Synthesis and Antiproliferative Activity of Some Novel Triazole Derivatives from Dehydroabietic Acid
Abstract
:1. Introduction
2. Results and Discussion
| Compound | (IC50 ± SD, µM) b | |||
|---|---|---|---|---|
| MRC-5 | AGS | SK-MES-1 | J82 | |
| 1 | 85.8 ± 4.6 | 79.9 ± 4.8 | 77.3 ± 4.7 | 46.3 ± 2.3 |
| 2 | >100 | 90.2 ± 5.4 | >100 | 76.3 ± 5.9 |
| 3 | >100 | 94.4 ± 5.4 | >100 | >100 |
| 4 | 50.2 ± 3.1 | 66.0 ± 4.4 | 28.9 ± 2.1 | 28.1 ± 1.7 |
| 5 | 17.1 ± 0.6 | 44.5 ± 1.9 | 6.1 ± 0.3 | 83.3 ± 3.3 |
| 6 | >100 | >100 | >100 | >100 |
| 7 | >100 | 36.7 ± 1.5 | 39.7 ± 2.7 | 25.6 ± 1.9 |
| 8 | >100 | 35.9 ± 1.7 | 37.0 ± 2.6 | 26.8 ± 1.8 |
| 9 | 73.3 ± 3.7 | 47.7 ± 2.9 | 43.6 ± 2.9 | 49.9 ± 2.9 |
| 10 | >100 | 58.9 ± 3.4 | 42.7 ± 2.9 | 33.9 ± 1.7 |
| 11 | >100 | >100 | >100 | >100 |
| 12 | >100 | >100 | >100 | >100 |
| 13 | >100 | >100 | >100 | >100 |
| 14 | >100 | >100 | >100 | >100 |
| 15 | 82.9 ± 4.5 | 74.9 ± 3.7 | 84.4 ± 5.1 | >100 |
| 16 | 68.8 ± 4.5 | 56.9 ± 3.3 | 74.2 ± 5.4 | 75.0 ± 6.1 |
| Etoposide c | 0.33 ± 0.02 | 0.58 ± 0.02 | 1.83 ± 0.09 | 3.49 ± 0.16 |
3. Experimental
3.1. General Procedures
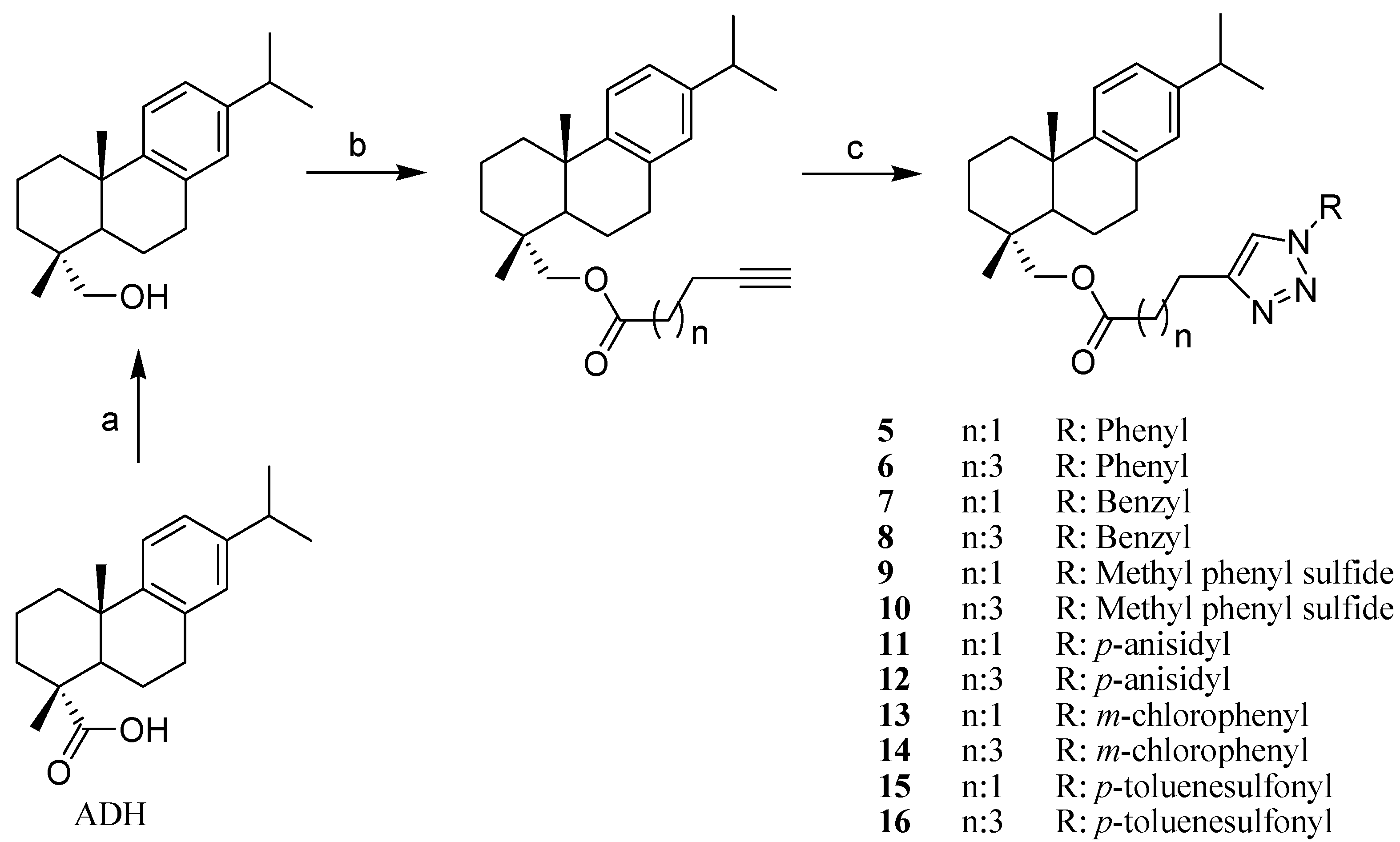
3.2. Obtention of Dehydroabietic Acid Derivatives
3.2.1. Preparation of Alkynyl Esters
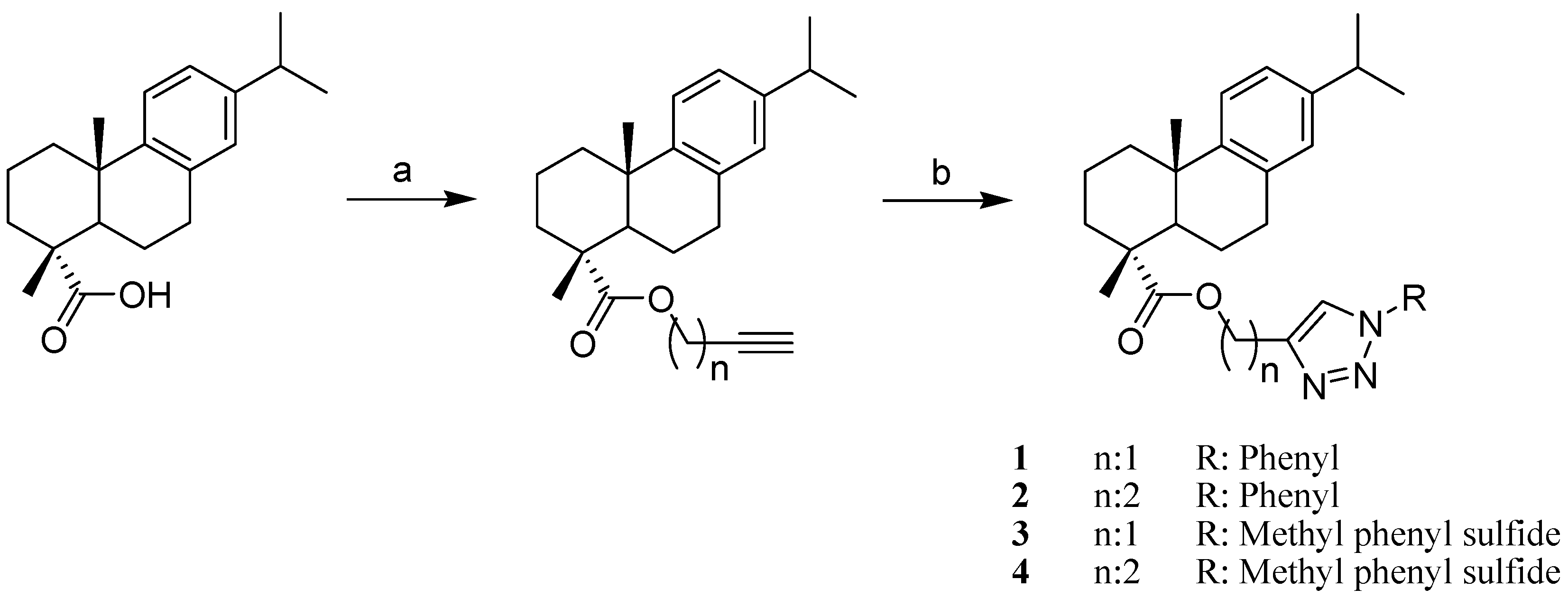
3.2.2. General Procedure for the Synthesis of Triazole 1–16
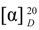 +27 (c 0.016, CHCl3); IR νmax (film) 2928, 2870, 1720, 1461, 1245, 755 cm−1; 1H-NMR (CDCl3): δ 8.02 (1H, s, H-3'), 7.72 (2H, d, J = 7.8 Hz, H-2'' and H-6''), 7.54 (2H, t, J = 7.8 Hz, H-3'' and H-5''), 7.46 (1H, t, J = 7.3 Hz, H-4''), 7.14 (1H, d, J = 8.2 Hz, H-11), 6.98 (1H, dd, J = 8.2, 1.5 Hz, H-12), 6.84 (1H, d, J = 1.5 Hz, H-14), 5.30 (2H, s, H-1'), 2.88 (2H, m, H-7), 2.79 (1H, m, H-15), 2.28 (1H, brd, J = 12.7 Hz, H-1β), 2.24 (1H, dd, J = 12.2, 1.2 Hz, H-5), 1.28 (3H, s, H-19), 1.21 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20);13C-NMR (CDCl3): δ 178.96 (C-18), 147.15 (C-9), 146.13 (C-13), 144.93 (C-2'), 137.34 (C-1''), 135.02 (C-8), 130.21 (2C, C-3'' and C-5''), 129.34 (C-4''), 127.32 (C-14), 124.57 (C-11), 124.33 (C-12), 121.06 (2C, C-2'' and C-6''), 123.64 (C-3'), 58.12 (C-1'), 48.04 (C-4), 45.24 (C-5), 38.29, 37.34, 36.82, 33.85, 30.38, 25.57, 24.38 (2C, C-16 and C-17), 22.09, 18.92, 16.91; EIMS m/z 458.2462 [M+H]+ (calcd for C29H36N3O2, 458.2807).
+27 (c 0.016, CHCl3); IR νmax (film) 2928, 2870, 1720, 1461, 1245, 755 cm−1; 1H-NMR (CDCl3): δ 8.02 (1H, s, H-3'), 7.72 (2H, d, J = 7.8 Hz, H-2'' and H-6''), 7.54 (2H, t, J = 7.8 Hz, H-3'' and H-5''), 7.46 (1H, t, J = 7.3 Hz, H-4''), 7.14 (1H, d, J = 8.2 Hz, H-11), 6.98 (1H, dd, J = 8.2, 1.5 Hz, H-12), 6.84 (1H, d, J = 1.5 Hz, H-14), 5.30 (2H, s, H-1'), 2.88 (2H, m, H-7), 2.79 (1H, m, H-15), 2.28 (1H, brd, J = 12.7 Hz, H-1β), 2.24 (1H, dd, J = 12.2, 1.2 Hz, H-5), 1.28 (3H, s, H-19), 1.21 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20);13C-NMR (CDCl3): δ 178.96 (C-18), 147.15 (C-9), 146.13 (C-13), 144.93 (C-2'), 137.34 (C-1''), 135.02 (C-8), 130.21 (2C, C-3'' and C-5''), 129.34 (C-4''), 127.32 (C-14), 124.57 (C-11), 124.33 (C-12), 121.06 (2C, C-2'' and C-6''), 123.64 (C-3'), 58.12 (C-1'), 48.04 (C-4), 45.24 (C-5), 38.29, 37.34, 36.82, 33.85, 30.38, 25.57, 24.38 (2C, C-16 and C-17), 22.09, 18.92, 16.91; EIMS m/z 458.2462 [M+H]+ (calcd for C29H36N3O2, 458.2807). 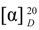 +17 (c 0.025, CHCl3); IR νmax (film) 2930, 2865, 1726, 1470, 1245, 761 cm−1; 1H-NMR (CDCl3): δ 7.79 (1H, s, H-4'), 7.63 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.50 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.3 Hz, H-4''), 7.16 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.2 Hz, H-12), 6.84 (1H, brs, H-14), 4.43 (2H, t, J = 6.6 Hz, H-1'), 3.15 (2H, t, J = 6.6 Hz, H-2'), 2.83 (2H, m, H-7), 2.71 (1H, m, H-15), 2.29 (1H, brd, J = 12.2 Hz, H-1β), 2.24 (1H, dd, J = 12.2, 1.2, H-5), 1.26 (3H, s, H-19), 1.21 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.19 (3H, s, H-20); 13C-NMR (CDCl3): δ 178.35 (C-18), 146.73 (C-9), 145.68 (C-13), 144.90 (C-3'), 137.03 (C-1''), 134.49 (C-8), 129.71 (2C, C-3'' and C-5''), 128.56 (C-4''), 126.85 (C-14), 124.11 (C-11), 123.90 (C-12), 120.37 (2C, C-2'' and C-6''), 119.70 (C-4'), 63.10 (C-1'), 47.62 (C-4), 44.83 (C-5), 37.96, 36.89, 36.60, 33.38, 29.99, 25.59, 25.14, 23.92 (2C, C-16 and C-17), 21.70, 18.52, 16.50; EIMS m/z 472.2695 [M+H]+ (calcd for C30H38N3O2, 472.2964).
+17 (c 0.025, CHCl3); IR νmax (film) 2930, 2865, 1726, 1470, 1245, 761 cm−1; 1H-NMR (CDCl3): δ 7.79 (1H, s, H-4'), 7.63 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.50 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.3 Hz, H-4''), 7.16 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.2 Hz, H-12), 6.84 (1H, brs, H-14), 4.43 (2H, t, J = 6.6 Hz, H-1'), 3.15 (2H, t, J = 6.6 Hz, H-2'), 2.83 (2H, m, H-7), 2.71 (1H, m, H-15), 2.29 (1H, brd, J = 12.2 Hz, H-1β), 2.24 (1H, dd, J = 12.2, 1.2, H-5), 1.26 (3H, s, H-19), 1.21 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.19 (3H, s, H-20); 13C-NMR (CDCl3): δ 178.35 (C-18), 146.73 (C-9), 145.68 (C-13), 144.90 (C-3'), 137.03 (C-1''), 134.49 (C-8), 129.71 (2C, C-3'' and C-5''), 128.56 (C-4''), 126.85 (C-14), 124.11 (C-11), 123.90 (C-12), 120.37 (2C, C-2'' and C-6''), 119.70 (C-4'), 63.10 (C-1'), 47.62 (C-4), 44.83 (C-5), 37.96, 36.89, 36.60, 33.38, 29.99, 25.59, 25.14, 23.92 (2C, C-16 and C-17), 21.70, 18.52, 16.50; EIMS m/z 472.2695 [M+H]+ (calcd for C30H38N3O2, 472.2964). 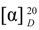 +33 (c 0.013, CHCl3); IR νmax (film) 2922, 2852, 1714, 1442, 1238, 742 cm−1; 1H-NMR (CDCl3): δ 7.59 (1H, s, H-3'), 7.29 (5H, m, Ph), 7.11 (1H, d, J = 8.1 Hz, H-11), 6.96 (1H, brd, J = 8.1 Hz, H-12), 6.81 (1H, brs, H-14), 5.50 (2H, s, CH2S), 5.16 (2H, s, H-1'), 2.81 (2H, m, H-7), 2.72 (1H, m, H-15), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 2.14 (1H, dd, J = 12.2, 1.5, Hz, H-5), 1.22 (3H, s, H-19), 1.17 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.16 (3H, s, H-20); 13C-NMR (CDCl3): δ 178.90 (C-18), 147.05 (C-9), 146.12 (C-13), 143.92 (C-2'), 134.91 (C-8), 132.63 (2C, C-2'' and C-6''), 131.98 (C-1''), 129.88 (2C, C-3'' and C-5''), 129.18 (C-4''), 127.24 (C-14), 124.50 (C-11), 124.30 (C-12), 123.66 (C-3'), 57.93 (C-1'), 54.35 (CH2S), 47.96 (C-4), 45.18 (C-5), 38.25, 37.26, 36.78, 33.97, 30.31, 25.46, 24.29 (2C, C-16 and C-17), 22.03, 18.34, 16.77; EIMS m/z 504.2641 [M+H]+ (calcd for C30H38N3O2S, 504.2684).
+33 (c 0.013, CHCl3); IR νmax (film) 2922, 2852, 1714, 1442, 1238, 742 cm−1; 1H-NMR (CDCl3): δ 7.59 (1H, s, H-3'), 7.29 (5H, m, Ph), 7.11 (1H, d, J = 8.1 Hz, H-11), 6.96 (1H, brd, J = 8.1 Hz, H-12), 6.81 (1H, brs, H-14), 5.50 (2H, s, CH2S), 5.16 (2H, s, H-1'), 2.81 (2H, m, H-7), 2.72 (1H, m, H-15), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 2.14 (1H, dd, J = 12.2, 1.5, Hz, H-5), 1.22 (3H, s, H-19), 1.17 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.16 (3H, s, H-20); 13C-NMR (CDCl3): δ 178.90 (C-18), 147.05 (C-9), 146.12 (C-13), 143.92 (C-2'), 134.91 (C-8), 132.63 (2C, C-2'' and C-6''), 131.98 (C-1''), 129.88 (2C, C-3'' and C-5''), 129.18 (C-4''), 127.24 (C-14), 124.50 (C-11), 124.30 (C-12), 123.66 (C-3'), 57.93 (C-1'), 54.35 (CH2S), 47.96 (C-4), 45.18 (C-5), 38.25, 37.26, 36.78, 33.97, 30.31, 25.46, 24.29 (2C, C-16 and C-17), 22.03, 18.34, 16.77; EIMS m/z 504.2641 [M+H]+ (calcd for C30H38N3O2S, 504.2684). 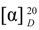 +14 (c 0.014, CHCl3); IR νmax (film) 2928, 2867, 1722, 1440, 1242, 749 cm−1; 1H-NMR (CDCl3): δ 7.36 (1H, s, H-4'), 7.29 (5H, m, Ph), 7.14 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 5.45 (2H, s, CH2S), 4.29 (2H, t, J = 6.6 Hz, H-1'), 3.03 (2H, t, J = 6.6 Hz, H-2'), 2.82 (2H, m, H-7), 2.70 (1H, m, H-15), 2.28 (1H, brd, J = 12.7 Hz, H-1β), 2.12 (1H, dd, J = 12.2, 1.5 Hz, H-5), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-19), 1.18 (3H, s, H-20); 13C-NMR (CDCl3): δ 178.24 (C-18), 146.77 (C-9), 145.74 (C-13), 144.81 (C-3'), 134.54 (C-8), 131.92 (C-1''), 131.79 (2C, C-2'' and C-6''), 129.24 (2C, C-3'' and C-5''), 128.50 (C-4''), 126.87 (C-14), 124.11 (C-11), 123.93 (C-12), 120.95 (C-4'), 63.15 (C-1'), 53.46 (CH2S), 47.55 (C-4), 44.82 (C-5), 37.96, 36.85, 36.50, 33.92, 29.91, 25.59, 25.08, 23.97 (2C, C-16 and C-17), 21.57, 18.49, 16.44; EIMS m/z 518.2164 [M+H]+ (calcd for C31H40N3O2S, 518.2841).
+14 (c 0.014, CHCl3); IR νmax (film) 2928, 2867, 1722, 1440, 1242, 749 cm−1; 1H-NMR (CDCl3): δ 7.36 (1H, s, H-4'), 7.29 (5H, m, Ph), 7.14 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 5.45 (2H, s, CH2S), 4.29 (2H, t, J = 6.6 Hz, H-1'), 3.03 (2H, t, J = 6.6 Hz, H-2'), 2.82 (2H, m, H-7), 2.70 (1H, m, H-15), 2.28 (1H, brd, J = 12.7 Hz, H-1β), 2.12 (1H, dd, J = 12.2, 1.5 Hz, H-5), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-19), 1.18 (3H, s, H-20); 13C-NMR (CDCl3): δ 178.24 (C-18), 146.77 (C-9), 145.74 (C-13), 144.81 (C-3'), 134.54 (C-8), 131.92 (C-1''), 131.79 (2C, C-2'' and C-6''), 129.24 (2C, C-3'' and C-5''), 128.50 (C-4''), 126.87 (C-14), 124.11 (C-11), 123.93 (C-12), 120.95 (C-4'), 63.15 (C-1'), 53.46 (CH2S), 47.55 (C-4), 44.82 (C-5), 37.96, 36.85, 36.50, 33.92, 29.91, 25.59, 25.08, 23.97 (2C, C-16 and C-17), 21.57, 18.49, 16.44; EIMS m/z 518.2164 [M+H]+ (calcd for C31H40N3O2S, 518.2841). 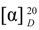 +20 (c 0.022, CHCl3); IR νmax (film) 2925, 2864, 1726, 1463, 1246, 761 cm−1; 1H-NMR (CDCl3): δ 7.78 (1H, s, H-5'), 7.70 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.53 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.3 Hz, H-4''), 7.16 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 3.98 (1H, d, J = 10.9 Hz, H-18) 3.75 (1H, d, J = 10.9 Hz, H-18), 3.11 (2H, t, J = 7.3 Hz, H-3'), 2.86 (2H, m, H-7), 2.81 (1H, m, H-15), 2.79 (2H, t, J = 7.3 Hz, H-2'), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.92 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.30 (C-1'), 147.51 (C-4'), 147.46 (C-9), 146.02 (C-13), 137.54 (C-1''), 135.06 (C-8), 130.11 (2C, C-3'' and C-5''), 128.96 (C-4''), 127.28 (C-14), 124.71 (C-11), 124.32 (C-12), 120.82 (2C, C-2'' and C-6''), 119.91 (C-5'), 73.16 (C-18), 44.78 (C-5), 38.65, 37.82, 37.20, 35.96, 34.03, 33.84, 30.65, 25.78, 24.39 (2C, C-16 and C-17), 21.46, 19.42, 18.91, 17.84; EIMS m/z 486.3360 [M+H]+ (calcd for C31H40N3O2, 486.3120).
+20 (c 0.022, CHCl3); IR νmax (film) 2925, 2864, 1726, 1463, 1246, 761 cm−1; 1H-NMR (CDCl3): δ 7.78 (1H, s, H-5'), 7.70 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.53 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.3 Hz, H-4''), 7.16 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 3.98 (1H, d, J = 10.9 Hz, H-18) 3.75 (1H, d, J = 10.9 Hz, H-18), 3.11 (2H, t, J = 7.3 Hz, H-3'), 2.86 (2H, m, H-7), 2.81 (1H, m, H-15), 2.79 (2H, t, J = 7.3 Hz, H-2'), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.92 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.30 (C-1'), 147.51 (C-4'), 147.46 (C-9), 146.02 (C-13), 137.54 (C-1''), 135.06 (C-8), 130.11 (2C, C-3'' and C-5''), 128.96 (C-4''), 127.28 (C-14), 124.71 (C-11), 124.32 (C-12), 120.82 (2C, C-2'' and C-6''), 119.91 (C-5'), 73.16 (C-18), 44.78 (C-5), 38.65, 37.82, 37.20, 35.96, 34.03, 33.84, 30.65, 25.78, 24.39 (2C, C-16 and C-17), 21.46, 19.42, 18.91, 17.84; EIMS m/z 486.3360 [M+H]+ (calcd for C31H40N3O2, 486.3120). 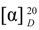 +25 (c 0.014, CHCl3); IR νmax (film) 2930, 2864, 1726, 1461, 1248, 761 cm−1; 1H-NMR (CDCl3): δ 7.70 (1H, s, H-7'), 7.71 (2H, d, J = 7.4 Hz, H-2'' and H-6''), 7.53 (2H, t, J = 7.4 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.4 Hz, H-4''), 7.18 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.97 (1H, d, J = 10.9 Hz, H-18) 3.71 (1H, d, J = 10.9 Hz, H-18), 2.85 (2H, m, H-7), 2.83 (1H, m, H-15), 2.80 (2H, t, J = 7.3 Hz, H-5'), 2.36 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.23 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.13 (C-1'), 148.86 (C-6'), 147.54 (C-9), 146.03 (C-13), 137.66 (C-1''), 135.12 (C-8), 130.09 (2C, C-3'' and C-5''), 128.87 (C-4''), 127.29 (C-14), 124.71 (C-11), 124.31 (C-12), 120.83 (2C, C-2'' and C-6''), 119.33 (C-7'), 72.84 (C-18), 44.68 (C-5), 38.70, 37.84, 37.20, 35.99, 34.47, 33.83, 30.66, 29.19, 25.78, 25.74, 24.94, 24.38 (2C, C-16 and C-17), 19.39, 18.95, 17.91; EIMS m/z 514.3686 [M+H]+ (calcd for C31H44N3O2, 514.3433).
+25 (c 0.014, CHCl3); IR νmax (film) 2930, 2864, 1726, 1461, 1248, 761 cm−1; 1H-NMR (CDCl3): δ 7.70 (1H, s, H-7'), 7.71 (2H, d, J = 7.4 Hz, H-2'' and H-6''), 7.53 (2H, t, J = 7.4 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.4 Hz, H-4''), 7.18 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.97 (1H, d, J = 10.9 Hz, H-18) 3.71 (1H, d, J = 10.9 Hz, H-18), 2.85 (2H, m, H-7), 2.83 (1H, m, H-15), 2.80 (2H, t, J = 7.3 Hz, H-5'), 2.36 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.23 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.13 (C-1'), 148.86 (C-6'), 147.54 (C-9), 146.03 (C-13), 137.66 (C-1''), 135.12 (C-8), 130.09 (2C, C-3'' and C-5''), 128.87 (C-4''), 127.29 (C-14), 124.71 (C-11), 124.31 (C-12), 120.83 (2C, C-2'' and C-6''), 119.33 (C-7'), 72.84 (C-18), 44.68 (C-5), 38.70, 37.84, 37.20, 35.99, 34.47, 33.83, 30.66, 29.19, 25.78, 25.74, 24.94, 24.38 (2C, C-16 and C-17), 19.39, 18.95, 17.91; EIMS m/z 514.3686 [M+H]+ (calcd for C31H44N3O2, 514.3433). 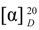 +16 (c 0.039, CHCl3); IR νmax (film) 2934, 2867, 1726, 1458, 1240, 727 cm−1; 1H-NMR (CDCl3): δ 7.36 (1H, s, H-5'), 7.34 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.24 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.22 (1H, t, J = 7.3 Hz, H-4''), 7.17 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 5.43 (2H, s, CH2Ph), 3.94 (1H, d, J = 10.9 Hz, H-18) 3.71 (1H, d, J = 10.9 Hz, H-18), 3.00 (2H, t, J = 7.3 Hz, H-3'), 2.85 (2H, m, H-7), 2.80 (1H, m, H-15), 2.70 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.90 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.24 (C-1'), 147.47 (C-9), 147.24 (C-4'), 146.02 (C-13), 135.24 (C-1''), 135.07 (C-8), 129.48 (2C, C-3'' and C-5''), 129.06 (C-4''), 128.40 (2C, C-2'' and C-6''), 127.29 (C-14), 124.71 (C-11), 124.33 (C-12), 121.44 (C-5'), 73.06 (C-18), 54.40 (CH2Ph), 44.77 (C-5), 38.66, 37.83, 37.19, 35.93, 34.04, 33.84, 30.65, 25.79, 24.40 (2C, C-16 and C-17), 21.51, 19.41, 18.92, 17.83; EIMS m/z 500.2639 [M+H]+ (calcd for C32H42N3O2, 500.3277).
+16 (c 0.039, CHCl3); IR νmax (film) 2934, 2867, 1726, 1458, 1240, 727 cm−1; 1H-NMR (CDCl3): δ 7.36 (1H, s, H-5'), 7.34 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.24 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.22 (1H, t, J = 7.3 Hz, H-4''), 7.17 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 5.43 (2H, s, CH2Ph), 3.94 (1H, d, J = 10.9 Hz, H-18) 3.71 (1H, d, J = 10.9 Hz, H-18), 3.00 (2H, t, J = 7.3 Hz, H-3'), 2.85 (2H, m, H-7), 2.80 (1H, m, H-15), 2.70 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.90 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.24 (C-1'), 147.47 (C-9), 147.24 (C-4'), 146.02 (C-13), 135.24 (C-1''), 135.07 (C-8), 129.48 (2C, C-3'' and C-5''), 129.06 (C-4''), 128.40 (2C, C-2'' and C-6''), 127.29 (C-14), 124.71 (C-11), 124.33 (C-12), 121.44 (C-5'), 73.06 (C-18), 54.40 (CH2Ph), 44.77 (C-5), 38.66, 37.83, 37.19, 35.93, 34.04, 33.84, 30.65, 25.79, 24.40 (2C, C-16 and C-17), 21.51, 19.41, 18.92, 17.83; EIMS m/z 500.2639 [M+H]+ (calcd for C32H42N3O2, 500.3277). 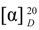 +10 (c 0.091, CHCl3); IR νmax (film) 2934, 2867, 1729, 1458, 1220, 751 cm−1; 1H-NMR (CDCl3): δ 7.36 (1H, s, H-7'), 7.34 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.25 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.22 (1H, t, J = 7.3 Hz, H-4''), 7.19 (1H, d, J = 8.1 Hz, H-11), 7.00 (1H, brd, J = 8.1 Hz, H-12), 6.90 (1H, brs, H-14), 5.48 (2H, s, CH2Ph), 3.95 (1H, d, J = 10.9 Hz, H-18) 3.70 (1H, d, J = 10.9 Hz, H-18), 2.85 (2H, m, H-7), 2.80 (1H, m, H-15), 2.69 (2H, t, J = 7.3 Hz, H-5'), 2.32 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.23 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.22 (3H, s, H-20), 0.94 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.13 (C-1'), 148.63 (C-6'), 147.55 (C-9), 146.02 (C-13), 135.36 (C-1''), 135.13 (C-8), 129.47 (2C, C-3'' and C-5''), 129.03 (C-4''), 128.37 (2C, C-2'' and C-6''), 127.30 (C-14), 124.73 (C-11), 124.32 (C-12), 121.04 (C-7'), 72.80 (C-18), 54.38 (CH2Ph), 44.68 (C-5), 38.71, 37.84, 37.19, 35.97, 34.45, 33.83, 30.67, 29.23, 25.81, 24.97, 24.41 (2C, C-16 and C-17), 19.39, 18.95, 17.91; EIMS m/z 528.4121 [M+H]+ (calcd for C34H46N3O2, 528.3590).
+10 (c 0.091, CHCl3); IR νmax (film) 2934, 2867, 1729, 1458, 1220, 751 cm−1; 1H-NMR (CDCl3): δ 7.36 (1H, s, H-7'), 7.34 (2H, d, J = 7.6 Hz, H-2'' and H-6''), 7.25 (2H, t, J = 7.6 Hz, H-3'' and H-5''), 7.22 (1H, t, J = 7.3 Hz, H-4''), 7.19 (1H, d, J = 8.1 Hz, H-11), 7.00 (1H, brd, J = 8.1 Hz, H-12), 6.90 (1H, brs, H-14), 5.48 (2H, s, CH2Ph), 3.95 (1H, d, J = 10.9 Hz, H-18) 3.70 (1H, d, J = 10.9 Hz, H-18), 2.85 (2H, m, H-7), 2.80 (1H, m, H-15), 2.69 (2H, t, J = 7.3 Hz, H-5'), 2.32 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.23 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.22 (3H, s, H-20), 0.94 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.13 (C-1'), 148.63 (C-6'), 147.55 (C-9), 146.02 (C-13), 135.36 (C-1''), 135.13 (C-8), 129.47 (2C, C-3'' and C-5''), 129.03 (C-4''), 128.37 (2C, C-2'' and C-6''), 127.30 (C-14), 124.73 (C-11), 124.32 (C-12), 121.04 (C-7'), 72.80 (C-18), 54.38 (CH2Ph), 44.68 (C-5), 38.71, 37.84, 37.19, 35.97, 34.45, 33.83, 30.67, 29.23, 25.81, 24.97, 24.41 (2C, C-16 and C-17), 19.39, 18.95, 17.91; EIMS m/z 528.4121 [M+H]+ (calcd for C34H46N3O2, 528.3590). 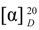 +7 (c 0.197, CHCl3); IR νmax (film) 2931, 2864, 1726, 1442, 1250, 749 cm−1; 1H-NMR (CDCl3): δ 7.37 (1H, s, H-5'), 7.31 (5H, m, Ph), 7.20 (1H, d, J = 8.1 Hz, H-11), 7.02 (1H, brd, J = 8.1 Hz, H-12), 6.92 (1H, brs, H-14), 5.51 (2H, s, CH2S), 4.00 (1H, d, J = 10.9 Hz, H-18) 3.73 (1H, d, J = 10.9 Hz, H-18), 3.02 (2H, t, J = 7.3 Hz, H-3'), 2.87 (2H, m, H-7), 2.82 (1H, m, H-15), 2.72 (2H, t, J = 7.3 Hz, H-2'), 2.28 (1H, brd, J = 12.7 Hz, H-1β), 1.25 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.23 (3H, s, H-20), 0.94 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.11 (C-1'), 147.47 (C-9), 147.37 (C-4'), 146.04 (C-13), 135.08 (C-8), 132.50 (3C, C-1'', C-2'' and C-6''), 129.86 (2C, C-3'' and C-5''), 128.99 (C-4''), 127.31 (C-14), 124.74 (C-11), 124.36 (C-12), 121.09 (C-5'), 73.00 (C-18), 53.96 (CH2S), 44.72 (C-5), 38.68, 37.84, 37.23, 35.94, 33.97, 33.86, 30.67, 25.83, 24.44 (2C, C-16 and C-17), 21.49, 19.41, 18.94, 17.89; EIMS m/z 532.3104 [M+H]+ (calcd for C32H42N3O2S, 532.2997).
+7 (c 0.197, CHCl3); IR νmax (film) 2931, 2864, 1726, 1442, 1250, 749 cm−1; 1H-NMR (CDCl3): δ 7.37 (1H, s, H-5'), 7.31 (5H, m, Ph), 7.20 (1H, d, J = 8.1 Hz, H-11), 7.02 (1H, brd, J = 8.1 Hz, H-12), 6.92 (1H, brs, H-14), 5.51 (2H, s, CH2S), 4.00 (1H, d, J = 10.9 Hz, H-18) 3.73 (1H, d, J = 10.9 Hz, H-18), 3.02 (2H, t, J = 7.3 Hz, H-3'), 2.87 (2H, m, H-7), 2.82 (1H, m, H-15), 2.72 (2H, t, J = 7.3 Hz, H-2'), 2.28 (1H, brd, J = 12.7 Hz, H-1β), 1.25 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.23 (3H, s, H-20), 0.94 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.11 (C-1'), 147.47 (C-9), 147.37 (C-4'), 146.04 (C-13), 135.08 (C-8), 132.50 (3C, C-1'', C-2'' and C-6''), 129.86 (2C, C-3'' and C-5''), 128.99 (C-4''), 127.31 (C-14), 124.74 (C-11), 124.36 (C-12), 121.09 (C-5'), 73.00 (C-18), 53.96 (CH2S), 44.72 (C-5), 38.68, 37.84, 37.23, 35.94, 33.97, 33.86, 30.67, 25.83, 24.44 (2C, C-16 and C-17), 21.49, 19.41, 18.94, 17.89; EIMS m/z 532.3104 [M+H]+ (calcd for C32H42N3O2S, 532.2997). 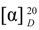 +11 (c 0.256, CHCl3); IR νmax (film) 2937, 2867, 1725, 1467, 1251, 749 cm−1; 1H-NMR (CDCl3): δ 7.32 (5H, m, Ph), 7.28 (1H, s, H-7'), 7.21 (1H, d, J = 8.1 Hz, H-11), 7.03 (1H, brd, J = 8.1 Hz, H-12), 6.92 (1H, brs, H-14), 5.59 (2H, s, CH2S), 4.00 (1H, d, J = 10.9 Hz, H-18), 3.74 (1H, d, J = 10.9 Hz, H-18), 2.87 (2H, m, H-7), 2.85 (1H, m, H-15), 2.70 (2H, t, J = 7.3 Hz, H-5'), 2.34 (2H, t, J = 7.3 Hz, H-2'), 2.32 (1H, brd, J = 12.9 Hz, H-1β), 1.25 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.24 (3H, s, H-20), 0.96 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.08 (C-1'), 148.69 (C-6'), 147.54 (C-9), 146.02 (C-13), 135.12 (C-8), 132.64 (2C, C-2'' and C-6''), 132.48 (C-1''), 129.86 (2C, C-3'' and C-5''), 129.02 (C-4''), 127.31 (C-14), 124.74 (C-11), 124.34 (C-12), 120.71 (C-7'), 72.80 (C-18), 54.04 (CH2S), 44.68 (C-5), 38.72, 37.85, 37.21, 35.99, 34.29, 33.87, 30.69, 29.14, 25.82, 25.70, 24.85, 24.43 (2C, C-16 and C-17), 19.41, 18.97, 17.94; EIMS m/z 560.4263 [M+H]+ (calcd for C34H46N3O2S, 560.3310).
+11 (c 0.256, CHCl3); IR νmax (film) 2937, 2867, 1725, 1467, 1251, 749 cm−1; 1H-NMR (CDCl3): δ 7.32 (5H, m, Ph), 7.28 (1H, s, H-7'), 7.21 (1H, d, J = 8.1 Hz, H-11), 7.03 (1H, brd, J = 8.1 Hz, H-12), 6.92 (1H, brs, H-14), 5.59 (2H, s, CH2S), 4.00 (1H, d, J = 10.9 Hz, H-18), 3.74 (1H, d, J = 10.9 Hz, H-18), 2.87 (2H, m, H-7), 2.85 (1H, m, H-15), 2.70 (2H, t, J = 7.3 Hz, H-5'), 2.34 (2H, t, J = 7.3 Hz, H-2'), 2.32 (1H, brd, J = 12.9 Hz, H-1β), 1.25 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.24 (3H, s, H-20), 0.96 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.08 (C-1'), 148.69 (C-6'), 147.54 (C-9), 146.02 (C-13), 135.12 (C-8), 132.64 (2C, C-2'' and C-6''), 132.48 (C-1''), 129.86 (2C, C-3'' and C-5''), 129.02 (C-4''), 127.31 (C-14), 124.74 (C-11), 124.34 (C-12), 120.71 (C-7'), 72.80 (C-18), 54.04 (CH2S), 44.68 (C-5), 38.72, 37.85, 37.21, 35.99, 34.29, 33.87, 30.69, 29.14, 25.82, 25.70, 24.85, 24.43 (2C, C-16 and C-17), 19.41, 18.97, 17.94; EIMS m/z 560.4263 [M+H]+ (calcd for C34H46N3O2S, 560.3310). 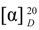 +21 (c 0.017, CHCl3); IR νmax (film) 2928, 2864, 1726, 1442, 1257, 833 cm−1; 1H-NMR (CDCl3): δ 7.70 (1H, s, H-5'), 7.59 (2H, d, J = 9.0 Hz, H-2'' and H-6''), 7.16 (1H, d, J = 8.1 Hz, H-11), 7.00 (2H, d, J = 9.0 Hz, H-3'' and H-5''), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 3.98 (1H, d, J = 10.9 Hz, H-18), 3.86 (3H, s, PhOMe), 3.75 (1H, d, J = 10.9 Hz, H-18), 3.10 (2H, t,J = 7.3 Hz, H-3'), 2.87 (2H, m, H-7), 2.82 (1H, m, H-15), 2.78 (2H, t, J = 7.3 Hz, H-2'), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.32 (C-1'), 160.07 (C-4''), 147.46 (C-9), 147.29 (C-4'), 146.01 (C-13), 135.06 (C-8), 131.05 (C-1''), 127.28 (C-14), 124.71 (C-11), 124.32 (C-12), 122.45 (2C, C-2'' and C-6''), 120.09 (C-5'), 115.11 (2C, C-3'' and C-5''), 73.15 (C-18), 56.03 (OMe), 44.79 (C-5), 38.66, 37.83, 37.20, 35.96, 34.08, 33.84, 30.65, 25.78, 24.40 (2C, C-16 and C-17), 21.47, 19.42, 18.92, 17.84; EIMS m/z 516.4582 [M+H]+ (calcd for C32H42N3O3, 516.3226).
+21 (c 0.017, CHCl3); IR νmax (film) 2928, 2864, 1726, 1442, 1257, 833 cm−1; 1H-NMR (CDCl3): δ 7.70 (1H, s, H-5'), 7.59 (2H, d, J = 9.0 Hz, H-2'' and H-6''), 7.16 (1H, d, J = 8.1 Hz, H-11), 7.00 (2H, d, J = 9.0 Hz, H-3'' and H-5''), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 3.98 (1H, d, J = 10.9 Hz, H-18), 3.86 (3H, s, PhOMe), 3.75 (1H, d, J = 10.9 Hz, H-18), 3.10 (2H, t,J = 7.3 Hz, H-3'), 2.87 (2H, m, H-7), 2.82 (1H, m, H-15), 2.78 (2H, t, J = 7.3 Hz, H-2'), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.32 (C-1'), 160.07 (C-4''), 147.46 (C-9), 147.29 (C-4'), 146.01 (C-13), 135.06 (C-8), 131.05 (C-1''), 127.28 (C-14), 124.71 (C-11), 124.32 (C-12), 122.45 (2C, C-2'' and C-6''), 120.09 (C-5'), 115.11 (2C, C-3'' and C-5''), 73.15 (C-18), 56.03 (OMe), 44.79 (C-5), 38.66, 37.83, 37.20, 35.96, 34.08, 33.84, 30.65, 25.78, 24.40 (2C, C-16 and C-17), 21.47, 19.42, 18.92, 17.84; EIMS m/z 516.4582 [M+H]+ (calcd for C32H42N3O3, 516.3226). 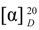 +12 (c 0.060, CHCl3); IR νmax (film) 2937, 2867, 1729, 1461, 1257, 830 cm−1; 1H-NMR (CDCl3): δ 7.61 (1H, s, H-7'), 7.60 (2H, d, J = 8.9 Hz, H-2'' and H-6''), 7.17 (1H, d, J = 8.1 Hz, H-11), 7.00 (2H, d, J = 9.0 Hz, H-3'' and H-5''), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.97 (1H, d, J = 10.9 Hz, H-18), 3.86 (3H, s, PhOMe), 3.71 (1H, d, J = 10.9 Hz, H-18), 2.86 (2H, m, H-7), 2.81 (1H, m, H-15), 2.78 (2H, t, J = 7.3 Hz, H-5'), 2.35 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.15 (C-1'), 160.01 (C-4''), 148.66 (C-6'), 147.54 (C-9), 146.02 (C-13), 135.12 (C-8), 131.17 (C-1''), 127.29 (C-14), 124.71 (C-11), 124.30 (C-12), 122.49 (2C, C-2'' and C-6''), 119.54 (C-7'), 115.10 (2C, C-3'' and C-5''), 72.84 (C-18), 56.03 (OMe), 44.68 (C-5), 38.69, 37.83, 37.19, 35.99, 34.47, 33.83, 30.66, 29.23, 25.79, 25.74, 24.95, 24.38 (2C, C-16 and C-17), 19.39, 18.94, 17.90; EIMS m/z 544.4256 [M+H]+ (calcd for C34H46N3O3, 544.3539).
+12 (c 0.060, CHCl3); IR νmax (film) 2937, 2867, 1729, 1461, 1257, 830 cm−1; 1H-NMR (CDCl3): δ 7.61 (1H, s, H-7'), 7.60 (2H, d, J = 8.9 Hz, H-2'' and H-6''), 7.17 (1H, d, J = 8.1 Hz, H-11), 7.00 (2H, d, J = 9.0 Hz, H-3'' and H-5''), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.97 (1H, d, J = 10.9 Hz, H-18), 3.86 (3H, s, PhOMe), 3.71 (1H, d, J = 10.9 Hz, H-18), 2.86 (2H, m, H-7), 2.81 (1H, m, H-15), 2.78 (2H, t, J = 7.3 Hz, H-5'), 2.35 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.15 (C-1'), 160.01 (C-4''), 148.66 (C-6'), 147.54 (C-9), 146.02 (C-13), 135.12 (C-8), 131.17 (C-1''), 127.29 (C-14), 124.71 (C-11), 124.30 (C-12), 122.49 (2C, C-2'' and C-6''), 119.54 (C-7'), 115.10 (2C, C-3'' and C-5''), 72.84 (C-18), 56.03 (OMe), 44.68 (C-5), 38.69, 37.83, 37.19, 35.99, 34.47, 33.83, 30.66, 29.23, 25.79, 25.74, 24.95, 24.38 (2C, C-16 and C-17), 19.39, 18.94, 17.90; EIMS m/z 544.4256 [M+H]+ (calcd for C34H46N3O3, 544.3539). 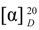 +19 (c 0.014, CHCl3); IR νmax (film) 2928, 2864, 1726, 1460, 1248, 776 cm−1; 1H-NMR (CDCl3): δ 7.78 (1H, s, H-5'), 7.76 (1H, s, H-2''), 7.61 (1H, d, J = 8.0 Hz, H-6''), 7.45 (1H, t, J = 8.0 Hz, H-5''), 7.39 (1H, d, J = 8.0 Hz, H-4''), 7.16 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 3.98 (1H, d, J = 10.9 Hz, H-18) 3.75 (1H, d, J = 10.9 Hz, H-18), 3.11 (2H, t, J = 7.3 Hz, H-3'), 2.86 (2H, m, H-7), 2.80 (1H, m, H-15), 2.78 (2H, t, J = 7.3 Hz, H-2'), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.92 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.23 (C-1'), 147.81 (C-4'), 147.45 (C-9), 146.03 (C-13), 138.36 (C-3''), 135.94 (C-1''), 135.04 (C-8), 131.20 (C-5''), 129.00 (C-4''), 127.28 (C-14), 124.71 (C-11), 124.33 (C-12), 121.02 (C-2''), 119.85 (C-5'), 118.72 (C-6''), 73.15 (C-18), 44.74 (C-5), 38.64, 37.82, 37.21, 35.96, 33.90, 33.84, 30.64, 25.77, 24.40 (2C, C-16 and C-17), 21.40, 19.41, 18.91, 17.84; EIMS m/z 520.2411 [M+H]+ (calcd for C31H39ClN3O2, 520.2731).
+19 (c 0.014, CHCl3); IR νmax (film) 2928, 2864, 1726, 1460, 1248, 776 cm−1; 1H-NMR (CDCl3): δ 7.78 (1H, s, H-5'), 7.76 (1H, s, H-2''), 7.61 (1H, d, J = 8.0 Hz, H-6''), 7.45 (1H, t, J = 8.0 Hz, H-5''), 7.39 (1H, d, J = 8.0 Hz, H-4''), 7.16 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.86 (1H, brs, H-14), 3.98 (1H, d, J = 10.9 Hz, H-18) 3.75 (1H, d, J = 10.9 Hz, H-18), 3.11 (2H, t, J = 7.3 Hz, H-3'), 2.86 (2H, m, H-7), 2.80 (1H, m, H-15), 2.78 (2H, t, J = 7.3 Hz, H-2'), 2.26 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.92 (3H, s, H-19); 13C-NMR (CDCl3): δ 173.23 (C-1'), 147.81 (C-4'), 147.45 (C-9), 146.03 (C-13), 138.36 (C-3''), 135.94 (C-1''), 135.04 (C-8), 131.20 (C-5''), 129.00 (C-4''), 127.28 (C-14), 124.71 (C-11), 124.33 (C-12), 121.02 (C-2''), 119.85 (C-5'), 118.72 (C-6''), 73.15 (C-18), 44.74 (C-5), 38.64, 37.82, 37.21, 35.96, 33.90, 33.84, 30.64, 25.77, 24.40 (2C, C-16 and C-17), 21.40, 19.41, 18.91, 17.84; EIMS m/z 520.2411 [M+H]+ (calcd for C31H39ClN3O2, 520.2731). 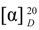 +17 (c 0.012, CHCl3); IR νmax (film) 2934, 2864, 1726, 1461, 1241, 782 cm−1; 1H-NMR (CDCl3): δ 7.76 (1H, s, H-2''), 7.69 (1H, s, H-5'), 7.63 (1H, d, J = 8.0 Hz, H-6''), 7.45 (1H, t, J = 8.0 Hz, H-5''), 7.39 (1H, d, J = 8.0 Hz, H-4''), 7.17 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.97 (1H, d, J = 10.9 Hz, H-18) 3.72 (1H, d, J = 10.9 Hz, H-18), 2.86 (2H, m, H-7), 2.83 (1H, m, H-15), 2.80 (2H, t, J = 7.3 Hz, H-5'), 2.35 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.94 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.06 (C-1'), 149.16 (C-6'), 147.55 (C-9), 146.04 (C-13), 138.48 (C-3''), 135.91 (C-1''), 135.10 (C-8), 131.16 (C-5''), 128.90 (C-4''), 127.28 (C-14), 124.70 (C-11), 124.30 (C-12), 121.03 (C-2''), 119.19 (C-7'), 118.75 (C-6''), 72.83 (C-18), 44.70 (C-5), 38.73, 37.84, 37.21, 36.00, 34.43, 33.83, 30.65, 29.10, 25.75, 25.68, 24.90, 24.36 (2C, C-16 and C-17), 19.40, 18.94, 17.90; EIMS m/z 548.4267 [M+H]+ (calcd for C33H43ClN3O2, 548.3044).
+17 (c 0.012, CHCl3); IR νmax (film) 2934, 2864, 1726, 1461, 1241, 782 cm−1; 1H-NMR (CDCl3): δ 7.76 (1H, s, H-2''), 7.69 (1H, s, H-5'), 7.63 (1H, d, J = 8.0 Hz, H-6''), 7.45 (1H, t, J = 8.0 Hz, H-5''), 7.39 (1H, d, J = 8.0 Hz, H-4''), 7.17 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.97 (1H, d, J = 10.9 Hz, H-18) 3.72 (1H, d, J = 10.9 Hz, H-18), 2.86 (2H, m, H-7), 2.83 (1H, m, H-15), 2.80 (2H, t, J = 7.3 Hz, H-5'), 2.35 (2H, t, J = 7.3 Hz, H-2'), 2.27 (1H, brd, J = 12.9 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.94 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.06 (C-1'), 149.16 (C-6'), 147.55 (C-9), 146.04 (C-13), 138.48 (C-3''), 135.91 (C-1''), 135.10 (C-8), 131.16 (C-5''), 128.90 (C-4''), 127.28 (C-14), 124.70 (C-11), 124.30 (C-12), 121.03 (C-2''), 119.19 (C-7'), 118.75 (C-6''), 72.83 (C-18), 44.70 (C-5), 38.73, 37.84, 37.21, 36.00, 34.43, 33.83, 30.65, 29.10, 25.75, 25.68, 24.90, 24.36 (2C, C-16 and C-17), 19.40, 18.94, 17.90; EIMS m/z 548.4267 [M+H]+ (calcd for C33H43ClN3O2, 548.3044). 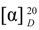 +22 (c 0.022, CHCl3); IR νmax (film) 2934, 2864, 1729, 1461, 1240, 816 cm−1; 1H-NMR (CDCl3): δ 7.97 (2H, d, J = 8.1 Hz, H-2'' and H-6''), 7.91 (1H, s, H-5'), 7.37 (2H, d, J = 8.1 Hz, H-3'' and H-5''), 7.17 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.94 (1H, d, J = 10.9 Hz, H-18) 3.72 (1H, d, J = 10.9 Hz, H-18), 3.02 (2H, t, J = 7.3 Hz, H-3'), 2.88 (2H, m, H-7), 2.81 (1H, m, H-15), 2.69 (2H, t, J = 7.3 Hz, H-2'), 2.43 (3H, s, PhMe), 2.27 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.90 (3H, s, H-19); 13C-NMR (CDCl3): δ 172.82 (C-1'), 147.59 (C-4'), 147.44 (C-9), 146.64 (C-4''), 146.04 (C-13), 135.04 (C-8), 133.59 (C-1''), 130.82 (2C, C-2'' and C-6''), 129.05 (2C, C-3'' and C-5''), 127.30 (C-14), 124.68 (C-11), 124.33 (C-12), 121.43 (C-5'), 73.25 (C-18), 44.76 (C-5), 38.65, 37.81, 37.17, 35.95, 33.84, 33.50, 30.62, 25.77, 24.39 (2C, C-16 and C-17), 21.18, 19.40, 18.89, 17.80; EIMS m/z 564.3065 [M+H]+ (calcd for C32H42N3O4S, 564.2896).
+22 (c 0.022, CHCl3); IR νmax (film) 2934, 2864, 1729, 1461, 1240, 816 cm−1; 1H-NMR (CDCl3): δ 7.97 (2H, d, J = 8.1 Hz, H-2'' and H-6''), 7.91 (1H, s, H-5'), 7.37 (2H, d, J = 8.1 Hz, H-3'' and H-5''), 7.17 (1H, d, J = 8.1 Hz, H-11), 6.99 (1H, brd, J = 8.1 Hz, H-12), 6.88 (1H, brs, H-14), 3.94 (1H, d, J = 10.9 Hz, H-18) 3.72 (1H, d, J = 10.9 Hz, H-18), 3.02 (2H, t, J = 7.3 Hz, H-3'), 2.88 (2H, m, H-7), 2.81 (1H, m, H-15), 2.69 (2H, t, J = 7.3 Hz, H-2'), 2.43 (3H, s, PhMe), 2.27 (1H, brd, J = 12.7 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.20 (3H, s, H-20), 0.90 (3H, s, H-19); 13C-NMR (CDCl3): δ 172.82 (C-1'), 147.59 (C-4'), 147.44 (C-9), 146.64 (C-4''), 146.04 (C-13), 135.04 (C-8), 133.59 (C-1''), 130.82 (2C, C-2'' and C-6''), 129.05 (2C, C-3'' and C-5''), 127.30 (C-14), 124.68 (C-11), 124.33 (C-12), 121.43 (C-5'), 73.25 (C-18), 44.76 (C-5), 38.65, 37.81, 37.17, 35.95, 33.84, 33.50, 30.62, 25.77, 24.39 (2C, C-16 and C-17), 21.18, 19.40, 18.89, 17.80; EIMS m/z 564.3065 [M+H]+ (calcd for C32H42N3O4S, 564.2896). 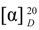 +30 (c 0.035, CHCl3); IR νmax (film) 2937, 2867, 1726, 1451, 1250, 816 cm−1; 1H-NMR (CDCl3): δ 7.93 (2H, d, J = 8.1 Hz, H-2'' and H-6''), 7.72 (1H, s, H-5'), 7.32 (2H, d, J = 8.1 Hz, H-3'' and H-5''), 7.18 (1H, d, J = 8.1 Hz, H-11), 7.01 (1H, brd, J = 8.1 Hz, H-12), 6.89 (1H, brs, H-14), 3.99 (1H, d, J = 10.9 Hz, H-18) 3.66 (1H, d, J = 10.9 Hz, H-18), 2.86 (2H, m, H-7), 2.83 (1H, m, H-15), 2.81 (2H, t,J = 7.3 Hz, H-5'), 2.43 (3H, s, PhMe), 2.24 (2H, t, J = 7.3 Hz, H-2'), 2.20 (1H, brd, J = 12.9 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.20 (C-1'), 148.65 (C-6'), 147.60 (C-9), 146.10 (C-13), 145.42 (C-4''), 135.18 (C-8), 133.20 (C-1''), 129.98 (2C, C-2'' and C-6''), 128.76 (2C, C-3'' and C-5''), 127.33 (C-14), 124.72 (C-11), 124.33 (C-12), 119.33 (C-7'), 72.73 (C-18), 44.58 (C-5), 38.74, 37.83, 37.23, 35.94, 34.38, 33.83, 30.64, 28.63, 25.77, 25.70, 24.82, 24.40 (2C, C-16 and C-17), 22.08, 19.36, 18.92, 17.90; EIMS m/z 592.3027 [M+H]+ (calcd for C34H46N3O4S, 592.3209).
+30 (c 0.035, CHCl3); IR νmax (film) 2937, 2867, 1726, 1451, 1250, 816 cm−1; 1H-NMR (CDCl3): δ 7.93 (2H, d, J = 8.1 Hz, H-2'' and H-6''), 7.72 (1H, s, H-5'), 7.32 (2H, d, J = 8.1 Hz, H-3'' and H-5''), 7.18 (1H, d, J = 8.1 Hz, H-11), 7.01 (1H, brd, J = 8.1 Hz, H-12), 6.89 (1H, brs, H-14), 3.99 (1H, d, J = 10.9 Hz, H-18) 3.66 (1H, d, J = 10.9 Hz, H-18), 2.86 (2H, m, H-7), 2.83 (1H, m, H-15), 2.81 (2H, t,J = 7.3 Hz, H-5'), 2.43 (3H, s, PhMe), 2.24 (2H, t, J = 7.3 Hz, H-2'), 2.20 (1H, brd, J = 12.9 Hz, H-1β), 1.22 (6H, d, J = 6.9 Hz, H-16 and H-17), 1.21 (3H, s, H-20), 0.93 (3H, s, H-19); 13C-NMR (CDCl3): δ 174.20 (C-1'), 148.65 (C-6'), 147.60 (C-9), 146.10 (C-13), 145.42 (C-4''), 135.18 (C-8), 133.20 (C-1''), 129.98 (2C, C-2'' and C-6''), 128.76 (2C, C-3'' and C-5''), 127.33 (C-14), 124.72 (C-11), 124.33 (C-12), 119.33 (C-7'), 72.73 (C-18), 44.58 (C-5), 38.74, 37.83, 37.23, 35.94, 34.38, 33.83, 30.64, 28.63, 25.77, 25.70, 24.82, 24.40 (2C, C-16 and C-17), 22.08, 19.36, 18.92, 17.90; EIMS m/z 592.3027 [M+H]+ (calcd for C34H46N3O4S, 592.3209). 3.3. Antiproliferative Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Wada, H.; Kodato, S.; Kawamori, M.; Morikawa, T.; Nakai, H.; Takeda, M.; Saito, S.; Onoda, Y.; Tamaki, H. Antiulcer activity of dehydroabietic acid derivatives. Chem. Pharm. Bull. 1985, 33, 1472–1487. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Astudillo, L.; Rodríguez, J.A.; Yañez, T.; Theoduloz, C.; Schmeda-Hirschmann, G. Gastroprotective and cytotoxic effect of dehydroabietic acid derivatives. Pharmacol. Res. 2005, 52, 429–437. [Google Scholar] [CrossRef]
- Gigante, B.; Silva, A.M.; Marcelo-Curto, M.J.; Feio, S.S.; Roseiro, J.; Reis, L.V. Structural effects on the bioactivity of dehydroabietic acid derivatives. Planta Med. 2002, 68, 680–684. [Google Scholar] [CrossRef]
- Tagat, J.R.; Nazareno, D.V.; Puar, M.S.; McCombie, S.W.; Ganguly, A.K. Synthesis and anti-herpes activity of some a-ring functionalized dehydroabietane derivatives. Bioorg. Med. Chem. Lett. 1994, 4, 1101–1104. [Google Scholar] [CrossRef]
- Tanaka, R.; Tokuda, H.; Ezaki, Y. Cancer chemopreventive activity of “rosin” constituents of Pinusspez, and their derivatives in two-stage mouses kin carcinogenesis test. Phytomedicine 2008, 15, 985–992. [Google Scholar] [CrossRef]
- Huang, X.-C.; Wang, M.; Pan, Y.-M.; Yao, G.-Y.; Wang, H.-S.; Tian, X.-Y.; Qin, J.-K.; Zhang, Y. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur. J. Med. Chem. 2013, 69, 508–520. [Google Scholar]
- Lin, C.-H.; Chuang, H.-S. Use of abietic acid and derivatives thereof for inhibiting cáncer. U.S. Patent 7015248 B2, 21 March 2006. [Google Scholar]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical–biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- De las Heras, F.G.; Alonso, R.; Alonso, G.J. Synthesis and cytostatic activity of N-glycosyl(halomethyl)-1,2,3-triazoles. A new type of alkylating agent. J. Med. Chem. 1979, 22, 496–501. [Google Scholar] [CrossRef]
- Duan, Y.-C.; Ma, Y.-C.; Zhang, E.; Shi, X.-J.; Wang, M.-M.; Ye, X.-W.; Liu, H.-M. Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur. J. Med. Chem. 2013, 62, 11–19. [Google Scholar] [CrossRef]
- Duan, Y.-C.; Zheng, Y.-C.; Li, X.-C.; Wang, M.-M.; Ye, X.-W.; Guan, Y.-Y.; Liu, G.-Z.; Zheng, J.-X.; Liu, H.-M. Design, synthesis and antiproliferative activity studies of novel 1,2,3-triazole-dithiocarbamate-urea hybrids. Eur. J. Med. Chem. 2013, 64, 99–110. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Zhuo, S.-T.; Li, C.-Y.; Xie, H.-T.; Li, D.; Tan, J.-H.; Ou, T.-M.; Huang, Z.-H.; Gu, L.-Q.; Huang, S.-L. Design, synthesis and biological evaluation of novel mansonone E derivatives prepared via CuAAC click chemistry as topoisomerase II inhibitors. Eur. J. Med. Chem. 2013, 68, 58–71. [Google Scholar] [CrossRef]
- Stefely, J.A.; Palchaudhuri, R.; Miller, P.A.; Peterson, R.J.; Moraski, G.C.; Hergenrother, P.J.; Miller, M.J. N-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)arylamide as a new scaffold that provides rapid access to antimicrotubule agents: Synthesis and evaluation of antiproliferative activity against select cancer cell lines. J. Med. Chem. 2010, 53, 3389–3395. [Google Scholar] [CrossRef]
- Soltis, M.J.; Yeh, H.J.; Cole, K.A.; Whittaker, N.; Wersto, R.P.; Kohn, E.C. Identification and characterization of human metabolites of CAI [5-amino-1-1(4'-chlorobenzoyl-3,5-dichlorobenzyl)-1,2,3-triazole-4-carboxamide). Drug Metab. Dispos. 1996, 24, 799–806. [Google Scholar]
- Johnson, E.A.; Marks, R.S.; Mandrekar, S.J.; Hillman, S.L.; Hauge, M.D.; Bauman, M.D.; Wos, E.J.; Moore, D.F.; Kugler, J.W.; Windschitl, H.E.; et al. Phase III randomized, double-blind study of maintenance CAI or placebo in patients with advanced non-small cell lung cancer (NSCLC) after completion of initial therapy (NCCTG 97-24-51). Lung Cancer 2008, 60, 200–207. [Google Scholar] [CrossRef]
- Pertino, M.W.; Theoduloz, C.; Bastías, M.; Schmeda-Hirschmann, G. Dimeric labdane diterpenes: Synthesis and antiproliferative effects. Molecules 2013, 18, 5936–5953. [Google Scholar] [CrossRef] [Green Version]
- Pertino, M.W.; Lopez, C.; Theoduloz, C.; Schmeda-Hirschmann, G. 1,2,3-Triazole-substituted oleanolic acid derivatives: Synthesis and antiproliferative activity. Molecules 2013, 18, 7661–7674. [Google Scholar] [CrossRef]
- Kádár, Z.; Molnár, J.; Schneider, G.; Zupkó, I.; Frank, E. A facile ‘click’ approach to novel 15β-triazolyl-5α-androstane derivatives, and an evaluation of their antiproliferative activities in vitro. Bioorg. Med. Chem. 2012, 20, 1396–1402. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Wang, Q.; Yuan, Y.; Zhang, H.; Qian, X. The novel anti-tumor agents of 4-triazol-1,8-naphthalimides: Synthesis, cytotoxicity, DNA intercalation and photocleavage. Eur. J. Med. Chem. 2011, 46, 1274–1279. [Google Scholar] [CrossRef]
- Yao, K.; Wang, J.; Zhang, W.; Lee, J.S.; Wang, C.; Chu, F.; He, X.; Tang, C. Degradable rosin-ester-caprolactone graft copolymers. Biomacromolecules 2011, 12, 2171–2177. [Google Scholar] [CrossRef]
- Halbrook, N.J.; Lawrence, R.V. The isolation of dehydroabietic acid from disproportionated rosin. J. Org. Chem. 1966, 31, 4246–4247. [Google Scholar] [CrossRef]
- OriginPro 8.1; OriginLab: Northampton, MA, USA, 2012.
- Sample Availability: Samples of the compounds 1–16 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pertino, M.W.; Verdugo, V.; Theoduloz, C.; Schmeda-Hirschmann, G. Synthesis and Antiproliferative Activity of Some Novel Triazole Derivatives from Dehydroabietic Acid. Molecules 2014, 19, 2523-2535. https://doi.org/10.3390/molecules19022523
Pertino MW, Verdugo V, Theoduloz C, Schmeda-Hirschmann G. Synthesis and Antiproliferative Activity of Some Novel Triazole Derivatives from Dehydroabietic Acid. Molecules. 2014; 19(2):2523-2535. https://doi.org/10.3390/molecules19022523
Chicago/Turabian StylePertino, Mariano Walter, Valery Verdugo, Cristina Theoduloz, and Guillermo Schmeda-Hirschmann. 2014. "Synthesis and Antiproliferative Activity of Some Novel Triazole Derivatives from Dehydroabietic Acid" Molecules 19, no. 2: 2523-2535. https://doi.org/10.3390/molecules19022523




