Highly Efficient and Diastereoselective Synthesis of New Pyrazolylpyrrolizine and Pyrazolylpyrrolidine Derivates by a Three-Component Domino Process
Abstract
:1. Introduction

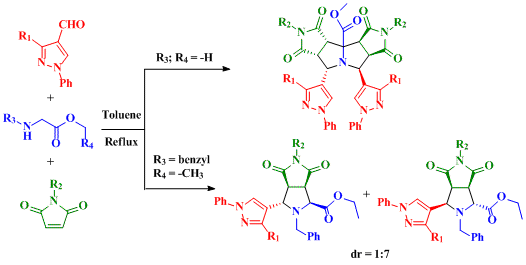
2. Results and Discussion
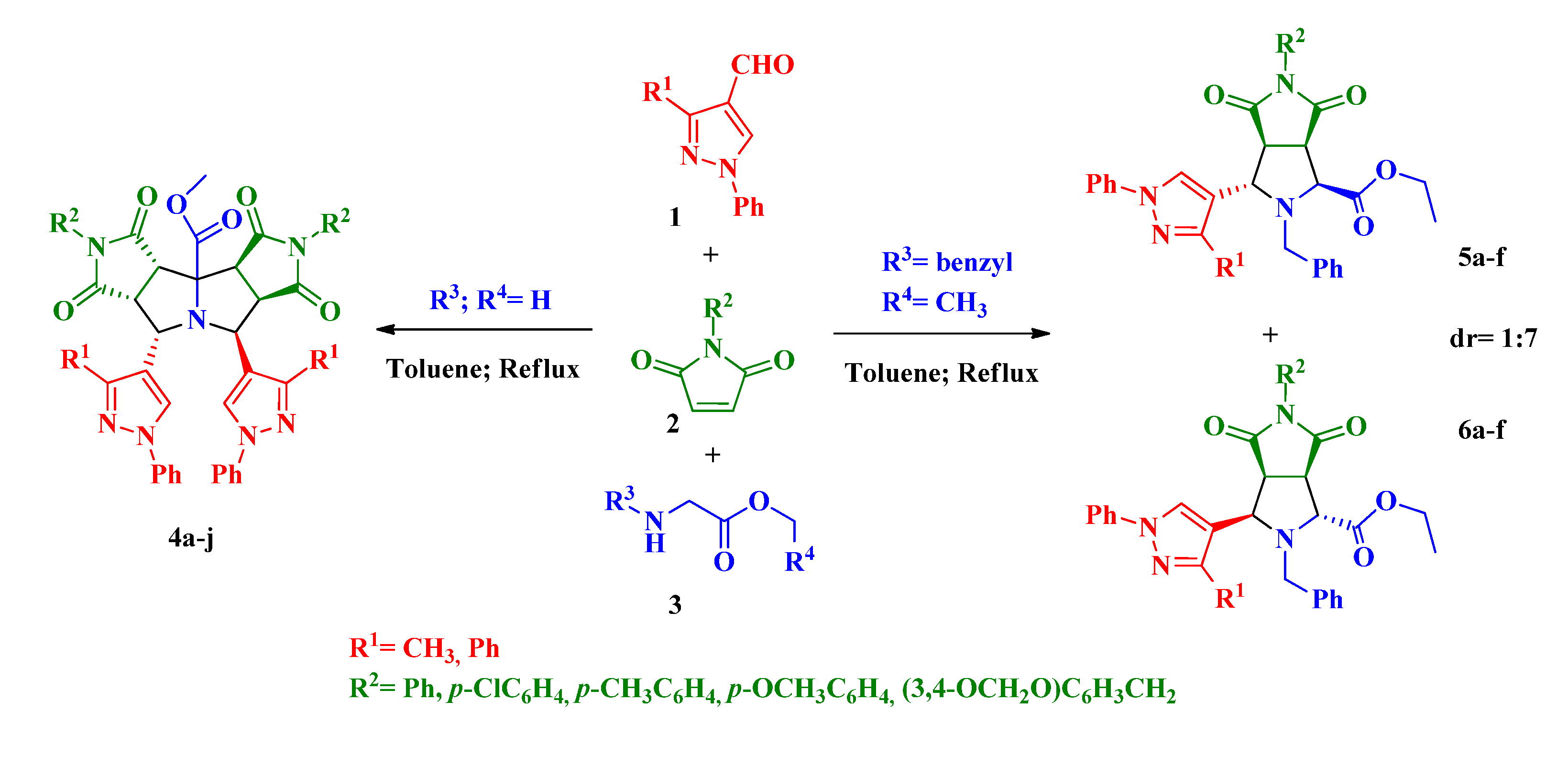
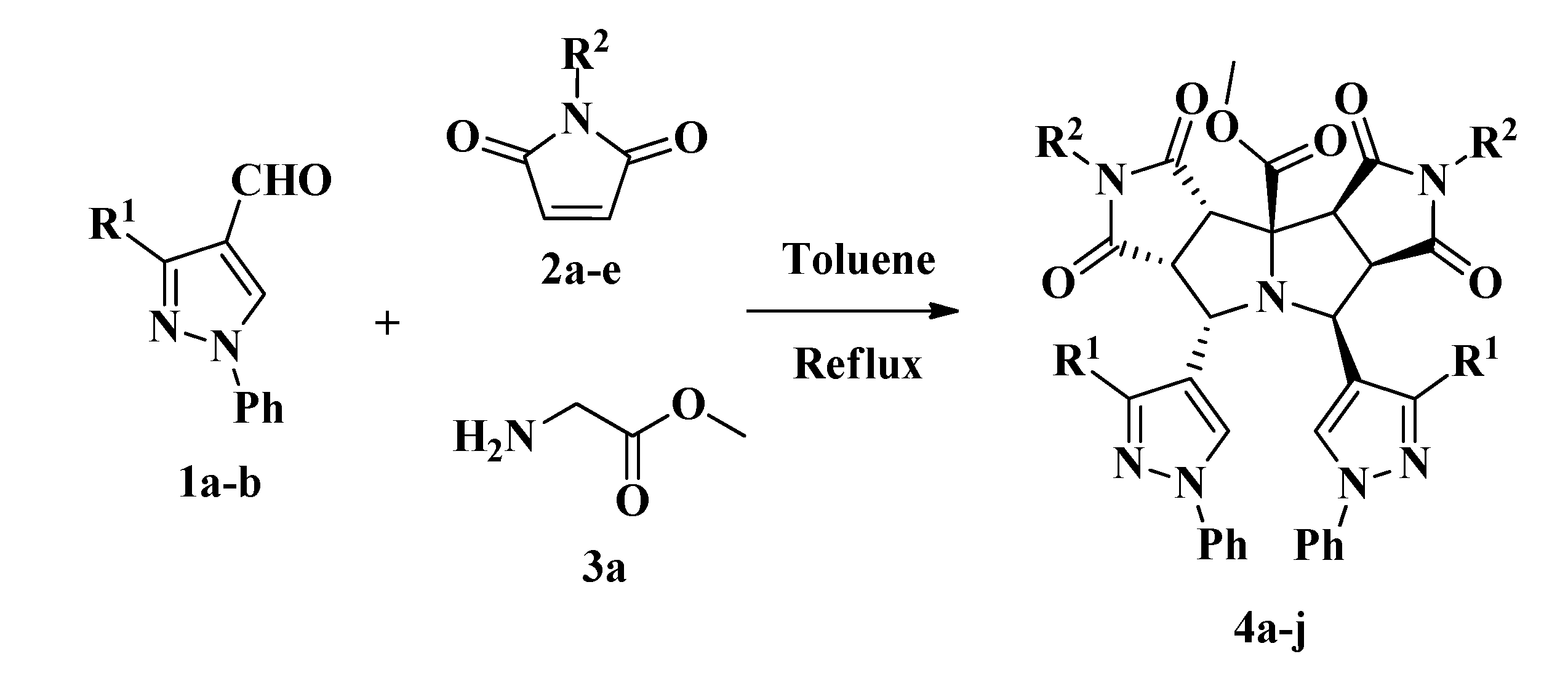
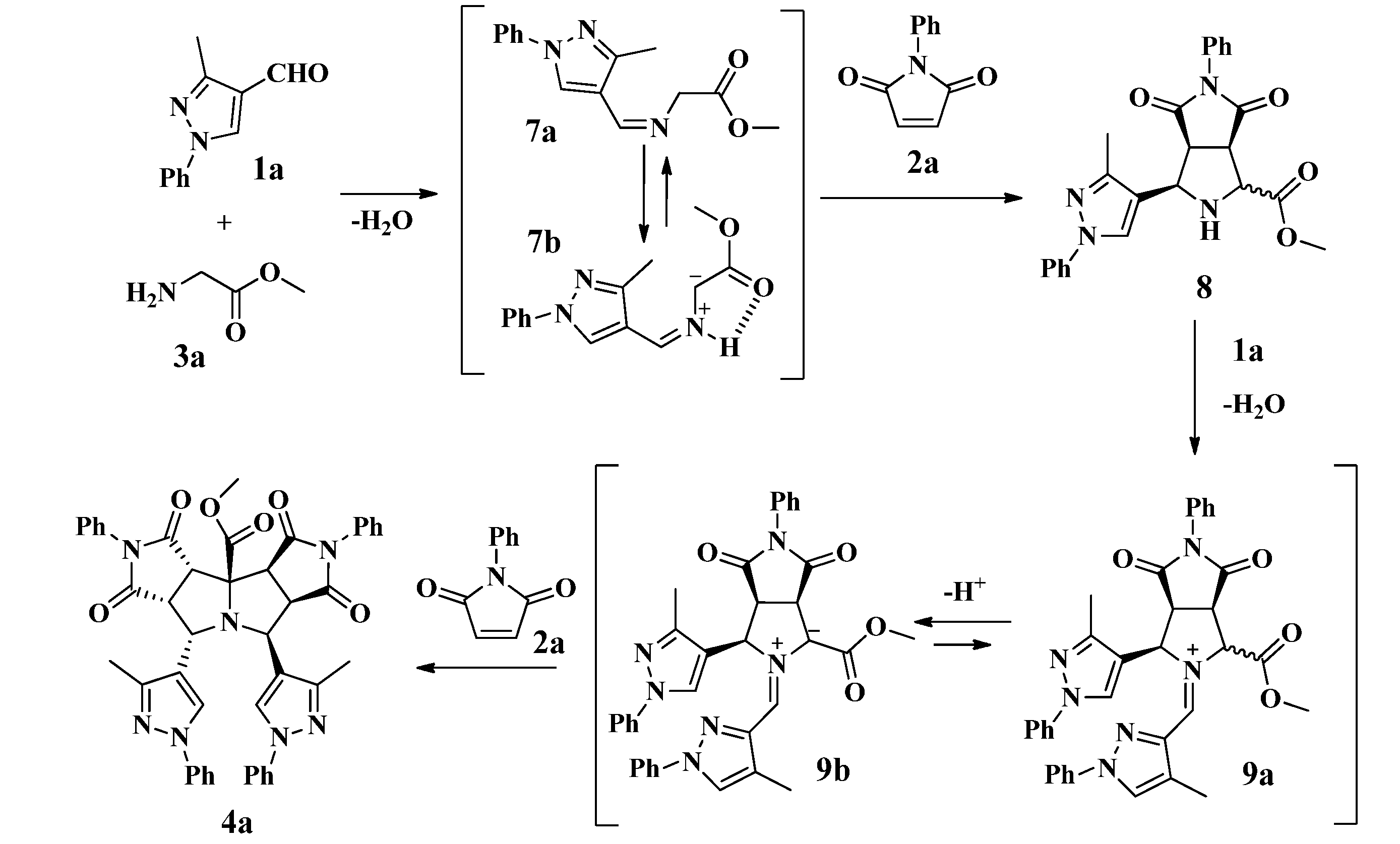
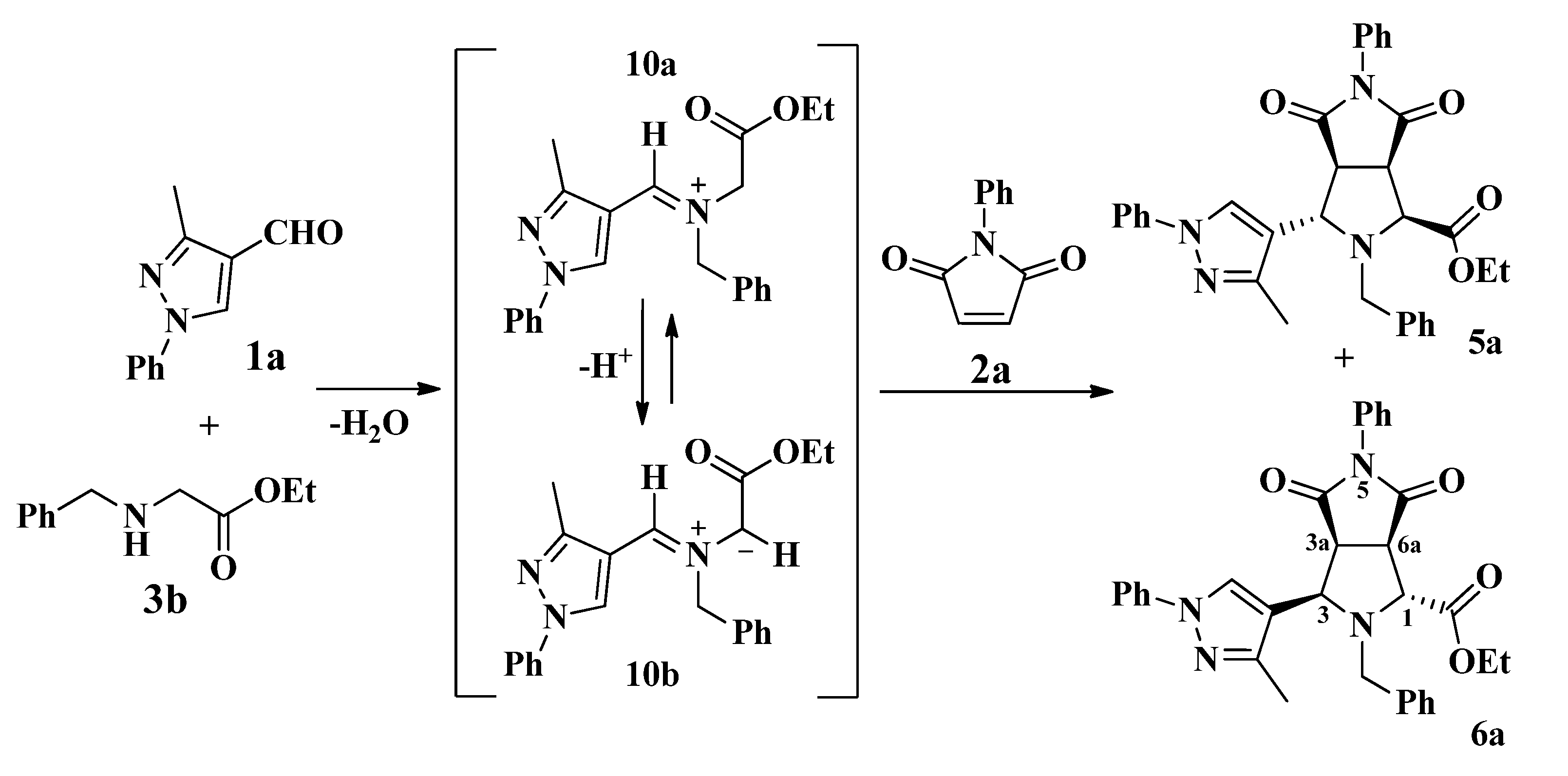
2.1. Synthesis of Pyrazolyldipyrrolo[3,4-a:3',4'-f]Pyrrolizines from Glycine Methyl Ester
| Entry | R1 | R2 | Yield (%) |
|---|---|---|---|
| 4a | -CH3 | C6H5 | 96 |
| 4b | -CH3 | p-ClC6H4 | 73 |
| 4c | -CH3 | p-CH3C6H4 | 84 |
| 4d | -CH3 | p-CH3OC6H4 | 90 |
| 4e | -CH3 | (3,4-OCH2O)C6H3CH2 | 78 |
| 4f | C6H5 | C6H5 | 81 |
| 4g | C6H5 | p-ClC6H4 | 75 |
| 4h | C6H5 | p-CH3C6H4 | 93 |
| 4i | C6H5 | p-CH3OC6H4 | 94 |
| 4j | C6H5 | (3,4-OCH2O)C6H3CH2 | 75 |

2.2. Synthesis of Pyrazolylpyrrolo[3,4-c]Pyrroles from N-benzylglicine Ethyl Ester

| Entry | R1 | R2 | d:r a (5:6) | Yield (%) 5 + 6 |
|---|---|---|---|---|
| 1 | -CH3 | C6H5 | 1:7 | 95 |
| 2 | -CH3 | p-ClC6H4 | 1:7 | 82 |
| 3 | -CH3 | p-CH3OC6H4 | 1:7 | 96 |
| 4 | C6H5 | C6H5 | 1:7 | 75 |
| 5 | C6H5 | p-ClC6H4 | 1:7 | 72 |
| 6 | C6H5 | p-CH3OC6H4 | 1:7 | 90 |
2.3. Theoretical Calculations


| Bond | X-Ray Length (Å) | Calculated Length (Å) | Angle Atoms | X-Ray Angle (°) | Calculated Angle (°) |
|---|---|---|---|---|---|
| C1-O1 | 1.210(2) | 1.213 | O1-C1-N2 | 124.61(17) | 125.02 |
| C1-N2 | 1.393(2) | 1.398 | O1-C1-C8a | 128.00(16) | 127.64 |
| C1-C8a | 1.508(3) | 1.512 | N2-C1-C8a | 107.36(14) | 110.73 |
| N2-C3 | 1.399(2) | 1.409 | C1-N2-C3 | 112.48(15) | 114.34 |
| C3-O3 | 1.209(2) | 1.211 | C1-N2-C21 | 122.84(14) | 120.21 |
| C3-C3a | 1.529(3) | 1.534 | C3-N2-C21 | 124.47(15) | 124.88 |
| C3c-C4 | 1.507(3) | 1.511 | O3-C3-N2 | 124.53(17) | 123.00 |
3. Experimental
3.1. General Information
3.2. Synthesis and Characterization Data for Pyrazolyldipyrrolo[3,4-a:3',4'-f]Pyrrolizines 4a–j
General Synthetic Procedure
3.3. Synthesis and Characterization Data for Pyrazolylpyrrolo[3,4-c]Pyrroles 5a–f and 6a–f.
General Synthetic Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sridharan, V.; Perumal, P.; Avendaño, C.; Menéndez, C. A New Three-Component Domino Synthesis of 1,4-Dihydropyridines. Tetrahedron 2007, 63, 4407–4413. [Google Scholar] [CrossRef]
- Ugi, I. Recent Progress in the Chemistry of Multicomponent Reactions. Pure Appl. Chem. 2001, 73, 187–191. [Google Scholar]
- Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Lieby-Muller, F.; Constantieux, T.; Rodriguez, J. Highly Efficient Access to Original Polycyclic Pyrrolopiperazine Scaffolds by a Three-Component Reaction with 1,3-Dicarbonyls. Synlett 2007, 8, 1323–1325. [Google Scholar]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V. A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Ramón, D.J.; Yus, M. Asymmetric Multicomponent Reactions (AMCRs): The New Frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar] [CrossRef]
- Ayerbe, M.; Arrieta, A.; Cossío, F. Stereocontrolled Synthesis of Highly Substituted Proline Esters via [3 + 2] Cycloaddition between N-Metalated Azomethine Ylides and Nitroalkenes. Origins of the Metal Effect on the Stereochemical Outcome. J. Org. Chem. 1998, 63, 1795–1805. [Google Scholar] [CrossRef]
- Garner, P.; Kaniskan, Ü. Synthesis of Highly Functionalized Pyrrolidines via a Mild One-Pot, Three-Component 1,3-Dipolar Cycloaddition Process. J. Org. Chem. 2005, 70, 10868–10871. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J. Enantioselective Synthesis of Proline Derivatives by 1,3-Dipolar Cycloadditions. Monatsh. Chem. 2011, 142, 659–680. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Wang, S.; Gentles, R.; Sowin, T.; Kati, W.; Muchmore, S.; Giranda, V.; Stewart, K.; Sham, H.; et al. Design, Synthesis, and Structural Analysis of Influenza Neuraminidase Inhibitors Containing Pyrrolidine Cores. J. Med. Chem. 2001, 44, 1192–1201. [Google Scholar] [CrossRef]
- Belskaya, N.; Bakulev, V.; Deryavina, T.; Subbotina, J.; Koddes, M.; Dehaen, W.; Toppet, S.; Robeyns, K.; van Meervely, L. 3-Alkylsulfanyl-2-Arylazo-3-(pyrrolidin-1-yl)-Acrylonitriles as Masked 1,3-Dipoles. Tetrahedron 2009, 65, 7662–7672. [Google Scholar] [CrossRef]
- Kathiravan, S.; Ramesh, E.; Raghunathan, R. Synthesis of pyrrolo[2,3-a]pyrrolizine and pyrrolizine[2,3-a]pyrrolizine Derived from Allyl Derivatives of Baylis–Hillman Adducts through Intramolecular 1,3-Dipolar Cycloaddition. Tetrahedron Lett. 2009, 50, 2389–2391. [Google Scholar] [CrossRef]
- Barker. G.; McGrath, J.; Klapars, A.; Stead, D.; Zhou, G.; Campos, K.; O’Brien, P. Enantioselective, Palladium-Catalyzed α-Arylation of N-Boc Pyrrolidine: In Situ React IR Spectroscopic Monitoring, Scope, and Synthetic Applications. J. Org. Chem. 2011, 76, 5936–5953. [Google Scholar] [CrossRef]
- Yao, S.; Gallenkamp, D.; Wölfel, K.; Lüke, B.; Schindler, M.; Scherkenbeck, J. Synthesis and SERCA Activities of Structurally Simplified Cyclopiazonic Acid Analogues. Bioorg. Med. Chem. 2011, 19, 4669–4678. [Google Scholar] [CrossRef]
- Deslandes, S.; Lamoral-Theys, D.; Frongia, C.; Chassaing, S.; Bruyùre, C.; Lozach, O.; Meijer, L.; Ducommun, B.; Kiss, R.; Delfourne, D. Synthesis and Biological Evaluation of Analogs of the Marine Alkaloids Granulatimide and Isogranulatimide. Eur. J. Med. Chem. 2012, 54, 626–636. [Google Scholar] [CrossRef]
- Eklund, E.; Pike, R.; Scheerer, J. Synthesis of 1-Aminopyrrolizidine Alkaloid (−)-Absouline by Stereoselective Aminoconjugate Addition. Tetrahedron Lett. 2012, 53, 4644–4647. [Google Scholar] [CrossRef]
- Kang, T.; Cheng, Y.; He, L.; Ye, J.; Liu, Q. Facile Synthesis of Highly Functional Pyrrolizidine Derivatives from β,γ-Unsaturated α-Keto Esters and Proline via a Tandem Cycloaddition. Tetrahedron Lett. 2012, 53, 2552–2555. [Google Scholar] [CrossRef]
- Toyooka, N.; Zhou, D.; Tezuka, Y.; Kadota, S.; Andriamaharavo, N.; Martin Garraffo, H.; Spande, T.; Daly, J. Efficient Enantio- and Diastereodivergent Synthesis of Poison-Frog Alkaloids 251O and Trans-223B. J. Org. Chem. 2009, 74, 6784–6791. [Google Scholar] [CrossRef]
- Stevens, K.; Tyrell, A.; Skerratt, S.; Robertson, J. Synthesis of NP25302. Org. Lett. 2011, 13, 5964–5967. [Google Scholar] [CrossRef]
- Georgiou, D.; Toutountzoglou, V.; Muir, K.; Hadjipavlou-Litina, D.; Elemes, J. Synthesis of Sulfur Containing Dihydro-Pyrrolo Derivatives and Their Biological Evaluation as Antioxidants. Bioorg. Med. Chem. 2012, 20, 5103–5109. [Google Scholar] [CrossRef]
- Rotstein, D.; Melville, C.; Padilla, F.; Cournoyer, D.; Lee, E.; Lemoine, R.; Petersen, A.; Setti, L.; Wanner, J.; Chen, L.; et al. Novel hexahydropyrrolo[3,4-c]pyrrole CCR5 Antagonists. Bioorg. Med. Chem. 2010, 20, 3116–3119. [Google Scholar] [CrossRef]
- Zhang, K.; Tieke, B.; Forgie, J.; Vilela, F.; Parkinson, J.; Skabara, P. Cross-Linked Polymers Based on 2,3,5,6-Tetra-Substituted pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-Dione (DPP): Synthesis, Optical and Electronic Properties. Polymer 2010, 51, 6107–6114. [Google Scholar] [CrossRef]
- Quiroga, J.; Portilla, J.; Abonía, R.; Insuasty, B.; Nogueras, J.; Cobo, J. Synthesis of Novel 5-Amino-1-Aroylpyrazoles. Tetrahedron Lett. 2008, 49, 5943–5945. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Kumar, P.; Kaushik, P.; Kaushik, D.; Dhingra, Y.; Aneja, K. Synthesis and Biological Evaluation of Some Pyrazolylpyrazolines as Anti-Inflammatory–antimicrobial Agents. Eur. J. Med. Chem. 2010, 45, 2650–2655. [Google Scholar] [CrossRef]
- Chauhan, A.; Sharma, P.; Kaushik, N. Pyrazole: A Versatile Moiety. Int. J. ChemTech Res. 2011, 3, 11–17. [Google Scholar]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Hassan, H.; Habib, O.; Moawad, E.; El-Bana, G.; Defrawy, A. Synthesis of Some Novel Antioxidant and Anticorrosive Additives for Egyptian Gasoline Motor Oils. Lubr. Sci. 2011, 23, 119–138. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Pérez, A.; Valencia, A.; Abonía, R.; Insuasty, B. Catalyst Free Three-Component Synthesis of (±)-Pyrazolylpyrrolopyrroles by 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Lett. 2011, 52, 5471–5473. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Cobo, J.; Glidewell, C. Two Methyl 3-(1H-Pyrazol-4yl) octahydropyrrolo[3,4-c]pyrrole-1-Carboxylates Form Different Hydrogen-Bonded Sheets. Acta Cryst. 2013, 69, 915–919. [Google Scholar]
- Quiroga, J.; Portillo, S.; Pérez, A.; Gálvez, J.; Abonía, R.; Insuasty, B. An Efficient Synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a Three-Component Reaction of 5-Aminopyrazoles, Isatin, and Cyclic β-Diketones. Tetrahedron Lett. 2011, 52, 2664–2666. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Pantoja, D.; Abonía, R.; Insuasty, B. Microwave-Assisted Synthesis of pyrazolo[3,4-b]pyridine-Spirocycloalkanediones by Three-Component Reaction of 5-Aminopyrazole Derivatives, Paraformaldehyde and Cyclic β-Diketones. Tetrahedron Lett. 2010, 51, 4717–4719. [Google Scholar] [CrossRef]
- Cui, P.; Xu, L.; Shi, Z.; Gan, L. Synthesis of Decahydropyrrolo[2,1,5-cd]indolizine through Consecutive [2 + 3] Cycloadditions and 6-Exo-Trig Cyclization. J. Org. Chem. 2011, 76, 4210–4212. [Google Scholar] [CrossRef]
- Cui, P.; Xu, L.; Cheng, H.; Gan, L. Synthesis of decahydropyrrolo[2,1,5-cd]indolizine Derivatives through RuCl3/AgOTf Induced Alkene–alkene and Alkene–arene Double Cycloisomerizations. Tetrahedron 2012, 68, 152–158. [Google Scholar] [CrossRef]
- Lu, Q.; Song, G.; Jasinski, J.; Keeley, A.; Zhang, W. One-Pot Double [3 + 2] Cycloaddition for Diastereoselective Synthesis of Tetracyclic Pyrrolidine Compounds. Green. Chem. 2012, 14, 3010–3012. [Google Scholar] [CrossRef]
- Petrovskaia, O.; Taylor, B.; Hauze, D.; Carroll, P.; Joullié, M. Investigations of the Reaction Mechanisms of 1,2-Indanediones with Amino Acids. J. Org. Chem. 2001, 66, 7666–7675. [Google Scholar] [CrossRef]
- Bashiardes, G.; Safir, I.; Said-Mohamed, A.; Barbot, F.; Laduranty, J. Microwave-Assisted [3 + 2] Cycloadditions of Azomethine Ylides. Org. Lett. 2003, 5, 4915–4918. [Google Scholar] [CrossRef]
- Elboray, E.; Grigg, R.; Fishwick, C.; Kilner, C.; Sarker, M.; Aly, M.; Abbas, H. XY–ZH Compounds as Potential 1,3-Dipoles. Part 65: Atom Economic Cascade Synthesis of Highly Functionalized Pyrimidinylpyrrolidines. Tetrahedron 2011, 67, 5700–5710. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Cobo, J.; Glidewell, C. Methyl (3aRS,3cRS,6cSR,7RS,8RS,8aSR)-2,5-bis(4-Chlorophenyl)-7,9-bis(1,3-Diphenyl-1H-Pyrazol-4-yl)-1,3,4,6-Tetraoxododecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-Carboxylate Dimethylformamide Disolvate: A Three Dimensional Hydrogen-Bonded Framework. Acta Cryst. 2012, 68, 439–442. [Google Scholar]
- Kudryavtsev, K.; Irkha, V. Three-Component Synthesis of Polysubstituted Homoproline Analogs. Molecules 2005, 10, 755–761. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Karplus, M. Contact Electron‐Spin Coupling of Nuclear Magnetic Moments. J. Chem. Phys. 1959, 30, 11–15. [Google Scholar] [CrossRef]
- Kalgutkar, A.; Crews, B.C.; Marnett, L.J. Design, Synthesis, and Biochemical Evaluation of N-Substituted Maleimides as Inhibitors of Prostaglandin Endoperoxide Synthases†. J. Med. Chem. 1996, 39, 1692–1703. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–j, 5a–f and 6a–f are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quiroga, J.; Gálvez, J.; Abonia, R.; Insuasty, B.; Ortíz, A.; Cobo, J.; Nogueras, M. Highly Efficient and Diastereoselective Synthesis of New Pyrazolylpyrrolizine and Pyrazolylpyrrolidine Derivates by a Three-Component Domino Process. Molecules 2014, 19, 4284-4300. https://doi.org/10.3390/molecules19044284
Quiroga J, Gálvez J, Abonia R, Insuasty B, Ortíz A, Cobo J, Nogueras M. Highly Efficient and Diastereoselective Synthesis of New Pyrazolylpyrrolizine and Pyrazolylpyrrolidine Derivates by a Three-Component Domino Process. Molecules. 2014; 19(4):4284-4300. https://doi.org/10.3390/molecules19044284
Chicago/Turabian StyleQuiroga, Jairo, Jaime Gálvez, Rodrigo Abonia, Braulio Insuasty, Alejandro Ortíz, Justo Cobo, and Manuel Nogueras. 2014. "Highly Efficient and Diastereoselective Synthesis of New Pyrazolylpyrrolizine and Pyrazolylpyrrolidine Derivates by a Three-Component Domino Process" Molecules 19, no. 4: 4284-4300. https://doi.org/10.3390/molecules19044284
APA StyleQuiroga, J., Gálvez, J., Abonia, R., Insuasty, B., Ortíz, A., Cobo, J., & Nogueras, M. (2014). Highly Efficient and Diastereoselective Synthesis of New Pyrazolylpyrrolizine and Pyrazolylpyrrolidine Derivates by a Three-Component Domino Process. Molecules, 19(4), 4284-4300. https://doi.org/10.3390/molecules19044284