Polyolefin Backbone Substitution in Binders for Low Temperature Powder Injection Moulding Feedstocks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of Binder Composition
| Name | PE | PW | PEG1000 | PEG4000 | PEG6000 | SA | ɸm |
|---|---|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | |
| F1 | 20 | 20 | 10 | 39 | 10 | 1 | 84.7 |
| F2 | 10 | 30 | 10 | 39 | 10 | 1 | 85.2 |
| F3 | 20 | 20 | 10 | 39 | 10 | 1 | 85.2 |
| F4 | 40 | - | 20 | 39 | - | 1 | 85.2 |
| F5 | 40 | - | 10 | 25 | 20 | 5 | 85.2 |
| F6a | 40 | 10 | - | 20 | 29 | 1 | 85.2 |
| F6b | 85.5 | ||||||
| F6c | 85.9 | ||||||
| F6d | 86.5 | ||||||
| F7a | 40 | 10 | - | 10 | 39 | 1 | 85.2 |
| F7b | 85.5 | ||||||
| F7c | 85.8 | ||||||
| F8a | 50 | - | - | 10 | 39 | 1 | 85.2 |
| F8b | 85.5 | ||||||
| F8c | 85.8 | ||||||
| F9 | 20 | 30 | - | 20 | 29 | 1 | 85.5 |
| F10 | 30 | 30 | - | - | 39 | 1 | 85.2 |
| F11 | 35 | 30 | - | 15 | 19 | 1 | 85.2 |
| F12 | 30 | 40 | - | 12 | 13 | 5 | 85.6 |
| F13 | 31 | 33 | - | 15 | 16 | 5 | 85.2 |
| F14 | 20 | 30 | 10 | 15 | 22 | 3 | 85.5 |
| F15 | 25 | 36 | 10 | 10 | 16 | 3 | 85.2 |
| F16 | 20 | 10 | 20 | 20 | 27 | 3 | 85.2 |
| Name | CW wt.% | PW wt.% | PEG1000 wt.% | PEG4000 wt.% | PEG6000 wt.% | SA wt.% | ɸm wt.% |
|---|---|---|---|---|---|---|---|
| F17 | - | 45 | - | - | 50 | 5 | 84.7 |
| F18 | - | 40 | - | 15 | 40 | 5 | 84.2 |
| F19 | 25 | 30 | - | 20 | 20 | 5 | 84.5 |
| F20 | 35 | 20 | 10 | 10 | 20 | 5 | 84.2 |
| F21 | 40 | 10 | 10 | 12 | 25 | 3 | 84.2 |
| F22 | 40 | 10 | 10 | 14 | 25 | 1 | 85.2 |
2.2. Thermal Analysis of Feedstocks
| Feedstock | Peak 1 | Peak 2 | Peak 3 | Peak 4 | ||||
|---|---|---|---|---|---|---|---|---|
| [°C] | [wt.%] | [°C] | [wt.%] | [°C] | [wt.%] | [°C] | [wt.%] | |
| F1 | 185 | 0.36 | 223 | 11.61 | - | - | 455 | 4.98 |
| F2 | 180 | 0.19 | 215 | 11.92 | - | - | 447 | 4.8 |
| F3 | 175 | 0.51 | 215 | 10.09 | - | - | 465 | 5.77 |
| F4 | 171 | 0.33 | 223 | 10.15 | 348 | 4.33 | 469 | 2.11 |
| F5 | 175 | 0.21 | 238 | 9.35 | 349 | 4.53 | 473 | 2.2 |
| F6a | 176 | 0.15 | 251 | 8.82 | 352 | 4.97 | 473 | 2.18 |
| F6b | 184 | 0.15 | 234 | 7.77 | 338 | 4.9 | 472 | 2.21 |
| F6c | 192 | 0.17 | 243 | 8.47 | 348 | 4.17 | 470 | 1.82 |
| F6d | 192 | 0.14 | 230 | 7.8 | 338 | 4.42 | 457 | 1.95 |
| F7a | 189 | 0.11 | 242 | 8.11 | 356 | 3.92 | 462 | 1.94 |
| F7b | 197 | 0.31 | 240 | 8.62 | 341 | 4.14 | 471 | 2.14 |
| F7c | 194 | 0.19 | 238 | 8.21 | 335 | 4.34 | 453 | 2.1 |
| F8a | 181 | 0.15 | 232 | 7.67 | 348 | 6.38 | 454 | 2.67 |
| F8b | 185 | 0.19 | 237 | 7.34 | 349 | 5.1 | 446 | 2.23 |
| F8c | 188 | 0.18 | 234 | 6.9 | 344 | 5.3 | 450 | 2.34 |
| F9 | 180 | 0.23 | 204 | 7.51 | 352 | 4.48 | 459 | 1.29 |
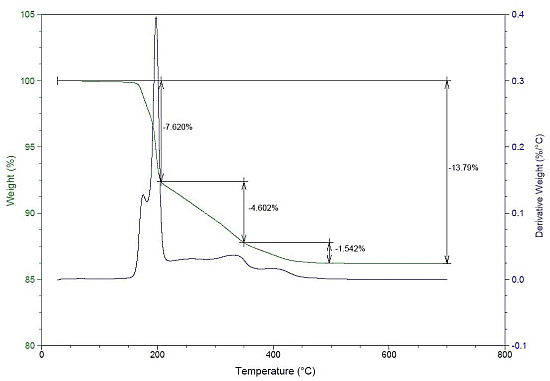
| F10 | 189 | 0.22 | 205 | 7.63 | 350 | 4.6 | 456 | 1.54 |
| F11 | 205 | 0.32 | - | - | 354 | 13.9 | 462 | 1.95 |
| F12 | 201 | 0.29 | 245 | 7.62 | 348 | 4.62 | 460 | 1.85 |
| F13 | 207 | 0.35 | 251 | 8.29 | 349 | 4.88 | 455 | 1.96 |
| F14 | 176 | 0.36 | 219 | 9.52 | - | - | 448 | 5.16 |
| F15 | 203 | 0.38 | 248 | 9.25 | 348 | 4.04 | 455 | 1.74 |
| F16 | 171 | 0.23 | 200 | 9.88 | - | - | 458 | 5 |
| F17 | 174 | 0.18 | 208 | 10.03 | - | - | 442 | 4.64 |
| F18 | 172 | 0.12 | 209 | 12.68 | - | - | 442 | 4.51 |
| F19 | 190 | 0.16 | 231 | 9.07 | - | - | 460 | 6.42 |
| F20 | 194 | 0.17 | 245 | 8.89 | 341 | 4.56 | 458 | 2.16 |
| F21 | 189 | 0.16 | - | - | 346 | 13.71 | 460 | 2.08 |
| F22 | 195 | 0.23 | 247 | 8.74 | 345 | 4.45 | 459 | 1.91 |

2.3. Thermal Analysis of Water Debinded Feedstocks
| Feedstock | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|
| [°C] | [wt.%] | [°C] | [wt.%] | [°C] | [wt.%] | |
| F2 | 210 | 0.93 | 225 | 5.75 | 487 | 4.86 |
| F6a | 200 | 2.43 | 364 | 11.4 | 475 | 1.91 |
| F7a | 170 | 2.48 | - | - | 477 | 13.52 |
| F9 | 170 | 1.33 | 250 | 6.1 | 480 | 3.56 |
| F10 | 220 | 0.16 | 229 | 5.77 | 490 | 4.44 |
| F14 | 199 | 0.89 | 220 | 6.57 | 494 | 4.64 |
| F16 | 175 | 1.17 | 252 | 10.1 | 449 | 4.19 |
| F22 | 208 | 1.8 | 230 | 6.37 | 467 | 5.35 |
3. Experimental
3.1. Materials
| Name | PE | PW | CW | PEG1000 | PEG4000 | PEG6000 | SA |
|---|---|---|---|---|---|---|---|
| Density [g/cm³] ISO 1133 | 0.918 | 0.9 | 0.97 | 1.09 | 1.41 | 1.21 | 0.85 |
| Melting Temperature [°C] | 108 | 58 | 84 | 32 | 62 | 62 | 70 |
| Molecular Weight [g/mol] | 250,000 | 400 | 1000 | 1000 | 4000 | 6000 | 284 |
| Viscosity [kPa.s] | 20 | 2 | 12 | 10 | 72 | 147 | 3 |
3.2. Preparation of Feedstocks
3.3. Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Onbattuvelli, V.P.; Vallury, S.; McCabe, T.; Park, S.J.; Atre, S.V. Properties of SiC and AlN feedstocks for the powder injection moulding of thermal management devices. PIM Int. 2010, 4, 64–70. [Google Scholar]
- Chung, C.; Rhee, B.; Cao, M.; Liu, C. Requirements of binder for powder injection molding. San Diego, USA, 11–14 June 1989; Gasbarre, T.G., Jandesca, W.F., Eds.; Metal Powder Industries Federation: Princeton, NJ, USA, 1989; pp. 67–78. [Google Scholar]
- Tseng, W.J.; Hsu, C.K. Cracking defect and porosity evolution during thermal debinding in ceramic injection moldings. Ceram. Int. 1999, 25, 461–466. [Google Scholar] [CrossRef]
- Zhang, J.; Edirisinghe, M.; Evans, J. A catalogue of ceramic injection moulding defects and their causes. Ind. Ceram. 1989, 9, 72–82. [Google Scholar]
- Hausnerova, B.; Vltavska, P.; Sedlacek, T. Pressure-Affected Flow Properties of Powder Injection Moulding Compounds. Powder Technol. 2009, 194, 192–196. [Google Scholar] [CrossRef]
- Onbattuvelli, V.P.; Enneti, R.K.; Park, S.; Atre, S.V. The effects of nanoparticle addition on binder removal from injection molded aluminum nitride. Int. J. Refract. Metals Hard Mater. 2013, 36, 77–84. [Google Scholar] [CrossRef]
- Hausnerova, B.; Marcanikova, L.; Filip, P.; Saha, P. Optimization of powder injection molding of feedstock based on aluminum oxide and multicomponent water-soluble polymer binder. Polym. Eng. Sci. 2011, 51, 1376–1382. [Google Scholar] [CrossRef]
- Trunec, M.; Cihlar, J. Thermal removal of multicomponent binder from ceramic injection mouldings. J. Eur. Ceram. Soc. 2002, 22, 2231–2241. [Google Scholar] [CrossRef]
- Krauss, V.A.; Oliveira, A.A.M.; Klein, A.N.; Al-Qureshi, H.A.; Fredel, M.C. A model for PEG removal from alumina injection moulded parts by solvent debinding. J. Mater. Process. Technol. 2007, 182, 268–273. [Google Scholar] [CrossRef]
- Voorhees, K.J.; Baugh, S.F.; Stevenson, D.N. The thermal degradation of poly(ethylene glycol)/poly(vinyl alcohol) binder in alumina ceramics. Thermochim. Acta 1996, 274, 187–207. [Google Scholar] [CrossRef]
- Huang, M.S.; Hsu, H.C. Effect of backbone polymer on properties of 316L stainless steel MIM compact. J. Mater. Process. Technol. 2009, 209, 5527–5535. [Google Scholar] [CrossRef]
- Hsu, K.C.; Lin, C.C.; Lo, G.M. Effect of wax composition on injection moulding of 304L stainless steel powder. Powder Metall. 1994, 37, 272–276. [Google Scholar] [CrossRef]
- Yang, W.W.; Hon, M.H. In situ evaluation of dimensional variations during water extraction from alumina injection-moulded parts. J. Eur. Ceram. Soc. 2000, 20, 851–858. [Google Scholar] [CrossRef]
- Yang, W.W.; Yang, K.Y.; Hon, M.H. Effects of PEG molecular weights on rheological behavior of alumina injection molding feedstocks. Mater. Chem. Phys. 2003, 78, 416–424. [Google Scholar] [CrossRef]
- Ren, S.B.; He, X.B.; Qu, X.H.; Humail, I.S.; Li, Y. Effects of binder compositions on characteristics of feedstocks of microsizedSiC ceramic injection moulding. Powder Metall. 2007, 50, 255–259. [Google Scholar] [CrossRef]
- Persson, H.; Hausnerova, B.; Nyborg, L.; Rigdahl, M. Rheological and thermal properties of a model system for PIM. Int. Polym. Proc. 2009, 24, 206–212. [Google Scholar] [CrossRef]
- Knapp, A.M.; Halloran, J.W. Binder removal from ceramic-filled thermoplastic blends. J. Am. Ceram. Soc. 2006, 89, 2776–2781. [Google Scholar] [CrossRef]
- Chartier, T.; Delhomme, E.; Baumard, J.F. Mechanisms of binder removal involved in supercritical debinding of injection moulded ceramics. J. Phys. III 1997, 7, 291–302. [Google Scholar]
- Maximenko, A.; Biest, O. Finite element modelling of binder removal from ceramic mouldings. J. Eur. Ceram. Soc. 1998, 18, 1001–1009. [Google Scholar] [CrossRef]
- Seeger, M.; Gritter, R.J. Thermal decomposition and volatilization of poly(α-olefins). J. Polym. Sci.: Polym. Chem. 1977, 15, 1393–1402. [Google Scholar] [CrossRef]
- Wright, J.K.; Evans, J.R.G. Kinetics of the oxidative degradation of ceramic injection-moulding vehicle. J. Mater. Sci. 1991, 26, 4897–4904. [Google Scholar] [CrossRef]
- Craig, R.G.; Eick, J.D.; Peyton, F.A. Properties of natural waxes used in dentistry. J. Dent. Res. 1965, 44, 1308–1316. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer 1997, 38, 317–323. [Google Scholar] [CrossRef]
Appendix

- Sample Availability: Samples of all compounds F1–F22 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hausnerova, B.; Kuritka, I.; Bleyan, D. Polyolefin Backbone Substitution in Binders for Low Temperature Powder Injection Moulding Feedstocks. Molecules 2014, 19, 2748-2760. https://doi.org/10.3390/molecules19032748
Hausnerova B, Kuritka I, Bleyan D. Polyolefin Backbone Substitution in Binders for Low Temperature Powder Injection Moulding Feedstocks. Molecules. 2014; 19(3):2748-2760. https://doi.org/10.3390/molecules19032748
Chicago/Turabian StyleHausnerova, Berenika, Ivo Kuritka, and Davit Bleyan. 2014. "Polyolefin Backbone Substitution in Binders for Low Temperature Powder Injection Moulding Feedstocks" Molecules 19, no. 3: 2748-2760. https://doi.org/10.3390/molecules19032748
APA StyleHausnerova, B., Kuritka, I., & Bleyan, D. (2014). Polyolefin Backbone Substitution in Binders for Low Temperature Powder Injection Moulding Feedstocks. Molecules, 19(3), 2748-2760. https://doi.org/10.3390/molecules19032748