New Thiophene and Flavonoid from Tagetes minuta Leaves Growing in Saudi Arabia
Abstract
:1. Introduction
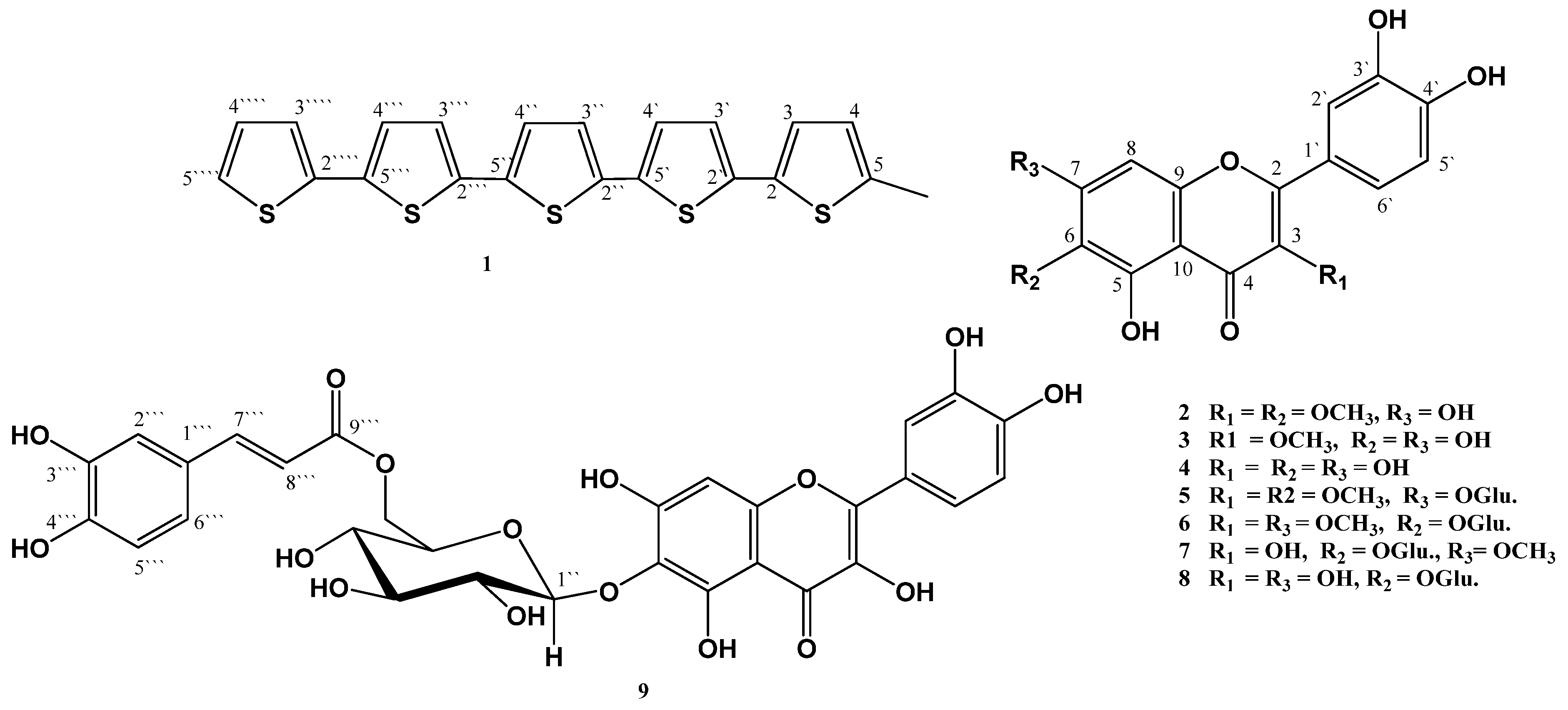
2. Results and Discussion
| No. | δH [mult., J (Hz)] | δC (mult.) | HMBC |
|---|---|---|---|
| 2 | - | 133.5 (C) | - |
| 3 | 6.93 d (3.5) | 125.9 (CH) | 2, 4, 5 |
| 4 | 6.64 brs | 124.0 (CH) | 2, 3, 5 |
| 5 | - | 137.3 (C) | - |
| 2' | - | 134.2 (C) | - |
| 3' | 6.97 d (3.5) | 121.2 (CH) | 2 |
| 4' | 6.96 d (3.5) | 122.3 (CH) | 2', 3', 2'' |
| 5' | - | 128. 9 (C) | - |
| 2'' | - | 134.7 (C) | - |
| 3'' | 6.98 d (3.5) | 121.5 (CH) | 4''', 2''' |
| 4'' | 7.02 d (3.5) | 122.3 (CH) | 2''' |
| 5'' | - | 126.1 (C) | - |
| 2''' | - | 135.1 (C) | - |
| 3''' | 6.99 d (3.5) | 125.8 (CH) | 4''', 5''', 2''' |
| 4''' | 7.12 d (3.5) | 121.6 (CH) | 2''', 2'''', 3''', 5''' |
| 5''' | - | 132.8 (C) | - |
| 2'''' | - | 135.3 (C) | - |
| 3'''' | 7.04 brs | 122.5 (CH) | 4'''', 5''' |
| 4'''' | 7.14 brd (3.5) | 121.7 (CH) | 2'''', 3'''' |
| 5'''' | 7.21 brd (5.5) | 123.0 (CH) | 3'''', 4'''' |
| 5-CH3 | 2.42 s | 15.4 (CH3) | 4, 5 |

| No. | δH [mult., J (Hz)] | δC (mult.) | HMBC |
|---|---|---|---|
| 2 | - | 148.1 (C) | - |
| 3 | - | 135.6 (C) | - |
| 4 | - | 176.1 (C) | - |
| 5 | - | 151.5 (C) | - |
| 6 | - | 129.6 (C) | - |
| 7 | - | 151.4 (C) | |
| 8 | 6. 59 s | 93.5 (CH) | 6, 7, 10 |
| 9 | - | 147.7 (C) | - |
| 10 | - | 105.1 (C) | - |
| 1' | - | 122.0 (C) | - |
| 2' | 7.74 brs | 115.5 (CH) | 2, 4', 6' |
| 3' | - | 145.0 (C) | - |
| 4' | - | 147.5 (C) | - |
| 5' | 6.88 d (7.0) | 115.3 (CH) | 3', 6' |
| 6' | 7.57 brd (7.0) | 119.9 (CH) | 2, 1', 4' |
| 1'' | 5.02 d (6.5) | 100.9 (CH) | 6 |
| 2'' | 3.77 dd (7.0, 9.0) | 73.1 (CH) | - |
| 3'' | 3.86 m | 75.7 (CH) | - |
| 4'' | 3.23 dd (9.0, 9.5) | 69.6 (CH) | - |
| 5'' | 4.35 m | 77.2 (CH) | - |
| 6'' | 4.41 dd (2.8, 12.0) 4.30 dd (7.0, 12.0) | 64.6 (CH2) | 9''' |
| 1''' | - | 125.2 (C) | - |
| 2''' | 6.96 d (1.5) | 115.7 (CH) | 6''', 7''' |
| 3''' | - | 148.3 (C) | - |
| 4''' | - | 145.3 (C) | - |
| 5''' | 6.77 d (6.8) | 115.3 (CH) | 3''' |
| 6''' | 6.93 dd (1.5, 6.8) | 120.5 (CH) | 4''', 5''', 7''', 8''' |
| 7''' | 7.43 d (16.0) | 145.5 (CH) | 9''' |
| 8''' | 6.23 d (16.0) | 113.4 (CH) | 1''', 9''' |
| 9''' | - | 166.5 (C) | - |
| 5-OH | 12.24 s | - | - |
| 7-OH | 10.89 s | - | - |
| 3'-OH | 9.35 s | - | - |
| 4'-OH | 9.35 s | - | - |
| 3-OH | 8.50 s | - | - |
| Comp. | % Activity |
|---|---|
| 2 | 81.1 |
| 3 | 82.4 |
| 4 | 91.6 |
| 5 | 68.3 |
| 6 | 69.1 |
| 7 | 71.3 |
| 8 | 83.0 |
| 9 | 89.1 |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Acid Hydrolysis of 9
3.6. Antimicrobial Assay
3.7. Antimalarial Assay
3.8. Antileishmanial Assay
3.9. Antioxidant Activity
4. Conclusions
Acknowledgments
Conflictts of Interest
References
- Loockerman, D.J.; Turner, B.L.; Jansen, R.K. Phylogenetic relationships within the Tageteae (Asteraceae) based on nuclear ribosomal ITS and chloroplast ndhF gene sequences. Syst. Bot. 2003, 28, 191–207. [Google Scholar]
- EL-Deeb, K.S.; Abbas, F.A.; El Fishawy, A.; Mossa, J.S. Chemical composition of the essential oil of Tagetes minuta growing in Saudi Arabia. Saudi Pharm. J. 2004, 12, 51–53. [Google Scholar]
- Leung, A.Y. Encyclopedia of Common Natural Ingredients. Essential Oils of Tagetes minuta from Brasil; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Senatore, F.; Napolitano, F.; Mohamed, M.A.H.; Harris, P.J.C.; Mn keni, P.N.S.; Henderson, J. Antibacterial activity of Tagetes minuta L. (Asteraceae) essential oil with different chemical composition. Flavour Fragance J. 2004, 19, 574–578. [Google Scholar] [CrossRef]
- Hadjiakhoondi, A.; Vatandoost, H.; Khanavi, M.; Abaee, M.R.; Karami, M. Biochemical investigation of different extracts and larvicidal activity of Tagetes minuta L. on Anopheles stephensi Larvae. Iran. J. Pharm. Sci. 2005, 1, 81–84. [Google Scholar]
- Shahzadi, I.; Hassan, A.; Khan, U.W.; Shah, M.M. Evaluating biological activities of the seed extracts from Tagetes minuta L. found in Northern Pakistan. J. Med. Plants Res. 2010, 4, 2108–2112. [Google Scholar]
- Lόpez, M.L.; Bonzani, N.E.; Zygadlo, J.A. Allelopathic potential of Tagetes minuta terpenes by a chemical, anatomical, and phytotoxic approach. Biochem. Syst. Ecol. 2009, 36, 882–890. [Google Scholar]
- Tereschuk, M.L.; Riera, M.V.Q.; Castro, G.R.; Abdala, L.R. Antimicrobial activity of flavonoids from leaves of Tagetes minuta. J. Ethnopharmacol. 1997, 56, 227–232. [Google Scholar] [CrossRef]
- Xu, L.-W.; Chen, J.; Qi, H.-Y.; Shi, Y.-P. Phytochemicals and their biological activities of plants in Tagetes L. Chin. Herb. Med. 2012, 4, 103–117. [Google Scholar]
- Bano, H.; Ahmed, S.W.; Azhar, I.; Ali, M.S.; Alam, N. Chemical constituents of Tagetes Patula L. Pak. J. Pharm. Sci. 2002, 15, 1–12. [Google Scholar]
- Melucci, M.; Barbarella, G.; Zambianchi, M.; di Pietro, P.; Bongini, A. Solution-phase microwave-assisted synthesis of un-substituted and modified α-quinque- and sexithiophenes. J. Org. Chem. 2004, 69, 4821–4828. [Google Scholar] [CrossRef]
- Harborne, J.B.; Mabry, H. The Flavonoids; Chapman & Hall: New York, NY, USA, 1975. [Google Scholar]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Sayed, H.M.; Mohamed, M.H.; Farag, S.F.; Mohamed, G.A.; Proksch, P. A new steroid glycoside and furochromones from Cyperus rotundus L. Nat. Prod. Res. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- El-Shanawany, M.A.; Sayed, H.M.; Ibrahim, S.R.M.; Fayed, M.A.A.; Radwan, M.M.; Ross, S.A. A new isoflavone from Blepharis ciliaris of an Egyptian origin. Med. Chem. Res. 2012, 19, 2346–2350. [Google Scholar]
- Parejo, I.; Bastida, J.; Viladomat, F.; Codina, C. Acylated quercetagetin glycosides with antioxidant activity from Tagetes maxima. Phytochemistry 2005, 66, 2356–2362. [Google Scholar] [CrossRef]
- Michels, G.; Mohamed, G.A.; Weber, N.; Chovolou, Y.; Kampkötter, A.; Wätjen, W.; Proksch, P. Effects of methylated derivatives of luteolin isolated from Cyperus alopecuroides in Rat H4IIE hepatoma cells. Basic Clin. Pharmacol. Toxicol. 2006, 98, 168–172. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Sayed, H.M. Phenolic constituents of Cucurbita pepo L. cv ‘Eskandrani’ (Summer Squash) flowers. Bull. Pharm. Sci. 2009, 32, 311–319. [Google Scholar] [CrossRef]
- Schmeda-Hirschmanna, G.; Tapia, A.; Theoduloz, C.; Rodriguez, J.; Lόpez, S.; Feresin, G.E. Free radical scavengers and antioxidants from Tagetes mendocina. Z. Naturforsch. 2004, 59c, 345–353. [Google Scholar]
- Harborne, J.B. The Flavonoids Advances in Research since 1980; Chapman & Hall: New York, NY, USA, 1988. [Google Scholar]
- Dugas, A.J., Jr.; Castañeda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure-activity relationships. J. Nat. Prod. 2000, 63, 327–331. [Google Scholar] [CrossRef]
- Bharate, S.B.; Khan, S.I.; Yunus, N.A.M.; Chauthe, S.K.; Jacob, M.R.; Tekwani, B.L.; Khan, I.A.; Singh, I.P. Anti-protozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg. Med. Chem. 2007, 15, 87–96. [Google Scholar] [CrossRef]
- Radwan, M.M.; Rodriguez-Guzman, R.; Manly, S.P.; Jacob, M.; Ross, S.A. Sepicanin A-A new geranyl flavanone from Artocarpus sepicanus with activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochem. Lett. 2009, 2, 141–143. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al-Musayeib, N.M. New constituents from the rhizomes of Egyptian Iris germanica L. Molecules 2012, 17, 2587–2598. [Google Scholar] [CrossRef]
- El-Shanawany, M.A.; Ross, S.A.; Ibrahim, S.R.M.; Mohamed, G.A.; Nafady, A.M. A new xanthone from the roots of Centaurium spicatum L. Phytochem. Lett. 2011, 4, 126–128. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Backheet, E.Y.; Khalifa, A.A.; Ibraheim, Z.Z.; Ross, S.A. Antiparasitic antioxidant phenylpropanoids and iridoid glycosides from Tecoma mollis. Fitoterapia 2012, 83, 500–507. [Google Scholar] [CrossRef]
- Al-Musayeib, N.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Ross, S.A. Lupeol-3-O-decanoate, a new triterpene ester from Cadaba farinosa Forssk. growing in Saudi Arabia. Med. Chem. Res. 2013, 22, 5297–5302. [Google Scholar] [CrossRef]
- Mohamed, G.A. Alliuocide A. A new antioxidant flavonoid from Allium cepa L. Phytopharmacology 2013, 4, 220–227. [Google Scholar]
- Mohamed, G.A.; Ibrahim, S.R.M.; Al-Musayeib, N.M.; Ross, S.A. New anti-inflammatory flavonoids from Cadaba glandulosa Forssk. Arch. Pharm. Res. 2014. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the isolated compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Musayeib, N.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Ross, S.A. New Thiophene and Flavonoid from Tagetes minuta Leaves Growing in Saudi Arabia. Molecules 2014, 19, 2819-2828. https://doi.org/10.3390/molecules19032819
Al-Musayeib NM, Mohamed GA, Ibrahim SRM, Ross SA. New Thiophene and Flavonoid from Tagetes minuta Leaves Growing in Saudi Arabia. Molecules. 2014; 19(3):2819-2828. https://doi.org/10.3390/molecules19032819
Chicago/Turabian StyleAl-Musayeib, Nawal M., Gamal A. Mohamed, Sabrin R. M. Ibrahim, and Samir A. Ross. 2014. "New Thiophene and Flavonoid from Tagetes minuta Leaves Growing in Saudi Arabia" Molecules 19, no. 3: 2819-2828. https://doi.org/10.3390/molecules19032819
APA StyleAl-Musayeib, N. M., Mohamed, G. A., Ibrahim, S. R. M., & Ross, S. A. (2014). New Thiophene and Flavonoid from Tagetes minuta Leaves Growing in Saudi Arabia. Molecules, 19(3), 2819-2828. https://doi.org/10.3390/molecules19032819





