Three New Clerodane Diterpenes from Polyalthia longifolia var. pendula
Abstract
:1. Introduction
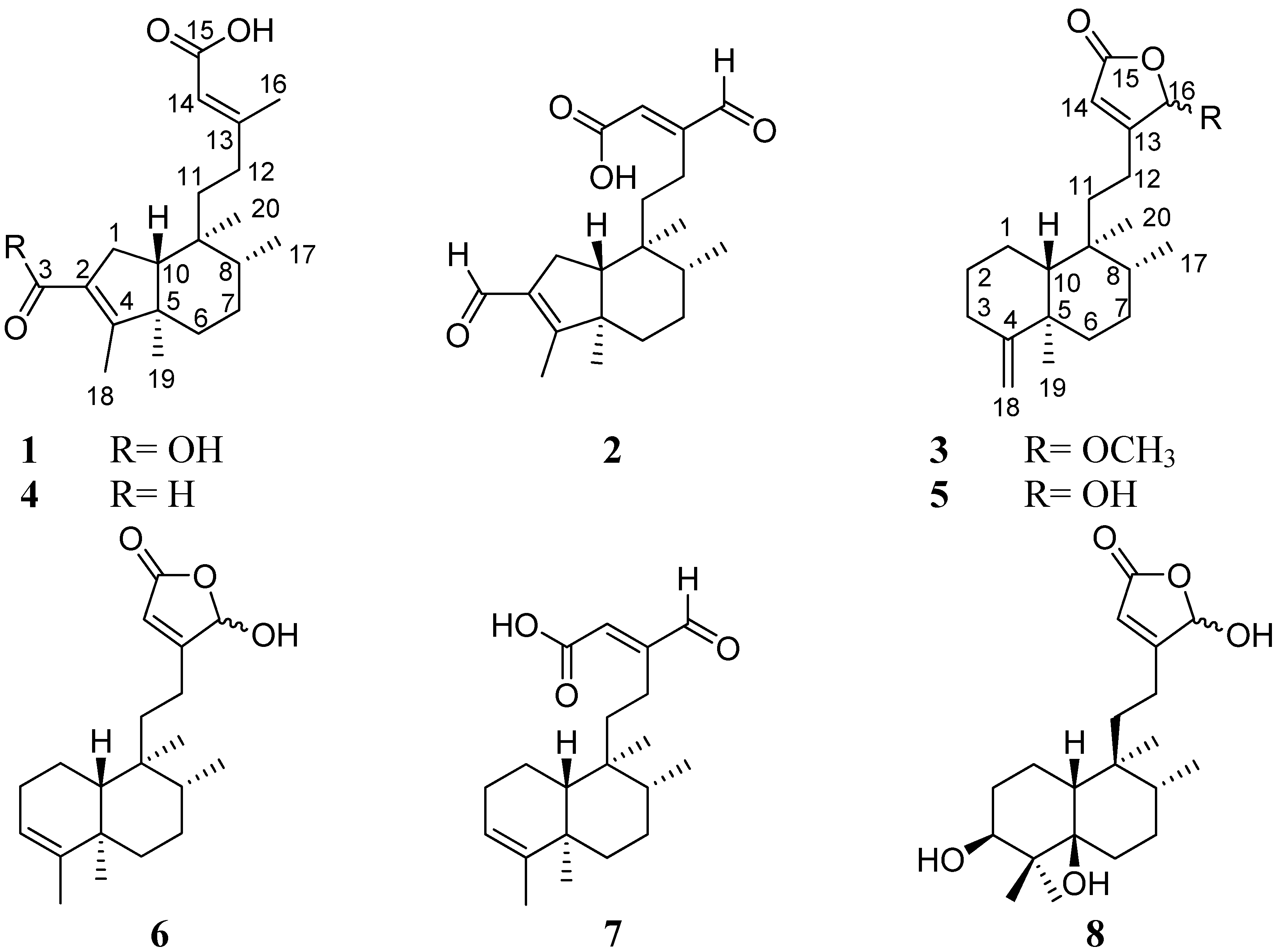
2. Results and Discussion
| No. | 1 a | 2 b | 3 c | ||||
|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | ||
| 1 | 29.3 | 2.30 dd (14.6, 6.0) | 25.5 | 2.64 m | 21.1 | 1.40–1.55 m | |
| 2.35 dd (14.6, 2.1) | 2.15 m | ||||||
| 2 | 126.7 | - | 137.3 | - | 32.4 | 2.05 m | |
| 2.24 m | |||||||
| 3 | 169.6 * | - | 189.8 | 9.95 s | 28.01/28.03 | 1.20 m | |
| 1.84 m | |||||||
| 4 | 164.9 | - | 174.9 | - | 159.0 | - | |
| 5 | 50.1 | - | 51.1 | - | 40.0 | - | |
| 6 | 34.4 | 1.37 td (11.5, 5.0) | 38.5 | 1.65 m | 36.8 | 1.45–1.58 m | |
| 1.67 m | 1.45 m | ||||||
| 7 | 28.1 | 1.57 m | 28.3 | 1.60 m | 26.9 | 1.40–1.55 m | |
| 1.60 m | |||||||
| 8 | 37.2 | 1.55 m | 37.1 | 1.64 m | 36.1 | 1.38–1.43 m | |
| 9 | 37.7 | - | 38.5 | - | 38.72/38.68 | - | |
| 10 | 54.1 | 1.69 dd (6.0, 2.1) | 53.8 | 1.71 m | 48.1 | 1.03 dt (12.0, 3.0 ) | |
| 11 | 38.1 | 1.47 td (8.5, 4.5) | 34.0 | 1.36 m | 34.00/34.02 | 1.40–1.60 m | |
| 12 | 34.5 | 2.07 m | 19.3 | 2.52 m | 20.7 | 2.02 m | |
| 2.12 m | 2.66 m | 2.23 m | |||||
| 13 | 160.2 | - | 154.7 | - | 168.9 | - | |
| 14 | 115.8 | 5.70 s | 135.3 | 6.49 s | 117.11/117.20 | 6.09 br s | |
| 15 | 169.4 * | - | 167.2 | - | 170.4 | - | |
| 16 | 17.7 | 2.15 s | 194.4 | 9.55 s | 104.0 | 5.91 s | |
| 17 | 14.0 | 0.87 d (6.0) | 14.9 | 0.92 d (6.5) | 15.8 | 0.77 d (6.5) | |
| 0.79 d (6.5) | |||||||
| 18 | 10.2 | 2.00 s | 9.9 | 2.08 s | 103.0 | 4.48 d (3.5) | |
| 19 | 15.9 | 0.95 s | 17.1 | 0.96 s | 20.5 | 1.02 s | |
| 20 | 17.1 | 0.92 s | 18.0 | 0.85 s | 17.7 | 0.74 s | |
| 16-OCH3 | 56.1 | 3.43 s/3.43 s | |||||
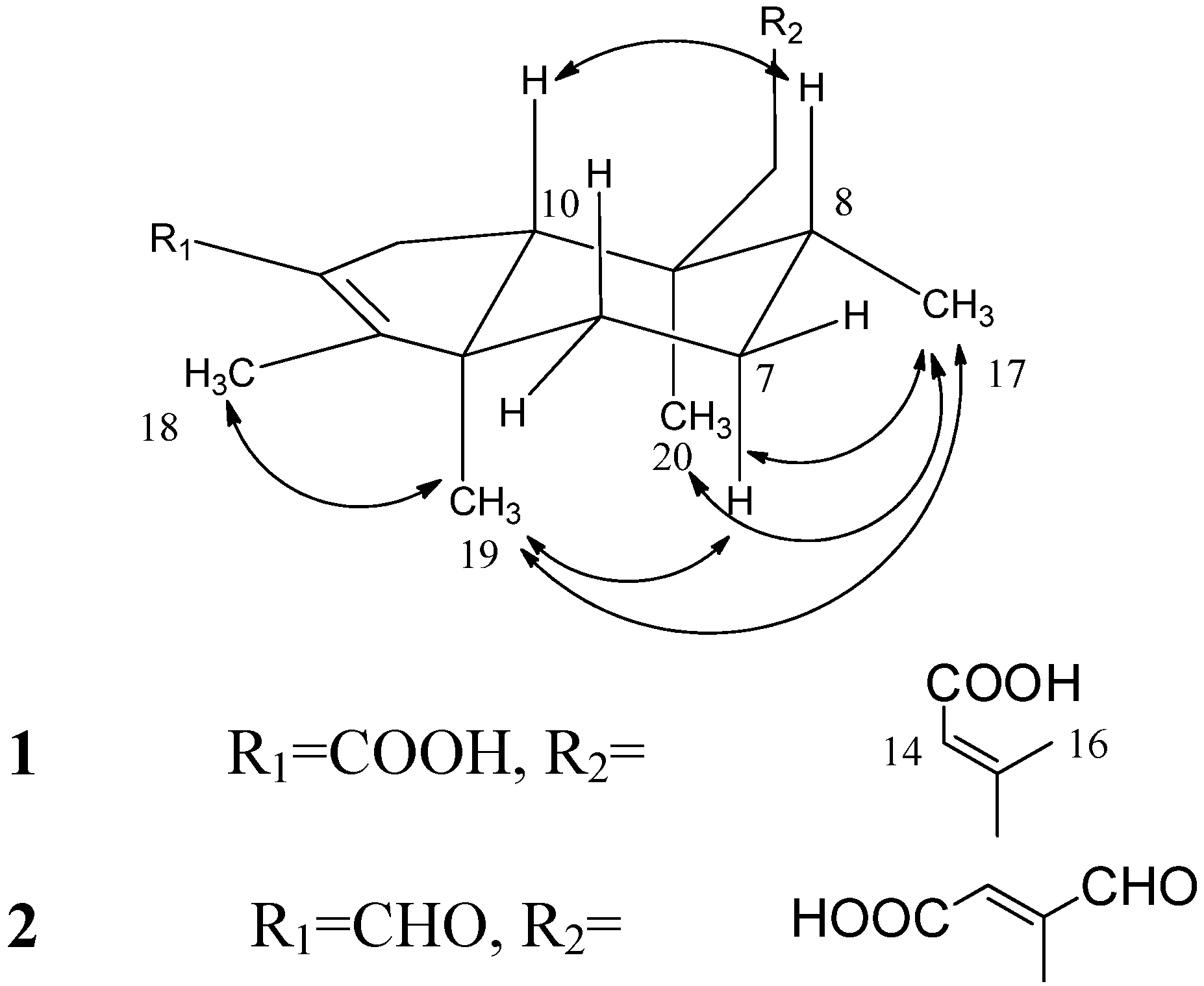
 ) and COSY (
) and COSY (  ) correlations of compounds 1–2.
) correlations of compounds 1–2.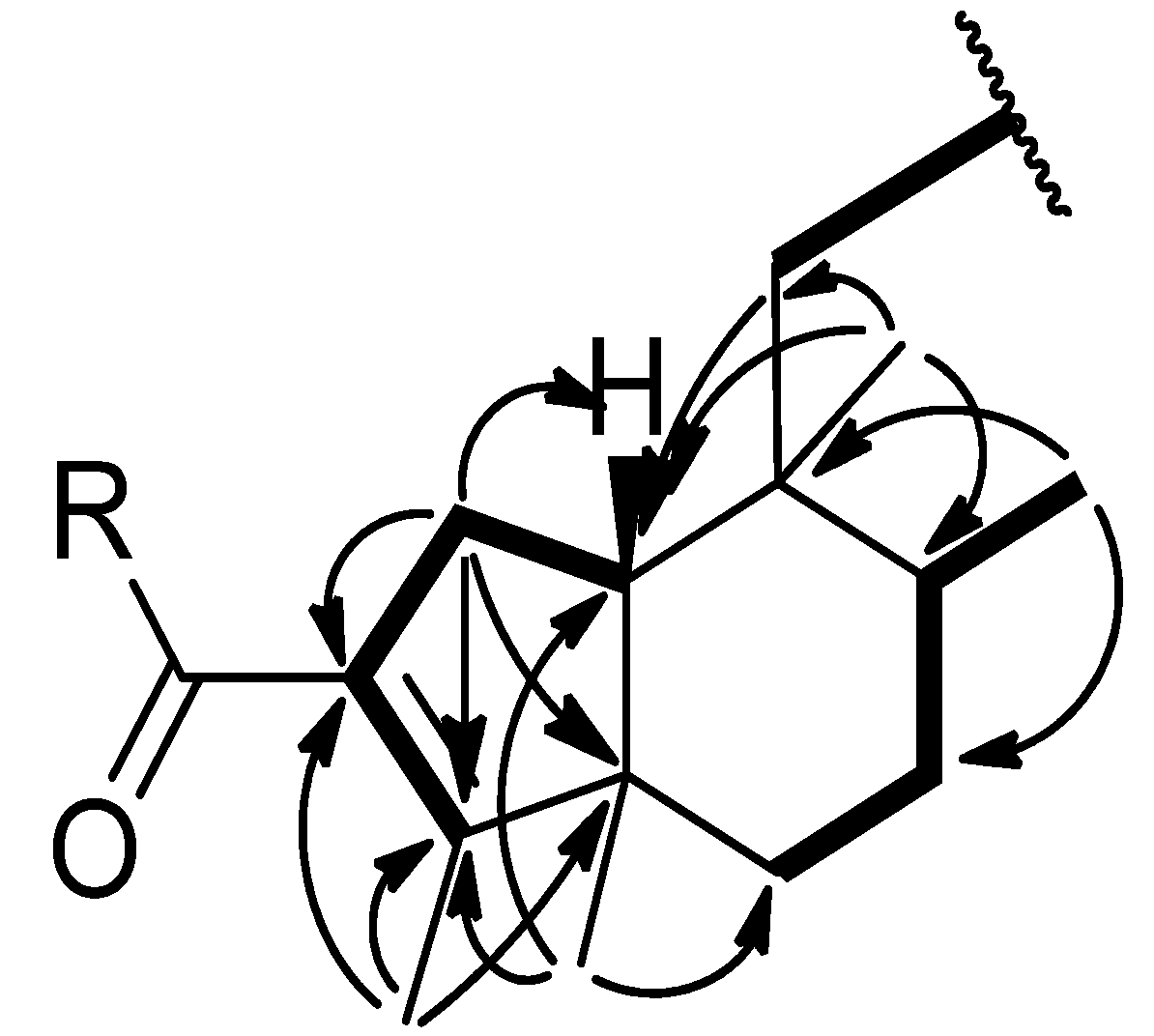

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
 +2.0 (c 0.10, CHCl3), UV λmax (log ε) (MeOH) nm: 220 (3.24). IR νmax (KBr) cm−1: 3,361, 2,957, 2,922, 1,684, 1,654, 1,437, 1,208, 1,142. ESIMS (positive mode) m/z: 357.3 [M+Na]+. ESIMS (negative mode) m/z: 333.3 [M-H]−. HR-FAB-MS m/z: 335.2226 [M+H]+ (calcd. for C20H31O4 335.2222). 1H-NMR (CD3OD, 500 MHz) and 13C-NMR (CD3OD, 125 MHz), see Table 1.
+2.0 (c 0.10, CHCl3), UV λmax (log ε) (MeOH) nm: 220 (3.24). IR νmax (KBr) cm−1: 3,361, 2,957, 2,922, 1,684, 1,654, 1,437, 1,208, 1,142. ESIMS (positive mode) m/z: 357.3 [M+Na]+. ESIMS (negative mode) m/z: 333.3 [M-H]−. HR-FAB-MS m/z: 335.2226 [M+H]+ (calcd. for C20H31O4 335.2222). 1H-NMR (CD3OD, 500 MHz) and 13C-NMR (CD3OD, 125 MHz), see Table 1. +13.3 (c 1.0, CHCl3), UV λmax (log ε) (MeOH) nm: 219 (2.78). IR νmax (KBr) cm−1: 2,924, 2,853, 1,687, 1,676, 1,639, 1,564, 1,383, 1,208, 1,183. ESIMS (positive mode) m/z: 355.2 [M+Na]+. ESIMS (negative mode) m/z: 331.3 [M-H]−. HR-FAB-MS m/z: 355.1880 [M+Na]+ (calcd for C20H28O4Na, 355.1885), 333.2060 [M+H]+ (calcd. for C20H29O4+, 333.2066). 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz), see Table 1.
+13.3 (c 1.0, CHCl3), UV λmax (log ε) (MeOH) nm: 219 (2.78). IR νmax (KBr) cm−1: 2,924, 2,853, 1,687, 1,676, 1,639, 1,564, 1,383, 1,208, 1,183. ESIMS (positive mode) m/z: 355.2 [M+Na]+. ESIMS (negative mode) m/z: 331.3 [M-H]−. HR-FAB-MS m/z: 355.1880 [M+Na]+ (calcd for C20H28O4Na, 355.1885), 333.2060 [M+H]+ (calcd. for C20H29O4+, 333.2066). 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz), see Table 1. −71.0 (c 0.1, MeOH), UV λmax (log ε) (MeOH) nm: 207 (4.32). IR νmax (KBr) cm−1: 2,955, 2,924, 2,853, 1,758, 1,680, 1,639, 1,456, 1,206. ESIMS (positive mode) m/z: 355.24 [M+Na]+. ESIMS (negative mode) m/z: 331.20 [M-H]−. HR-FAB-MS m/z: 355.2251 [M+Na]+ (calcd. for C21H32O3Na, 355.2249), 333.2427 [M+H]+ (calcd. for C21H33O3+, 333.2430). 1H-NMR (DMSO-d6, 500 MHz) and 13C-NMR (DMSO-d6, 125 MHz), see Table 1.
−71.0 (c 0.1, MeOH), UV λmax (log ε) (MeOH) nm: 207 (4.32). IR νmax (KBr) cm−1: 2,955, 2,924, 2,853, 1,758, 1,680, 1,639, 1,456, 1,206. ESIMS (positive mode) m/z: 355.24 [M+Na]+. ESIMS (negative mode) m/z: 331.20 [M-H]−. HR-FAB-MS m/z: 355.2251 [M+Na]+ (calcd. for C21H32O3Na, 355.2249), 333.2427 [M+H]+ (calcd. for C21H33O3+, 333.2430). 1H-NMR (DMSO-d6, 500 MHz) and 13C-NMR (DMSO-d6, 125 MHz), see Table 1.3.5. Cell Culture
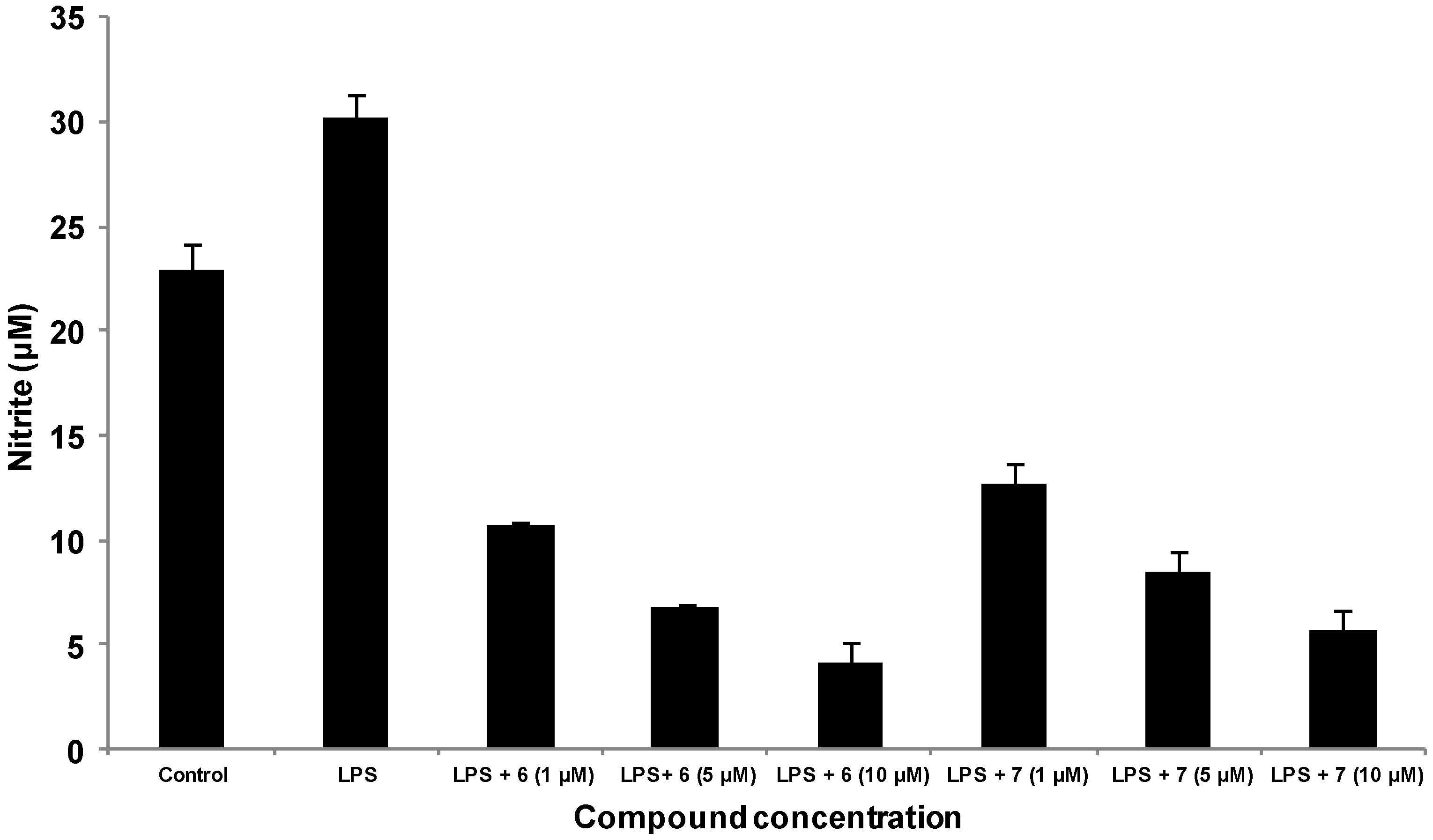
3.6. Detection of Nitric Oxide Expression by Griess Reaction
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, A.; Das, B.K.; Chatterjee, K.C.; Chandra, G. Antibacterial potentiality and phytochemical analysis of mature leaves of Polyalthia longifolia (Magnoliales: Annonaceae). South Pac. J. Nat. Sci. 2008, 26, 68–72. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmed, M.; Ahmed, S.I.; Azeem, M.; Khan, R.A.; Rasool, N.; Saleem, H.; Noor, F.; Faizi, S. Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia var. pendula. Phytother. Res. 2005, 19, 881–884. [Google Scholar] [CrossRef]
- Zhao, G.X.; Jung, J.H.; Smith, D.L.; Wood, K.V.; McLaughlin, J.L. Cytotoxic clerodane diterpenes from Polyalthia longifolia. Planta Med. 1991, 57, 380–383. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Shih, Y.C.; Hsieh, T.J.; Chia, Y.C.; Tseng, H.Y.; Chen, H.C.; Chen, S.J.; Hsu, M.C.; Wu, Y.C. Cytotoxic constituents of Polyalthia longifolia var. pendula. J. Nat. Prod. 2000, 63, 1475–1478. [Google Scholar] [CrossRef]
- Marthanda Murthy, M.; Subramanyam, M.; Hima Bindu, M. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005, 76, 336–339. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Shukla, P.K. Antimicrobial evaluation of clerodane diterpenes from Polyalthia longifolia var. pendula. Nat. Prod. Commun. 2009, 4, 327–330. [Google Scholar]
- Chang, F.R.; Hwang, T.L.; Yang, Y.L.; Li, C.E.; Wu, C.C.; Issa, H.H.; Hsieh, W.B.; Wu, Y.C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef]
- Misra, R.; Pandey, R.C.; Dev, S. Higher isoprenoids-IX: Diterpenoids from the oleoresin of Hardwickia pinnata Part 2: Kolavic, kolavenic, kolavenolic and kolavonic acids. Tetrahedron 1979, 35, 979–984. [Google Scholar] [CrossRef]
- Hara, N.; Asaki, H.; Fujimoto, Y.; Gupta, Y.K.; Singh, A.K.; Sahai, M. Clerodane and ent-halimane diterpenes from Polyalthia longifolia. Phytochemistry 1995, 38, 189–194. [Google Scholar] [CrossRef]
- Hao, X.J.; Yang, X.S.; Zhang, Z.; Shang, L.J. Clerodane diterpenes from Polyalthia cheliensis. Phytochemistry 1995, 39, 447–448. [Google Scholar] [CrossRef]
- Phadnis, A.P.; Patwardhan, S.A.; Dhaneshwar, N.N.; Tavale, S.S. Clerodane diterpenoids from Polyalthia longifolia. Phytochemistry 1988, 27, 2899–2901. [Google Scholar] [CrossRef]
- Tori, M.; Katto, A.; Sono, M. Nine new clerodane diterpenoids from rhizomes of Solidago altissima. Phytochemistry 1999, 52, 487–493. [Google Scholar] [CrossRef]
- Bomm, M.D.; Zukerman-Schpector, J.; Lopes, L.M.X. Rearranged (4→2)-abeo-clerodane and clerodane diterpenes from Aristolochia chamissonis. Phytochemistry 1999, 50, 455–461. [Google Scholar]
- Bohlmann, F.; Singh, P.; Singh, R.K.; Joshi, K.C.; Jakupovic, J. A diterpene with a new carbon skeleton from Solidago altissima. Phytochemistry 1985, 24, 1114–1115. [Google Scholar] [CrossRef]
- Kijjoa, A.; Pinto, M.M.M.; Pinho, P.M.M.; Tantisewie, B.; Herz, W. Clerodane derivatives from Polyalthia viridis. Phytochemistry 1990, 29, 653–655. [Google Scholar] [CrossRef]
- Manabe, S.; Nishino, C. Stereochemistry of cis-clerodane diterpenes. Tetrahedron 1986, 42, 3461–3470. [Google Scholar] [CrossRef]
- Kijjoa, A.; Pinto, M.M.M.; Pinho, P.M.M.; Herz, W. Clerodanes from Polyalthia viridis. Phytochemistry 1993, 34, 457–460. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987, 92, 639–646. [Google Scholar] [CrossRef]
- Garthwaite, J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991, 14, 60–67. [Google Scholar] [CrossRef]
- Stichtenoth, D.O.; Frolich, J.C. Nitric oxide and inflammatory joint diseases. Br. J. Rheumatol. 1998, 37, 246–257. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Park, W. Immunostimulatory effect of laminarin on RAW 264.7 mouse macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef]
- Wang, S.G.; Xu, Y.; Chen, J.D.; Yang, C.H.; Chen, X.H. Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 2013, 18, 12809–12819. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Tanna, A.; Nair, R.; Chanda, S. Assessment of anti-inflammatory and hepatoprotective potency of Polyalthia longifolia var. pendula leaf in Wistar albino rats. J. Nat. Med. 2009, 63, 80–85. [Google Scholar] [CrossRef]
- Shih, Y.T.; Hsu, Y.Y.; Chang, F.R.; Wu, Y.C.; Lo, Y.C. 6-Hydroxycleroda-3,13-dien-15,16-olide protects neuronal cells from lipopolysaccharide-induced neurotoxicity through the inhibition of microglia-mediated inflammation. Planta Med. 2010, 76, 120–127. [Google Scholar] [CrossRef]
- Lee, K.C.; Chang, H.H.; Chung, Y.H.; Lee, T.Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds in the manuscript are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, T.-H.; Cheng, Y.-Y.; Chen, C.-J.; Ng, L.-T.; Chou, L.-C.; Huang, L.-J.; Chen, Y.-H.; Kuo, S.-C.; El-Shazly, M.; Wu, Y.-C.; et al. Three New Clerodane Diterpenes from Polyalthia longifolia var. pendula. Molecules 2014, 19, 2049-2060. https://doi.org/10.3390/molecules19022049
Wu T-H, Cheng Y-Y, Chen C-J, Ng L-T, Chou L-C, Huang L-J, Chen Y-H, Kuo S-C, El-Shazly M, Wu Y-C, et al. Three New Clerodane Diterpenes from Polyalthia longifolia var. pendula. Molecules. 2014; 19(2):2049-2060. https://doi.org/10.3390/molecules19022049
Chicago/Turabian StyleWu, Tung-Ho, Yung-Yi Cheng, Chao-Jung Chen, Lean-Teik Ng, Li-Chen Chou, Li-Jiau Huang, Yung-Husan Chen, Sheng-Chu Kuo, Mohamed El-Shazly, Yang-Chang Wu, and et al. 2014. "Three New Clerodane Diterpenes from Polyalthia longifolia var. pendula" Molecules 19, no. 2: 2049-2060. https://doi.org/10.3390/molecules19022049
APA StyleWu, T.-H., Cheng, Y.-Y., Chen, C.-J., Ng, L.-T., Chou, L.-C., Huang, L.-J., Chen, Y.-H., Kuo, S.-C., El-Shazly, M., Wu, Y.-C., Chang, F.-R., & Liaw, C.-C. (2014). Three New Clerodane Diterpenes from Polyalthia longifolia var. pendula. Molecules, 19(2), 2049-2060. https://doi.org/10.3390/molecules19022049







