Cinchona Alkaloid Derivative-Catalyzed Enantioselective Synthesis via a Mannich-Type Reaction and Antifungal Activity of β-Amino Esters Bearing Benzoheterocycle Moieties
Abstract
:1. Introduction
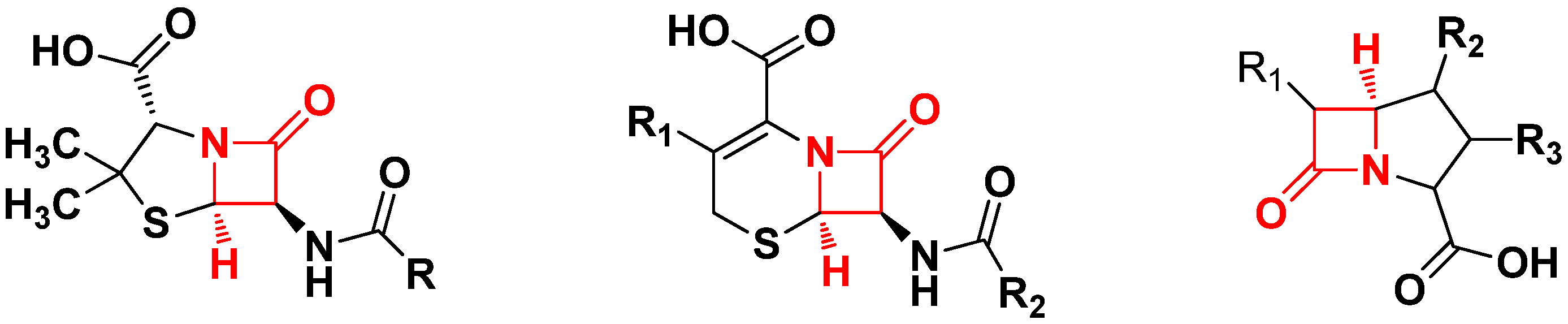
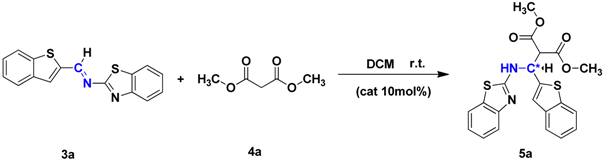
2. Results and Discussion
2.1. Optimization of Reaction Conditions
| Entry | Temperature (°C) | Solvent | Catalyst (mol%) | Time (h) | Yield a (%) | ee b (%) |
|---|---|---|---|---|---|---|
| 1 | r.t. | THF | 10 | 72 | 34 | 30 |
| 2 | Reflux | THF | 10 | 2 | - | - |
| 3 | r.t. | PhMe | 10 | 72 | 52 | 34 |
| 4 | Reflux | PhMe | 10 | 12 | 54 | 26 |
| 5 | r.t. | Acetone | 10 | 72 | 38 | 20 |
| 6 | r.t. | DCM | 10 | 72 | 61 | 78 |
| 7 | 35 | DCM | 10 | 24 | 67 | 56 |
| 8 | r.t. | DCM | 5 | 96 | 48 | 76 |
| 9 | r.t. | DCM | 20 | 72 | 72 | 78 |
| Entry | Products | R | R1 | Time (h) a | Yield (%) b | ee (%) c |
|---|---|---|---|---|---|---|
| 1 | 5ac | 6-H | −CH3 | 72 | 82 | 56 |
| 2 | 5bc | 6-H | −C2H5 | 72 | 80 | 80 |
| 3 | 5cc | 6-H | −C3H7 | 96 | 78 | 76 |
| 4 | 5dc | 6-H | −CH2C6H5 | 96 | 76 | 92 |
| 5 | 5ec | 6-Cl | −CH3 | 72 | 85 | 64 |
| 6 | 5fc | 6-Cl | −C2H5 | 72 | 86 | 82 |
| 7 | 5gc | 6-Cl | −C3H7 | 96 | 82 | 80 |
| 8 | 5hc | 6-Cl | −CH2C6H5 | 96 | 78 | >99 |
| 9 | 5ic | 6-OCH3 | −CH3 | 72 | 81 | 80 |
| 10 | 5jc | 6-OCH3 | −C2H5 | 72 | 80 | 80 |
| 11 | 5kc | 6-OCH3 | −C3H7 | 96 | 79 | 70 |
| 12 | 5lc | 6-OCH3 | −CH2C6H5 | 96 | 75 | 89 |
| 13 | 5mc | 6-CH3 | −CH3 | 72 | 81 | 78 |
| 14 | 5nc | 6-CH3 | −C2H5 | 72 | 76 | 76 |
| 15 | 5oc | 6-CH3 | −C3H7 | 96 | 76 | 80 |
| 16 | 5pc | 6-CH3 | −CH2C6H5 | 96 | 72 | 86 |
2.2. Antifungal Activity
| Entry | Compound | Inhibition rate a (%) | |||||
|---|---|---|---|---|---|---|---|
| G. zeae | C. mandshurica | F. oxysporum | P. sasakii | P. infestans | S. sclerotiorum | ||
| 1 | 5dr | 16.00 ± 0.1 | 9.76 ± 0.5 | 47.04 ± 1.1 | 38.16 ± 1.84 | 13.06 ± 1.5 | 37.42 ± 0.62 |
| 2 | 5br | 11.67 ± 0.4 | 10.18 ± 0.7 | 37.17 ± 1.1 | 30.04 ± 2.31 | 9.70 ± 0.8 | 21.29 ± 0.76 |
| 3 | 5cr | 6.03 ± 0.7 | 10.18 ± 0.8 | 19.74 ± 1.5 | 18.37 ± 1.18 | 4.1 ± 0.7 | 3.33 ± 0.5 |
| 4 | 5dr | 40.67 ± 0.9 | 41.44 ± 0.7 | 60.53 ± 2.2 | 10.11 ± 1.22 | 1.12 ± 0.7 | 6.67 ± 0.6 |
| 5 | 5er | 3.33 ± 0.5 | 11.66 ± 0.5 | 25.99 ± 1.1 | 16.01 ± 2.31 | 2.33 ± 0.8 | 3.20 ± 0.4 |
| 6 | 5fr | 8.67 ± 0.5 | 16.45 ± 0.7 | 30.59 ± 0.7 | 18.37 ± 1.23 | 2.33 ± 0.8 | 2.30 ± 1.3 |
| 7 | 5gr | 4.20 ± 0.5 | 13.13 ± 1.1 | 16.78 ± 2.3 | 9.80 ± 0.69 | 1.03 ± 0.8 | 5.67 ± 0.8 |
| 8 | 5hr | 6.30 ± 0.7 | 11.66 ± 1.6 | 13.82 ± 2.3 | 13.18 ± 1.26 | 3.33 ± 0.5 | 3.33 ± 0.5 |
| 9 | 5ir | 9.67 ± 0.9 | 18.97 ± 1.0 | 42.76 ± 2.6 | 11.21 ± 2.10 | 6.67 ± 0.6 | 21.29 ± 0.76 |
| 10 | 5jr | 11.67 ± 0.6 | 12.71 ± 1.0 | 16.78 ± 1.7 | 13.07 ± 0.84 | 3.20 ± 0.4 | 3.33 ± 0.5 |
| 11 | 5kr | 4.67 ± 0.7 | 11.23 ± 1.2 | 16.45 ± 1.5 | 15.90 ± 1.24 | 2.30 ± 1.3 | 6.67 ± 0.6 |
| 12 | 5lr | 0.67 ± 0.6 | 17.18 ± 1.1 | 10.53 ± 2.1 | 3.33 ± 0.5 | 5.67 ± 0.8 | 3.20 ± 0.4 |
| 13 | 5mr | 1.02 ± 0.7 | 10.87 ± 0.8 | 10.86 ± 1.2 | 6.67 ± 0.6 | 3.33 ± 0.5 | 2.30 ± 1.3 |
| 14 | 5nr | 4.33 ± 0.8 | 10.50 ± 0.8 | 14.14 ± 0.5 | 3.20 ± 0.4 | 6.67 ± 0.6 | 5.67 ± 0.8 |
| 15 | 5or | 4.33 ± 0.8 | 19.36 ± 1.5 | 14.14 ± 1.9 | 2.30 ± 1.3 | 3.20 ± 0.4 | 3.33 ± 0.5 |
| 16 | 5pr | 3.33 ± 0.8 | 19.39 ± 0.3 | 29.93 ± 1.0 | 5.67 ± 0.8 | 2.30 ± 1.3 | 6.67 ± 0.6 |
| 17 | Hymexazole b | 55.54 ± 3.9 | 49.61 ± 7.8 | 56.12 ± 4.1 | 51.21 ± 5.9 | 68.22 ± 2.4 | 77.51 ± 3.9 |
| 18 | DMSO | 0 | 0 | 0 | 0 | 0 | 0 |
3. Experimental
3.1. Instruments and Chemicals
3.2. Synthesis
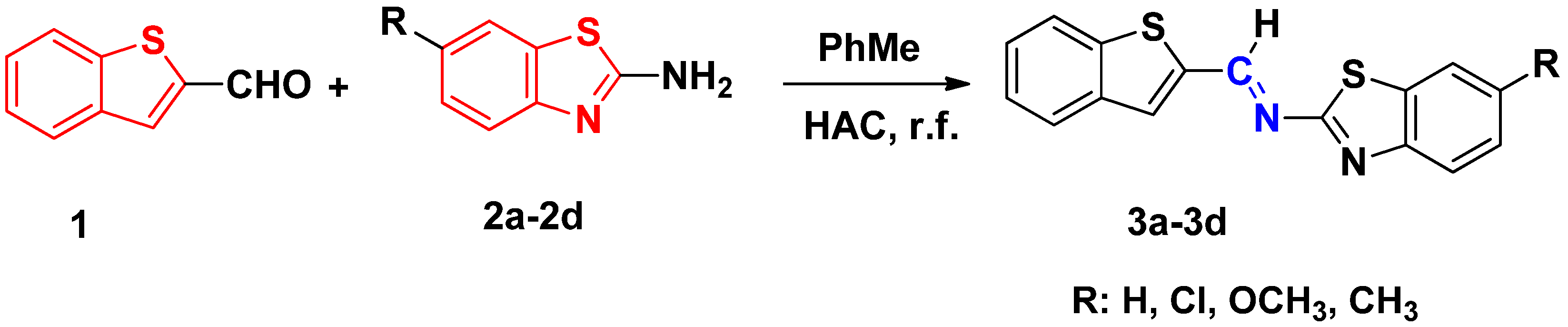
3.2.1. General Methods for Preparation of 3a–d
3.2.2. Characterization of 3a–d
3.2.3. General Method for the Preparation of 5a–p
3.2.4. Characterization of 5a–p
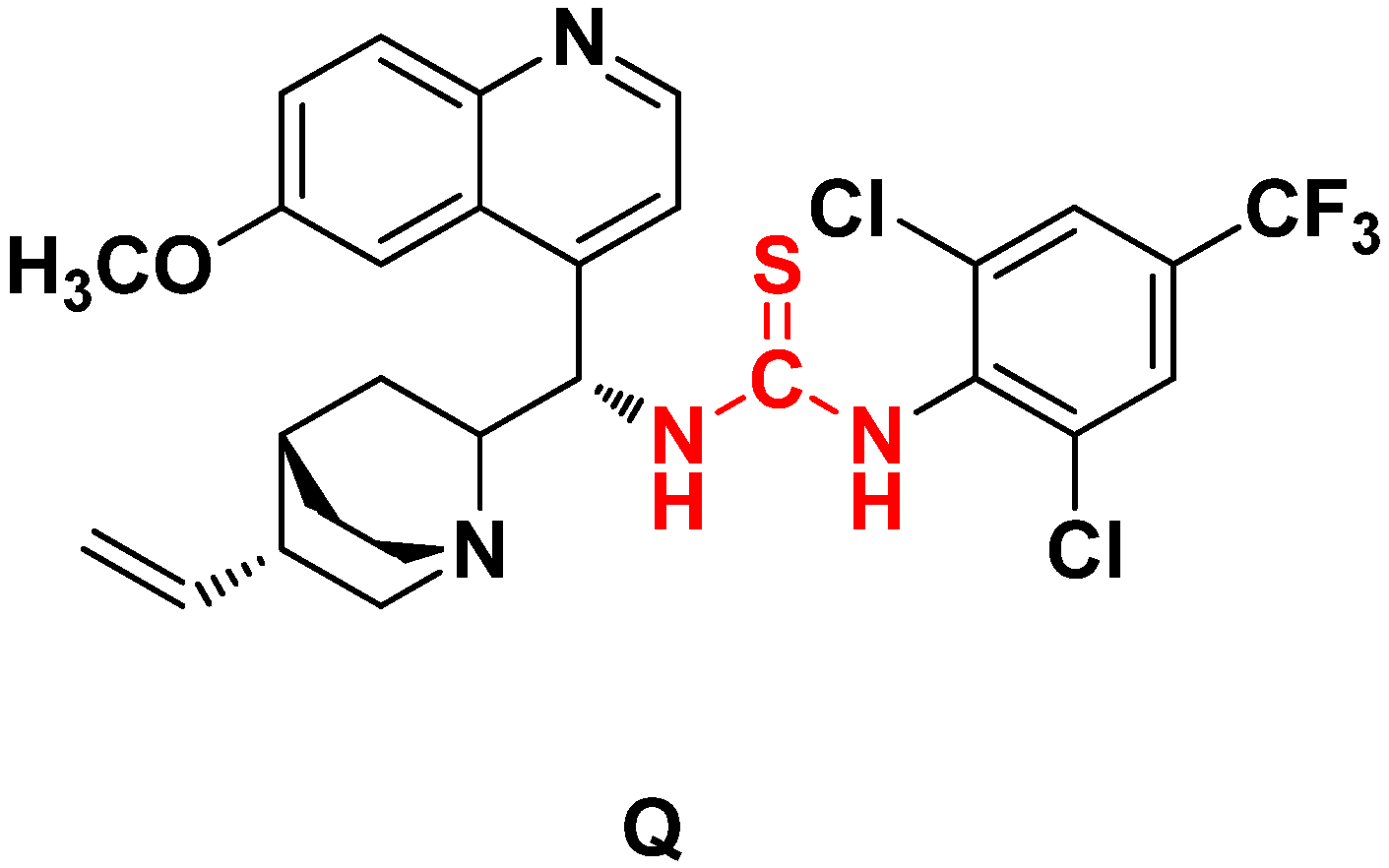
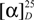 = +75.36 (c = 0.069 g/100 mL, CHCl3).
= +75.36 (c = 0.069 g/100 mL, CHCl3).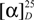 = +123.44 (c = 0.064 g/100 mL, CHCl3).
= +123.44 (c = 0.064 g/100 mL, CHCl3).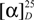 = +110.94 (c = 0.064 g/100 mL, CHCl3).
= +110.94 (c = 0.064 g/100 mL, CHCl3).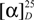 = +106.17 (c = 0.081 g/100 mL, CHCl3).
= +106.17 (c = 0.081 g/100 mL, CHCl3).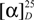 = +140.00 (c = 0.060 g/100 mL, CHCl3).
= +140.00 (c = 0.060 g/100 mL, CHCl3).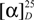 = +147.54 (c = 0.061 g/100 mL, CHCl3).
= +147.54 (c = 0.061 g/100 mL, CHCl3).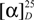 = +124.62 (c = 0.065 g/100 mL, CHCl3).
= +124.62 (c = 0.065 g/100 mL, CHCl3).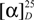 = +160.81 (c = 0.074 g/100 mL, CHCl3).
= +160.81 (c = 0.074 g/100 mL, CHCl3).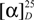 = +136.73 (c = 0.049 g/100 mL, CHCl3).
= +136.73 (c = 0.049 g/100 mL, CHCl3).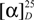 = +160.46 (c = 0.043 g/100 mL, CHCl3).
= +160.46 (c = 0.043 g/100 mL, CHCl3).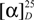 = +138.18 (c = 0.055 g/100 mL, CHCl3).
= +138.18 (c = 0.055 g/100 mL, CHCl3).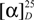 = +125 (c = 0.036 g/100 mL, CHCl3).
= +125 (c = 0.036 g/100 mL, CHCl3).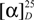 = +125.93 (c = 0.054 g/100 mL, CHCl3).
= +125.93 (c = 0.054 g/100 mL, CHCl3).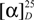 = +204.17 (c = 0.024 g/100 mL, CHCl3).
= +204.17 (c = 0.024 g/100 mL, CHCl3).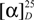 = +78.12 (c = 0.064 g/100 mL, CHCl3).
= +78.12 (c = 0.064 g/100 mL, CHCl3).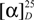 = +168.96 (c = 0.029 g/100 mL, CHCl3).
= +168.96 (c = 0.029 g/100 mL, CHCl3).3.3. Antifungal Activity Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steer, D.L.; Lew, R.A.; Perlmutter, P.; Smith, A.I.; Aguilar, M.I. The use of β-amino acids in the design of protease and peptidase inhibitors. Lett. Peptide Sci. 2002, 8, 241–246. [Google Scholar] [CrossRef]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry-exodus or revival. Angew. Chem. Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef]
- Cole, D.C. Recent stereoselective synthetic approaches to β-amino acids. Tetrahedron 1994, 50, 9517–9582. [Google Scholar] [CrossRef]
- Juaristi, E.; Quintana, D.; Escalante, J. Enantioselective synthesis of β-amino acids. Aldrichim. Acta 1994, 27, 3–11. [Google Scholar]
- Fisher, J.F.; Meroueh, S.O.; Mobashery, S. Bacterial resistance to β-lactam antibiotics: Compelling opportunism, compelling opportunity. Chem. Rev. 2005, 105, 395–424. [Google Scholar] [CrossRef]
- Yoakim, C.; Ogilvie, W.W.; Cameron, D.R.; Chabot, C.; Guse, I.; Haché, B.; Naud, J.; O’Meara, J.A.; Plante, R.; Déziel, R. β-Lactam derivatives as inhibitors of human cytomegalovirus protease. J. Med. Chem. 1998, 41, 2882–2891. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. β-Lactams: Versatile building blocks for the stereoselective synthesis of non-β-lactam products. Chem. Rev. 2007, 107, 4437–4492. [Google Scholar] [CrossRef]
- Xie, J.; Soleilhac, J.M.; Schmidt, C.; Peyroux, J.; Roques, B.P.; Fournié-Zaluski, M.C. New kelatorphan-related inhibitors of enkephalin metabolism: Improved antinociceptive properties. J. Med. Chem. 1989, 32, 1497–1503. [Google Scholar] [CrossRef]
- Salzmann, T.N.; Ratcliffe, R.W.; Christensen, B.G.; Bouffard, F.A. A stereocontrolled synthesis of (+)-thienamycin. J. Am. Chem. Soc. 1980, 102, 6161–6163. [Google Scholar] [CrossRef]
- Goody, R.S.; Alexandrov, K.; Engelhard, M. Combining chemical and biological techniques to produce modified proteins. ChemBioChem 2002, 3, 399–403. [Google Scholar] [CrossRef]
- Wang, L.; Schultz, P.G. Expanding the genetic code. Chem. Commun. 2002, 7, 1–11. [Google Scholar]
- Dockichev, T.V.; Latypova, D.R.; Shakirov, R.R.; Biglova, R.Z.; Talipov, R.F. Catalyzed synthesis of β-amino acids esters. Russ. J. Org. Chem. 2010, 46, 755–757. [Google Scholar] [CrossRef]
- Boys, M.L.; Cain-Janicki, K.J.; Doubleday, W.W.; Farid, P.N.; Kar, M.; Nugent, S.T.; Behling, J.R.; Pilipauskas, D.R. One-pot process for the preparation of a β-alkynyl β-amino acid ester. Org. Process Res. Dev. 1997, 1, 233–239. [Google Scholar] [CrossRef]
- Clark, J.D.; Weisenburger, G.A.; Anderson, D.K.; Colson, P.J.; Edney, A.D.; Gallagher, D.J.; Kleine, P.; Knable, C.M.; Lantz, M.K.; Moore, C.M.V.; et al. Pilot plant preparation of an αvβ3 integrin antagonist. Part 1. Process research and development of a (S)-β-amino acid ester intermediate: Synthesis via a scalable, diastereoselective imino-reformatsky reaction. Org. Process Res. Dev. 2004, 8, 51–61. [Google Scholar] [CrossRef]
- Evans, C.D.; Mahon, M.F.; Andrews, P.C.; Muir, J.; Bull, S.D. Intramolecular ester enolate-imine cyclization reactions for the asymmetric synthesis of polycyclic β-lactams and cyclic β-amino acid derivatives. Org. Lett. 2011, 13, 6276–6279. [Google Scholar] [CrossRef]
- Tang, P.T.; Ellman, J.A. Asymmetric synthesis of β-amino acid derivatives in corporating a broad range of substitution patterns by enolate additions to tert-butanesulfinyl imines. J. Org. Chem. 2002, 67, 7819–7832. [Google Scholar] [CrossRef]
- Liu, M.; Sibi, M.P. Recent advances in the stereoselective synthesis of β-amino acids. Tetrahedron 2002, 58, 7991–8035. [Google Scholar] [CrossRef]
- Liu, X.F.; Li, H.M.; Deng, L. Highly enantioselective amination of α-substituted α-cyanoacetates with chiral catalysts accessibl from both quinine and quinidine. Org. Lett. 2005, 7, 167–169. [Google Scholar] [CrossRef]
- Lou, S.; Taoka, B.M.; Ting, A.; Schaus, S.E. Asymmetric mannich reactions of β-keto esters with acyl imines catalyzed by cinchona alkaloids. J. Am. Chem. Soc. 2005, 127, 11256–11257. [Google Scholar] [CrossRef]
- Ting, A.; Lou, S.; Schaus, S.E. Highly diastereoselective asymmetric mannich reactions of 1,3-dicarbonyls with acyl imines. Org. Lett. 2006, 8, 2003–2006. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Song, J.; Hong, R.; Li, H.M.; Deng, L. Asymmetric friedel-crafts reaction of indoles with imines by an organic catalyst. J. Am. Chem. Soc. 2006, 128, 8156–8157. [Google Scholar] [CrossRef]
- Zhou, X.; Shang, D.J.; Zhang, Q.; Lin, L.L.; Liu, X.H.; Feng, X.M. Enantioselective three-component kabachnik-fields reaction catalyzed by chiral scandium(III)-N,N'-dioxide complexes. Org. Lett. 2009, 11, 1401–1404. [Google Scholar] [CrossRef]
- Uraguchi, D.; Sorimachi, K.; Terada, M. Organocatalytic asymmetric aza-friedel-crafts alkylation of furan. J. Am. Chem. Soc. 2004, 126, 11804–11805. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Deng, L. The mannich reaction of malonates with simple imines catalyzed by bifunctional cinchona alkaloids: Enantioselective synthesis of β-amino acids. J. Am. Chem. Soc. 2006, 128, 6048–6049. [Google Scholar] [CrossRef]
- Ting, A.; Schaus, S.E. Organocatalytic asymmetric mannich reactions: New methodology, catalyst design, and synthetic applications. Eur. J. Org. Chem. 2007, 35, 5797–5815. [Google Scholar] [CrossRef]
- Verkade, J.M.M.; van Hemert, L.J.C.; Quaedflieg, P.J.L.M.; Rutjes, F.P.J.T. Oganocatalysed asymmetric mannich reactions. Chem. Soc. Rev. 2008, 37, 29–41. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Highly enantioselective thiourea-catalyzed nitro-mannich reactions. Angew. Chem. Int. Ed. 2005, 44, 466–468. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged chiral catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Vachal, P.; Jacobsen, E.N. Structure-based analysis and optimization of a highly enantioselective catalyst for the strecker reaction. J. Am. Chem. Soc. 2002, 124, 10012–10014. [Google Scholar] [CrossRef]
- Vachal, P.; Jacobsen, E.N. Enantioselective catalytic addition of HCN to ketoimines. Catalytic synthesis of quaternary amino acids. Org. Lett. 2000, 2, 867–870. [Google Scholar] [CrossRef]
- Sigman, M.S.; Vachal, P.; Jacobsen, E.N. A general catalyst for the asymmetric strecker reaction. Angew. Chem. Int. Ed. 2000, 39, 1279–1281. [Google Scholar] [CrossRef]
- Sigman, M.S.; Jacobsen, E.N. Schiff base catalysts for the asymmetric strecker reaction identified and optimized from parallel synthetic libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Xu, X.; Furukawa, T.; Okino, T.; Miyabe, H.; Takemoto, Y. Bifunctional-thiourea-catalyzed diastereo- and enantioselective aza-henry reaction. Chem. Eur. J. 2006, 12, 466–476. [Google Scholar] [CrossRef]
- Okino, T.; Nakamura, S.; Furukawa, T.; Takemoto, Y. Enantioselective aza-henry reaction catalyzed by a bifunctional organocatalyst. Org. Lett. 2004, 6, 625–627. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Cordaro, M.; Risitano, F.; Scala, A.; Rescifina, A.; Chiacchio, U.; Grassi, G. Self-catalyzed mannich-type reaction of enolizable cyclic 1,3-dicarbonyls to acyclic nitrones: An entry to functionalized β-enamino diones. J. Org. Chem. 2013, 78, 3972–3979. [Google Scholar] [CrossRef]
- Eva, V.; Lucia, L.; Miroslav, V.; Radovan, S. Asymmetric mannich reactions catalyzed by proline and 4-hydroxyprolice derived organocatalysts in the presence of water. Tetrahedron Asymm. 2013, 24, 548–552. [Google Scholar] [CrossRef]
- Sawada, Y.; Yanai, T.; Nakagawa, H. Synthesis and insecticidal activity of benzoheterocycil analofues of N`-benzoheterocyclic analogues of N`-benzoyl-N-(tert-butyl)benzohydrazide: Patr 1. Design of benzoheterocyclic analogues. Pest Manag. Sci. 2003, 59, 25–35. [Google Scholar] [CrossRef]
- Khanitha, P.; Kazuki, K.; Hiroki, T. Synthesis of three classes of rhodacyanine dyes and ebalitation of their in vitro and in vivo antimalarial activity. Bioorg. Med. Chem. 2006, 14, 8550–8563. [Google Scholar] [CrossRef]
- Charris, J.; Monasterios, M.; Domingguez, J. Synthesis of some 5-nitro-2-furfurylidene derivatives and their antibacterial and antifungal activities. Heterocycl. Commun. 2002, 8, 275–280. [Google Scholar]
- Helal, M.H.M.; Salem, M.A.; El-Gaby, M.S.A.; Aljahdali, M. Synthesis and biological evaluation of some novel thiazole compounds as potential anti-inflammatory agents. Eur. J. Med. Chem. 2013, 65, 517–526. [Google Scholar] [CrossRef]
- Sidoova, E.; Loos, D.; Bujdakova, H. New anticandidous 2-alkylthio-6-aminobenzothiazoles. Molecules 1997, 2, 36–42. [Google Scholar] [CrossRef]
- Hassan, M.; Chohan, Z.H.; Supuran, C.T. Antibacterial Co (II) and Ni (II) complexes of benzothiazole-dereved Schiff bases. Synth. Reactiv. Inorg. Met. Org. Chem. 2002, 32, 1445–1461. [Google Scholar] [CrossRef]
- Bai, S.; Liang, X.P.; Song, B.A.; Bhadury, P.S.; Hu, D.Y.; Yang, S. Asymmetric mannich reactions catalyzed by cinchona alkaloid thiourea: Enantioselective one-pot synthesis of novel β-amino ester derivatives. Tetrahedron Asymm. 2011, 22, 518–523. [Google Scholar] [CrossRef]
- Li, L.; Song, B.A.; Bhadury, P.S.; Zhang, Y.P.; Hu, D.Y.; Yang, S. Enantioselective synthesis of β-amino esters bearing a benzothiazole moiety via a mannich-type reaction catalyzed by a cinchona alkaloid derivative. Eur. J. Org. Chem. 2011, 2011, 4743–4746. [Google Scholar]
- Li, W.H.; Song, B.A.; Bhadury, P.S.; Li, L.; Wang, Z.C.; Zhang, X.Y.; Hu, D.Y.; Chen, Z.; Zhang, Y.P.; Bai, S.; et al. Chiral cinchona alkaloid-derived thiourea catalyst for enantioselective synthesis of novel b-amino esters by mannich reaction. Chirality 2012, 24, 223–231. [Google Scholar] [CrossRef]
- He, M.; Pan, Z.X.; Bai, S.; Zhang, Y.P.; Jin, L.H.; Hu, D.Y.; Yang, S.; Song, B.A. Enantioselective synthesis of β-amino esters bearing a quinazoline moiety via a mannich-type reaction catalyzed by a cinchona alkaloid derivative. Sci. China Chem. 2013, 56, 321–328. [Google Scholar]
- Mahran, M.A.; El-nassry, S.M.F.; Allam, S.R.; El-zawawy, L.A. Synthesis of some new benzothiazole derivatives as potential antimicrobial and antiparasitic agents. Pharmazie 2003, 58, 527–530. [Google Scholar]
- Kamal, A.; Valli, V.S.Y.; Khan, M.N.A.; Farheen, S.; Reddy, M.K. Recent advances on structural modifications of benzothizaoles and their conjugate systems as potential chemotherapeutics. Exp. Opin. Investig. Drugs 2012, 21, 619–635. [Google Scholar] [CrossRef]
- Victor, F.; Raisa, R.; Claudia, R.B.G.; Thatyana, R.A.V. Chemistry and biological activities of 1,3-benzothiazoles. Mini Rev. Org. Chem. 2012, 9, 44–53. [Google Scholar] [CrossRef]
- Chattapadhyay, T.K.; Dureja, P. Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J. Agric. Food Chem. 2006, 54, 2129–2133. [Google Scholar]
- Xu, W.M.; He, J.; He, M.; Han, F.F.; Chen, X.H.; Pan, Z.X.; Wang, J.; Tong, M.G. Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 2011, 16, 9129–9141. [Google Scholar]
- Sample Availability: Some samples of the compounds 5a–p are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiao, H.; Wu, F.; Shi, L.; Chen, Z.; Su, S.; Tang, C.; Wang, H.; Li, Z.; Li, M.; Shi, Q. Cinchona Alkaloid Derivative-Catalyzed Enantioselective Synthesis via a Mannich-Type Reaction and Antifungal Activity of β-Amino Esters Bearing Benzoheterocycle Moieties. Molecules 2014, 19, 3955-3972. https://doi.org/10.3390/molecules19043955
Xiao H, Wu F, Shi L, Chen Z, Su S, Tang C, Wang H, Li Z, Li M, Shi Q. Cinchona Alkaloid Derivative-Catalyzed Enantioselective Synthesis via a Mannich-Type Reaction and Antifungal Activity of β-Amino Esters Bearing Benzoheterocycle Moieties. Molecules. 2014; 19(4):3955-3972. https://doi.org/10.3390/molecules19043955
Chicago/Turabian StyleXiao, Han, Fang Wu, Li Shi, Zhiwei Chen, Shihu Su, Chenghao Tang, Hongtao Wang, Zhining Li, Meichuan Li, and Qingcai Shi. 2014. "Cinchona Alkaloid Derivative-Catalyzed Enantioselective Synthesis via a Mannich-Type Reaction and Antifungal Activity of β-Amino Esters Bearing Benzoheterocycle Moieties" Molecules 19, no. 4: 3955-3972. https://doi.org/10.3390/molecules19043955