbs, P.E. Impact of low concentrations of tributyltin (tbt) on marine organisms: A review. In Metal Toxicology: Concepts and Applications; Newman, M.C., McIntosh, A.W., Eds.; Lewis Publishers: Chelsea, MI, USA, 1991; pp. 323–361. [Google Scholar]
- Barroso, C.M.; Moreira, M.H. Spatial and temporal changes of tbt pollution along the portuguese coast: Inefficacy of the eec directive 89/677. Mar. Pollut. Bull. 2002, 44, 480–486. [Google Scholar] [CrossRef]
- Chong, A.M.Y. Toxicity of Organotin, and Its Biosorption and Biodegradation by Microalgae. Ph.D. Thesis, City University of Hong Kong, Hong Kong, 2001. [Google Scholar]
- Tam, N.F.Y.; Chong, A.M.Y.; Wong, Y.S. Removal of Tributyltin (tbt) by Free and Immobilized Chlorella Sorokiniana. In Proceedings of the 4th World Water Congress and Exhibition, Marrakech, Morocco, 19–24th September 2004; HKUST Scholarly Publications: Marrakech, Morocco, 2004. [Google Scholar]
- Tam, N.F.Y.; Chong, A.; Wong, Y.S. Removal of Tributyltin (tbt) from Wastewater by Microalgae; WIT Press: Boston, MA, USA, 2003; pp. 261–271. [Google Scholar]
- Luan, T.G.; Jin, J.; Chan, S.M.N.; Wong, Y.S.; Tam, N.F.Y. Biosorption and biodegradation of tributyltin (tbt) by alginate immobilized chlorella vulgaris beads in several treatment cycles. Process Biochem. 2006, 41, 1560–1565. [Google Scholar] [CrossRef]
- Hoch, M.; Alonso-Azcarate, J.; Lischick, M. Assessment of adsorption behavior of dibutyltin (dbt) to clay-rich sediments in comparison to the highly toxic tributyltin (tbt). Environ. Pollut. 2003, 123, 217–227. [Google Scholar] [CrossRef]
- Burton, E.D.; Phillips, I.R.; Hawker, D.W. Sorption and desorption behavior of tributyltin with natural sediments. Environ. Sci. Technol. 2004, 38, 6694–6700. [Google Scholar] [CrossRef]
- Brändli, R.C.; Breedveld, G.D.; Cornelissen, G. Tributyltin sorption to marine sedimentary black carbon and to amended activated carbon. Environ. Toxicol. Chem. 2009, 28, 503–508. [Google Scholar]
- Hoch, M.; Weerasooriya, R. Modeling interactions at the tributyltin–kaolinite interface. Chemosphere 2005, 59, 743–752. [Google Scholar] [CrossRef]
- Weidenhaupt, A.; Arnold, C.; Müller, S.R.; Haderlein, S.B.; Schwarzenbach, R.P. Sorption of organotin biocides to mineral surfaces. Environ. Sci. Technol. 1997, 31, 2603–2609. [Google Scholar] [CrossRef]
- Arnold, C.G.; Ciani, A.; Müller, S.R.; Amirbahman, A.; Schwarzenbach, R.P. Association of triorganotin compounds with dissolved humic acids. Environ. Sci. Technol. 1998, 32, 2976–2983. [Google Scholar] [CrossRef]
- Fang, L.; Borggaard, O.K.; Marcussen, H.; Holm, P.E.; Bruun Hansen, H.C. The ph-dependent adsorption of tributyltin to charcoals and soot. Environ. Pollut. 2010, 158, 3642–3649. [Google Scholar] [CrossRef]
- Feber, K. Benzidine and Related Diaminobiphenyls; Encyclopedia of Chemical Technology: New York, NY, USA, 1978; Volume 3, p. 772. [Google Scholar]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Qureshi, I.; Qazi, M.A.; Bhatti, A.A.; Memon, S.; Sirajuddin; Yilmaz, M. An efficient calix[4]arene appended resin for the removal of arsenic. Desalination 2011, 278, 98–104. [Google Scholar] [CrossRef]
- Aksoy, T.; Erdemir, S.; Yildiz, H.B.; Yilmaz, M. Novel water-soluble calix[4,6]arene appended magnetic nanoparticles for the removal of the carcinogenic aromatic amines. Water Air Soil Pollut. 2012, 223, 4129–4139. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Akoz, E.; Memon, S.; Yilmaz, M. Synthesis of amino-substituted p-tert-butylcalix[4]arene for the removal of chicago sky blue and tropaeolin 000 azo dyes from aqueous environment. Water Air Soil Pollut. 2013, 224, 1–9. [Google Scholar]
- Ertul, Ş.; Bayrakci, M.; Yilmaz, M. Removal of chromate and phosphate anion from aqueous solutions using calix[4]aren receptors containing proton switchable units. J. Hazard. Mater. 2010, 181, 1059–1065. [Google Scholar] [CrossRef]
- Furer, V.; Borisoglebskaya, E.; Zverev, V.; Kovalenko, V. Dft and ir spectroscopic analysis of p-tert-butylthiacalix[4]arene. Spectrochim. Acta Part A 2006, 63, 207–212. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, C.; Ji, Y.; Nie, R.; Zhou, P.; Zhang, H. Preparation, characterization and application of p-tert-butyl-calix[4]arene-sba-15 mesoporous silica molecular sieves. J. Hazard. Mater. 2010, 178, 680–685. [Google Scholar] [CrossRef]
- Su, B.-L.; Ma, X.-C.; Xu, F.; Chen, L.-H.; Fu, Z.-Y.; Moniotte, N.; Maamar, S.B.; Lamartine, R.; Vocanson, F. Sba-15 mesoporous silica coated with macrocyclic calix[4]arene derivatives: Solid extraction phases for heavy transition metal ions. J. Colloid Interface Sci. 2011, 360, 86–92. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Hong, X.; Zhu, Y. Dicyano-functionalized mcm-41 anchored-palladium complexes as recoverable catalysts for heck reaction. J. Mol. Catal. A: Chem. 2004, 210, 143–148. [Google Scholar] [CrossRef]
- Broekhoff, J.C.P. Mesopore Determination From Nitrogen Sorption Isotherms: Fundamentals, Scope, Limitations. In Studies in Surface Science and Catalysis; Delmon, B., Grange, P., Jacobs, P., Poncelet, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1979; Volume 3, pp. 663–684. [Google Scholar]
- Lim, M.H.; Stein, A. Comparative studies of grafting and direct syntheses of inorganic-organic hybrid mesoporous materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]
- Caps, V.; Tsang, S.C. Heterogenisation of os species on mcm-41 structure for epoxidation of trans-stilbene. Appl. Catal. A 2003, 248, 19–31. [Google Scholar] [CrossRef]
- Sakthivel, A.; Hijazi, A.K.; Hanzlik, M.; Chiang, A.S.T.; Kühn, F.E. Heterogenization of [cu(ncch3)6][b(c6f5)4]2 and its application in catalytic olefin aziridination. Appl. Catal. A 2005, 294, 161–167. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Unger, M.A.; MacIntyre, W.G.; Huggett, R.J. Sorption behavior of tributyltin on estuarine and freshwater sediments. Environ. Toxicol. Chem. 1988, 7, 907–915. [Google Scholar]
- Langston, W.; Pope, N. Determinants of tbt adsorption and desorption in estuarine sediments. Mar. Pollut. Bull. 1995, 31, 32–43. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interface Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef]
- Thompson, A.B.; Cope, S.J.; Swift, T.D.; Notestein, J.M. Adsorption of n-butanol from dilute aqueous solution with grafted calixarenes. Langmuir 2011, 27, 11990–11998. [Google Scholar] [CrossRef]
- Idris, S.A.; Alotaibi, K.M.; Peshkur, T.A.; Anderson, P.; Morris, M.; Gibson, L.T. Adsorption kinetic study: Effect of adsorbent pore size distribution on the rate of cr (vi) uptake. Microporous Mesoporous Mater. 2013, 165, 99–105. [Google Scholar]
- Arnold, C.G.; Weidenhaupt, A.; David, M.M.; Müller, S.R.; Haderlein, S.B.; Schwarzenbach, R.P. Aqueous speciation and 1-octanol-water partitioning of tributyl-and triphenyltin: Effect of ph and ion composition. Environ. Sci. Technol. 1997, 31, 2596–2602. [Google Scholar] [CrossRef]
- Hoch, M.; Alonso‐Azcarate, J.; Lischick, M. Adsorption behavior of toxic tributyltin to clay-rich sediments under various environmental conditions. Environ. Toxicol. Chem. 2002, 21, 1390–1397. [Google Scholar]
- Sayin, S.; Yilmaz, M. Synthesis of a new calixarene derivative and its immobilization onto magnetic nanoparticle surfaces for excellent extractants toward cr(vi), as(v), and u(vi). J. Chem. Eng. Data. 2011, 56, 2020–2029. [Google Scholar] [CrossRef]
- Memon, S.; Memon, N.; Latif, Y. An efficient calix[4]arene based silica sorbent for the removal of endosulfan from water. J. Hazard. Mater. 2011, 186, 1696–1703. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Solangi, I.B.; Sherazi, S.T.H.; Memon, S. A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 2011, 268, 83–89. [Google Scholar] [CrossRef]
- Durmaz, M.; Alpaydin, S.; Sirit, A.; Yilmaz, M. Chiral schiff base derivatives of calix[4]arene: Synthesis and complexation studies with chiral and achiral amines. Tetrahedron: Asymmetry 2006, 17, 2322–2327. [Google Scholar] [CrossRef]
- Cotoruelo, L.M.; Marqués, M.D.; Díaz, F.J.; Rodríguez-Mirasol, J.; Rodríguez, J.J.; Cordero, T. Adsorbent ability of lignin-based activated carbons for the removal of p-nitrophenol from aqueous solutions. Chem. Eng. J. 2012, 184, 176–183. [Google Scholar] [CrossRef]
- Tan, I.; Ahmad, A.L.; Hameed, B. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Ramu, A.; Kannan, N.; Srivathsan, S.A. Adsorption of carboxylic acids on fly ash and activated carbon. Indian J. Environ. Health 1992, 34, 192–199. [Google Scholar]
- Al Mardini, F.; Legube, B. Effect of the adsorbate (bromacil) equilibrium concentration in water on its adsorption on powdered activated carbon. Part 1. Equilibrium parameters. J. Hazard. Mater. 2009, 170, 744–753. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A. Equilibrium and kinetic studies on the adsorption of dark colored compounds from apple juice using adsorbent resin. J. Food Eng. 2002, 53, 221–227. [Google Scholar] [CrossRef]
- Makha, M.; Raston, C.L. Direct synthesis of calixarenes with extended arms: P-phenylcalix[4,5,6,8]arenes and their water-soluble sulfonated derivatives. Tetrahedron Lett. 2001, 42, 6215–6217. [Google Scholar] [CrossRef]
- Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and characterization of mesoporous silica functionalized with calix [4] arene derivatives. Int. J. Mol. Sci. 2012, 13, 13726–13736. [Google Scholar] [CrossRef]
- Yang, C.-H. Statistical mechanical study on the freundlich isotherm equation. J. Colloid Interface Sci. 1998, 208, 379–387. [Google Scholar] [CrossRef]
- Chang, C.Y.; Tsai, W.T.; Ing, C.H.; Chang, C.F. Adsorption of polyethylene glycol (peg) from aqueous solution onto hydrophobic zeolite. J. Colloid Interface Sci. 2003, 260, 273–279. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hall, K.; Eagleton, L.; Acrivos, A.; Vermeulen, T. Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical basis for the dubinin-radushkevitch (dr) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(ii) ions onto sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Batch and column studies of biosorption of heavy metals by caulerpa lentillifera. Bioresour. Technol. 2008, 99, 2766–2777. [Google Scholar] [CrossRef]
- Wong, Y.; Szeto, Y.; Cheung, W.; McKay, G. Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Koble, R.A.; Corrigan, T.E. Adsorption isotherms for pure hydrocarbons. Ind. Eng. Chem. 1952, 44, 383–387. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alahmadi, S.; Mohamad, S.; Maah, M.J. Comparative Study of Tributyltin Adsorption onto Mesoporous Silica Functionalized with Calix[4]arene, p-tert-Butylcalix[4]arene and p-Sulfonatocalix[4]arene. Molecules 2014, 19, 4524-4547. https://doi.org/10.3390/molecules19044524
Alahmadi S, Mohamad S, Maah MJ. Comparative Study of Tributyltin Adsorption onto Mesoporous Silica Functionalized with Calix[4]arene, p-tert-Butylcalix[4]arene and p-Sulfonatocalix[4]arene. Molecules. 2014; 19(4):4524-4547. https://doi.org/10.3390/molecules19044524
Chicago/Turabian StyleAlahmadi, Sana, Sharifah Mohamad, and Mohd Jamil Maah. 2014. "Comparative Study of Tributyltin Adsorption onto Mesoporous Silica Functionalized with Calix[4]arene, p-tert-Butylcalix[4]arene and p-Sulfonatocalix[4]arene" Molecules 19, no. 4: 4524-4547. https://doi.org/10.3390/molecules19044524




