An Insecticidal Compound Produced by an Insect-Pathogenic Bacterium Suppresses Host Defenses through Phenoloxidase Inhibition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Insecticidal Effects of Metabolite Fractions
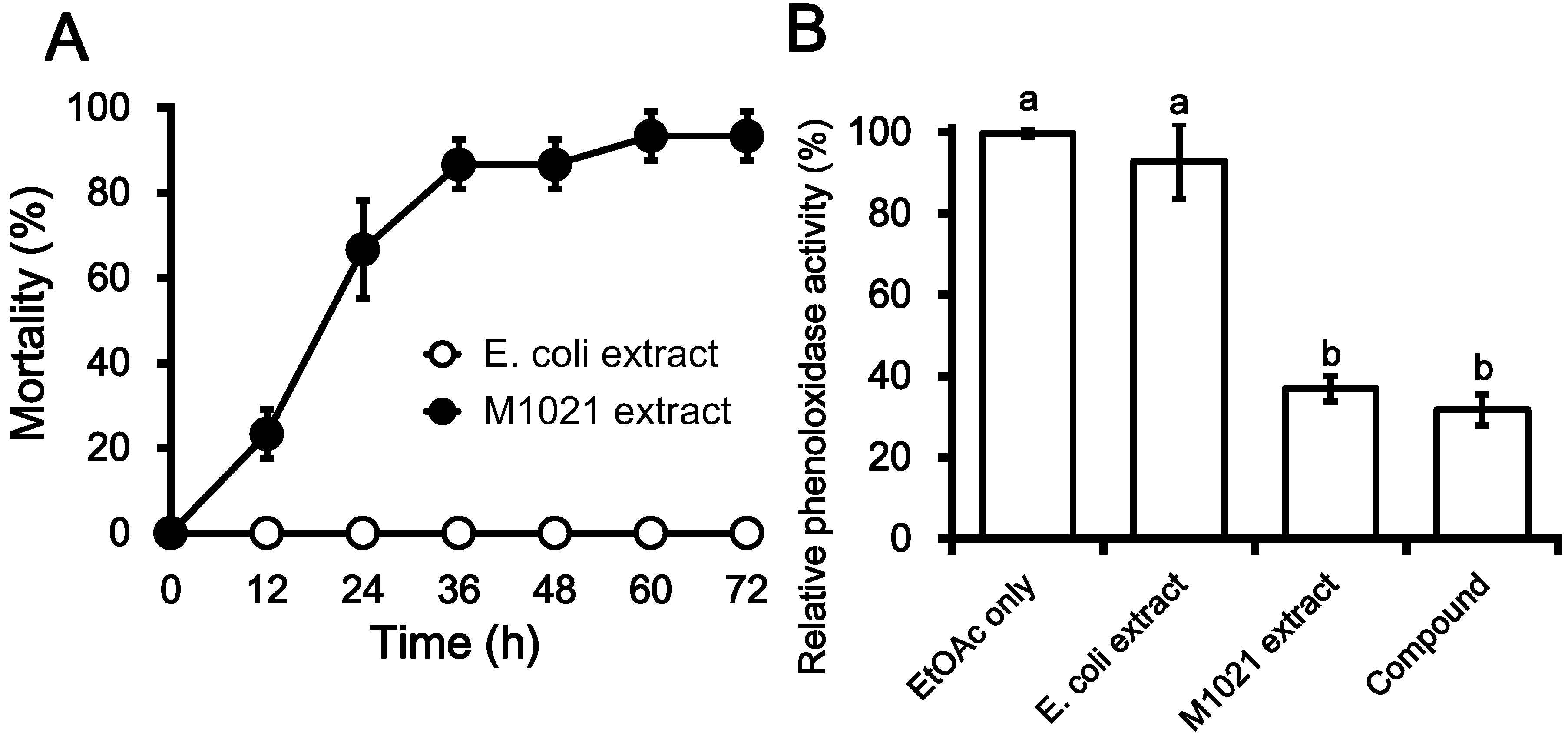
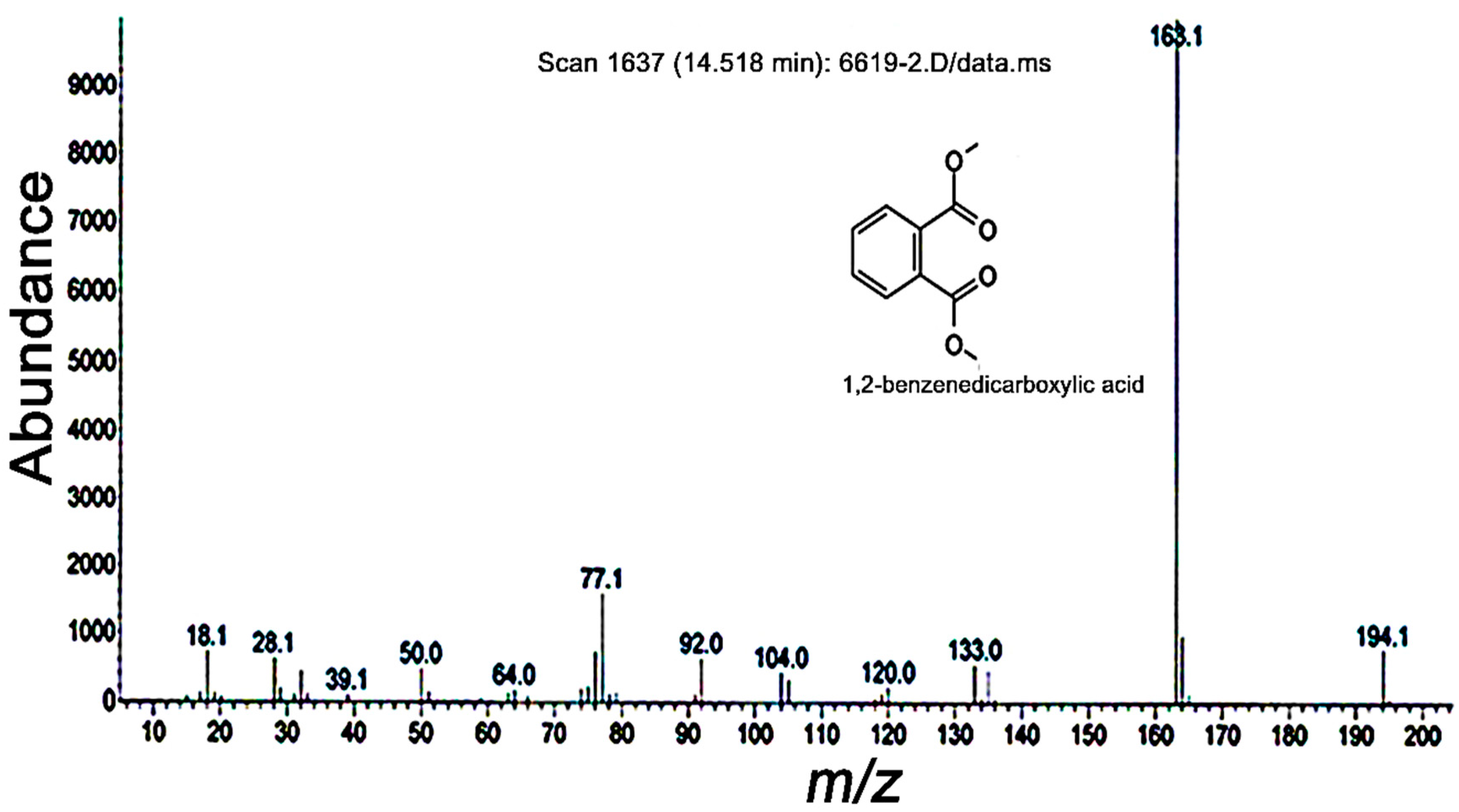
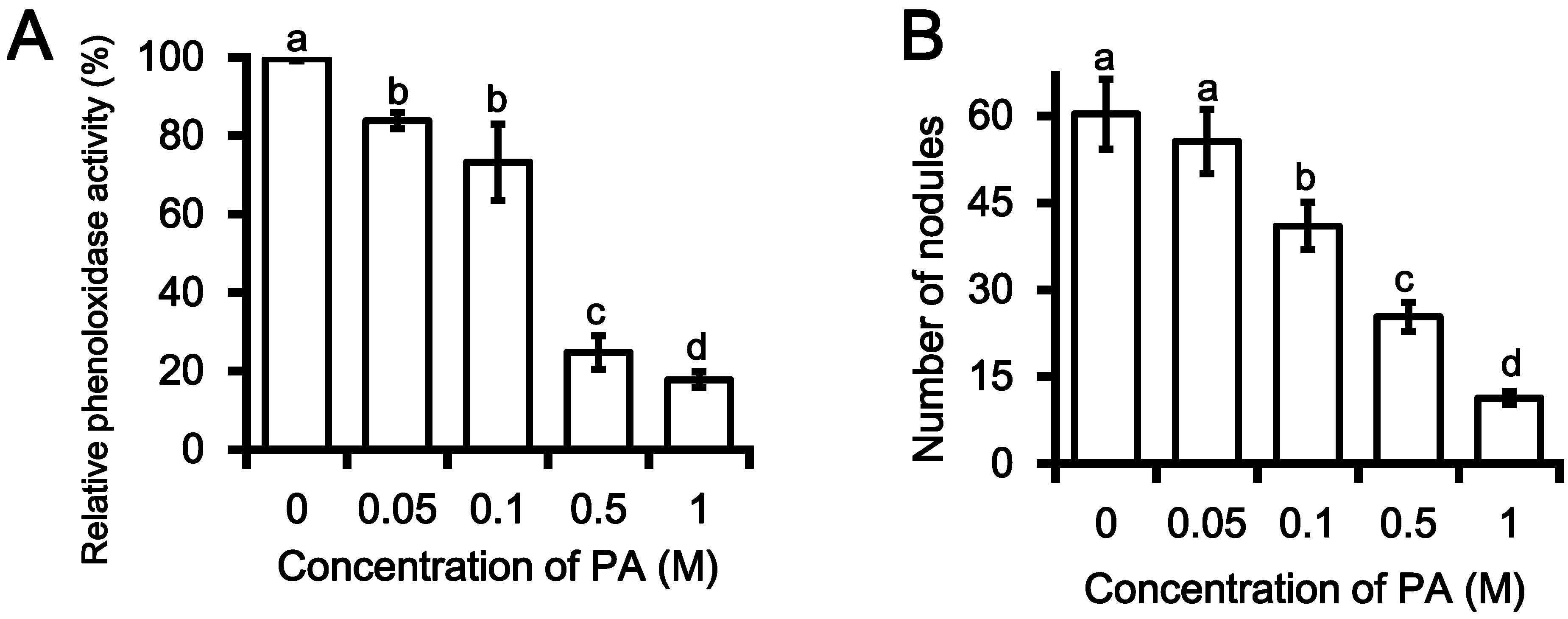
2.2. Inhibition PO Activity and Nodule Formation by PA
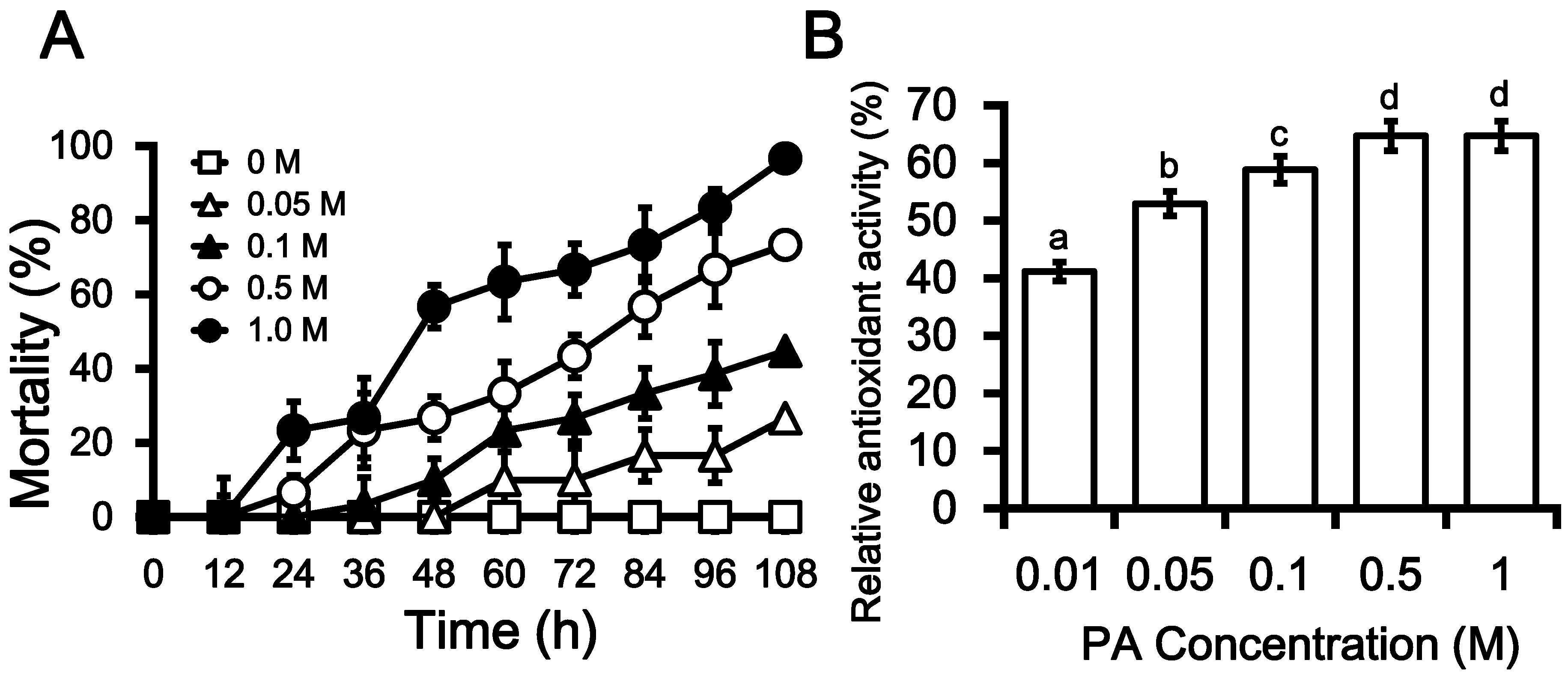
2.3. Insecticidal Activities of PA
2.4. Antioxidant Activity
2.5. Antimicrobial Activity
| Bacterial Strains | Phthalic Acid Concentration (M) | |
|---|---|---|
| MIC | IC50 | |
| Pantoea conspicua RSC-6 | 0.50 | 0.08 ± 0.01 |
| Bacillus aryabhattai RSC-7 | 0.10 | 0.14 ± 0.01 |
| Bacillus anthracis RSC-9 | 0.50 | 0.11 ± 0.02 |
| Enterobacter cowanii RSC-3 | 0.50 | 0.18 ± 0.03 |
| Citrobacter youngae RSC-5 | 0.10 | 0.015 ± 0.01 |
3. Experimental
3.1. Insect and Bacterial Growth Conditions
3.2. Bioassay Guided Fractionation of P. temperata M1021 Extract
Reverse-Phase High-Performance Liquid Chromatography Analysis
3.3. GC-MS and NMR Analyses
3.4. Assessment of PO and Nodule Inhibitions by PA
3.5. Insecticidal Bioassay
3.6. Antioxidant Activity
3.7. Antibacterial Activity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akhurst, R.J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes neoaplectana and heterorhabditis. J. Gen. Microbiol. 1980, 121, 303–309. [Google Scholar]
- Ullah, I.; Jang, E.K.; Kim, M.S.; Shin, J.H.; Park, G.S.; Khan, A.R.; Hong, S.J.; Jung, B.K.; Choi, J.; Park, Y.; et al. Identification and characterization of the insecticidal toxin “makes caterpillars floppy” in Photorhabdus temperata M1021 using a cosmid library. Toxins 2014, 6, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Park, G.S.; Khan, A.R.; Hong, S.J.; Jang, E.K.; Ullah, I.; Jung, B.K.; Choi, J.; Yoo, N.K.; Park, K.J.; Shin, J.H. Draft genome sequence of entomopathogenic bacterium photorhabdus temperata strain M1021, isolated from nematodes. Genome Announc. 2013, 1, 1–2. [Google Scholar]
- Duchaud, E.; Rusniok, C.; Frangeul, L.; Buchrieser, C.; Givaudan, A.; Taourit, S.; Bocs, S.; Boursaux-Eude, C.; Chandler, M.; Charles, J.F.; et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 2003, 21, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, N.R.; Sanchez-Contreras, M.; Eleftherianos, I.; Dowling, A.; Yang, G.; Wilkinson, P.; Parkhill, J.; Thomson, N.; Reynolds, S.E.; Bode, H.B.; et al. Rapid virulence annotation (RVA): Identification of virulence factors using a bacterial genome library and multiple invertebrate hosts. Proc. Natl. Acad. Sci. USA 2008, 105, 15967–15972. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, N.R.; Ciche, T.; Clarke, D. Photorhabdus and a host of hosts. Annu. Rev. Microbiol. 2009, 63, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.K.; Ullah, I.; Lim, J.H.; Lee, I.J.; Kim, J.G.; Shin, J.H. Physiological and molecular characterization of a newly identified entomopathogenic bacteria, Photorhabdus temperata M1021. J. Microbiol. Biotechnol. 2012, 22, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khan, A.R.; Jung, B.K.; Khan, A.L.; Lee, I.J.; Shin, J.H. Gibberellins synthesized by the entomopathogenic bacterium, Photorhabdus temperata M1021 as one of the factors of rice plant growth promotion. J. Plant Interact. 2014, 9, 775–782. [Google Scholar] [CrossRef]
- Seo, S.; Lee, S.; Hong, Y.; Kim, Y. Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata. Appl. Environ. Microbiol. 2012, 78, 3816–3823. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Boundy, S.; Joyce, S.A.; Aslam, S.; Marshall, J.W.; Cox, R.J.; Simpson, T.J.; Clarke, D.J.; ffrench-Constant, R.H.; Reynolds, S.E. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc. Natl. Acad. Sci. USA 2007, 104, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y. Three metabolites from an entomopathogenic bacterium, Xenorhabdus nematophila, inhibit larval development of Spodoptera exigua (lepidoptera: Noctuidae) by inhibiting a digestive enzyme, phospholipase A2. Insect Sci. 2011, 18, 282–288. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm Spodoptera exigua. Insect Biochem. Mol. Biol. 2008, 38, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.R.; Jiang, H.; Yu, X.Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 2004, 198, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Han, S.; Kim, Y. Identification of an entomopathogenic bacterium, Photorhabdus temperata subsp. temperata, in Korea. J. Asia Pac. Entomol. 2004, 7, 331–337. [Google Scholar] [CrossRef]
- Hu, K.; Webster, J.M. Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus–heterorhabditis infected Galleria mellonella larvae. FEMS Microbiol. Lett. 2000, 189, 219–223. [Google Scholar] [PubMed]
- Clarke, D.J. Photorhabdus: A model for the analysis of pathogenicity and mutualism. Cell Microbiol. 2008, 10, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Theodore, C.M.; King, J.B.; You, J.; Cichewicz, R.H. Production of cytotoxic glidobactins/luminmycins by Photorhabdus asymbiotica in liquid media and live crickets. J. Nat. Prod. 2012, 75, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- </i>Jang, E.K.; Ullah, I.; Kim, M.S.; Lee, K.Y.; Shin, J.H. Isolation and characterisation of the entomopathogenic bacterium, Photorhabdus temperata producing a heat stable insecticidal toxin. J. Plant Dis. Protect. 2011, 118, 178–184. [Google Scholar]
- Salvadori, J.D.M.; Defferrari, M.S.; Ligabue-Braun, R.; Yamazaki Lau, E.; Salvadori, J.R.; Carlini, C.R. Characterization of entomopathogenic nematodes and symbiotic bacteria active against Spodoptera frugiperda (lepidoptera: Noctuidae) and contribution of bacterial urease to the insecticidal effect. Biol. Control 2012, 63, 253–263. [Google Scholar] [CrossRef]
- Bussaman, P.; Sobanboa, S.; Grewal, P.S.; Chandrapatya, A. Pathogenicity of additional strains of Photorhabdus and Xenorhabdus (Enterobacteriaceae) to the mushroom mite Luciaphorus perniciosus (acari: Pygmephoridae). Appl. Entomol. Zool. 2009, 44, 293–299. [Google Scholar] [CrossRef]
- Abdel-Razek, A.S. Pathogenic effects of Xenorhabdus nematophilus and Photorhabdus luminescens (Enterobacteriaceae) against pupae of the diamondback moth, Plutella xylostella. J. Pest Sci. 2003, 76, 108–111. [Google Scholar]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase activity acts as a mosquito innate immune response against infection with semliki forest virus. PLoS Pathog. 2012, 8, 1–14. [Google Scholar] [CrossRef]
- Fukuda, D.; Haines, A.S.; Song, Z.; Murphy, A.C.; Hothersall, J.; Stephens, E.R.; Gurney, R.; Cox, R.J.; Crosby, J.; Willis, C.L.; et al. A natural plasmid uniquely encodes two biosynthetic pathways creating a potent anti-MRSA antibiotic. PLoS One 2011, 6, 1–9. [Google Scholar]
- Cerenius, L.; Babu, R.; Söderhäll, K.; Jiravanichpaisal, P. In vitro effects on bacterial growth of phenoloxidase reaction products. J. Invertebr. Pathol. 2010, 103, 21–23. [Google Scholar]
- Yokoo, S.; Tojo, S.; Ishibashi, N. Suppression of the prophenoloxidase cascade in the larval haemolymph of the turnip moth, agrotis segetum by an entomopathogenic nematode, Steinernema carpocapsae and its symbiotic bacterium. J. Insect Physiol. 1992, 38, 915–924. [Google Scholar] [CrossRef]
- Beck, M.H.; Strand, M.R. A novel polydnavirus protein inhibits the insect prophenoloxidase activation pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 19267–19272. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Scott, T.; Sugumaran, M.; Soderhall, K.; Law, J.H. Proenzyme of Manduca sexta phenol oxidase: Purification, activation, substrate specificity of the active enzyme, and molecular cloning. Proc. Natl. Acad. Sci. USA 1995, 92, 7764–7768. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The propo-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.M.; Li, J.; Chen, C.C.; Nappi, A.J. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, R.; Waterfield, N. An ABC guide to the bacterial toxin complexes. Adv. Appl. Microbiol. 2006, 58, 169–183. [Google Scholar] [PubMed]
- Feng, M.-L.; Li, Y.F.; Zhu, H.-J.; Zhao, L.; Xi, B.-B.; Ni, J.P. Synthesis, insecticidal activity, and structure-activity relationship of trifluoromethyl-containing phthalic acid diamide structures. J. Agric. Food Chem. 2010, 58, 10999–11006. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L., Jr.; Sanders, H.O. Toxicology of phthalic acid esters in aquatic organisms. Environ. Health Perspect. 1973, 3, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Zhao, J.; Gao, H.; Zhou, L.; Liu, Z.; Chen, Y.; Sui, P. Antimicrobial and antioxidant activities of the root bark essential oil of periploca sepium and its main component 2-hydroxy-4-methoxybenzaldehyde. Molecules 2010, 15, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Chalabaev, S.; Turlin, E.; Bay, S.; Ganneau, C.; Brito-Fravallo, E.; Charles, J.F.; Danchin, A.; Biville, F. Cinnamic acid, an autoinducer of its own biosynthesis, is processed via hca enzymes in photorhabdus luminescens. Appl. Environ. Microbiol. 2008, 74, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available available from the authors
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Lee, I.-J.; Shin, J.-H. An Insecticidal Compound Produced by an Insect-Pathogenic Bacterium Suppresses Host Defenses through Phenoloxidase Inhibition. Molecules 2014, 19, 20913-20928. https://doi.org/10.3390/molecules191220913
Ullah I, Khan AL, Ali L, Khan AR, Waqas M, Lee I-J, Shin J-H. An Insecticidal Compound Produced by an Insect-Pathogenic Bacterium Suppresses Host Defenses through Phenoloxidase Inhibition. Molecules. 2014; 19(12):20913-20928. https://doi.org/10.3390/molecules191220913
Chicago/Turabian StyleUllah, Ihsan, Abdul Latif Khan, Liaqat Ali, Abdur Rahim Khan, Muhammad Waqas, In-Jung Lee, and Jae-Ho Shin. 2014. "An Insecticidal Compound Produced by an Insect-Pathogenic Bacterium Suppresses Host Defenses through Phenoloxidase Inhibition" Molecules 19, no. 12: 20913-20928. https://doi.org/10.3390/molecules191220913
APA StyleUllah, I., Khan, A. L., Ali, L., Khan, A. R., Waqas, M., Lee, I.-J., & Shin, J.-H. (2014). An Insecticidal Compound Produced by an Insect-Pathogenic Bacterium Suppresses Host Defenses through Phenoloxidase Inhibition. Molecules, 19(12), 20913-20928. https://doi.org/10.3390/molecules191220913






