Sulfated Chitosan Oligosaccharides Suppress LPS-Induced NO Production via JNK and NF-κB Inactivation
Abstract
:1. Introduction
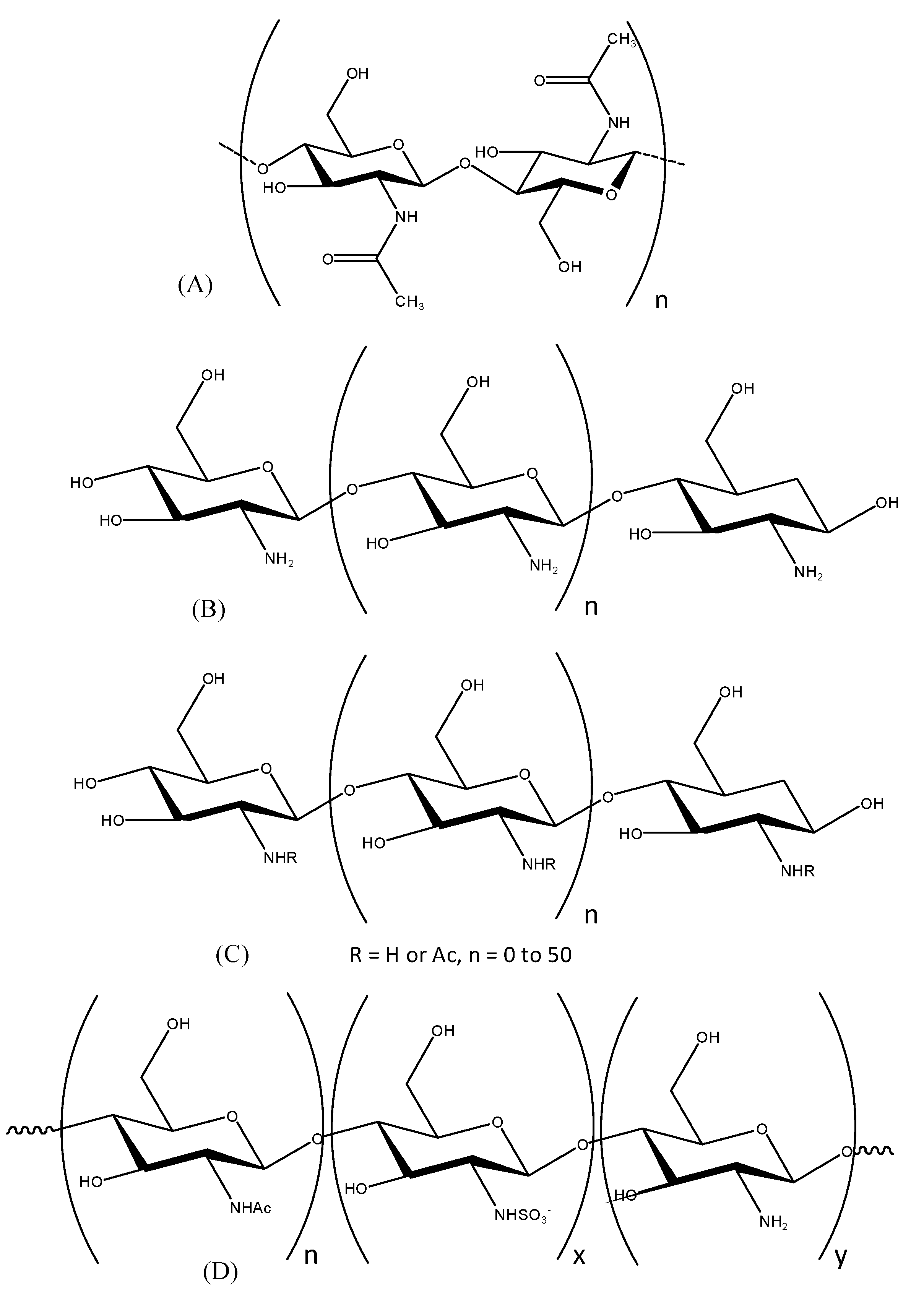
2. Results
2.1. Preparation of Chitooligosaccharides (COSs) and Sulfated COSs (S-COSs)
2.2. Effects of Sulfated Chitosan Oligosaccharide (S-COS) Pretreatment on Cell Viability of LPS-Induced Macrophages
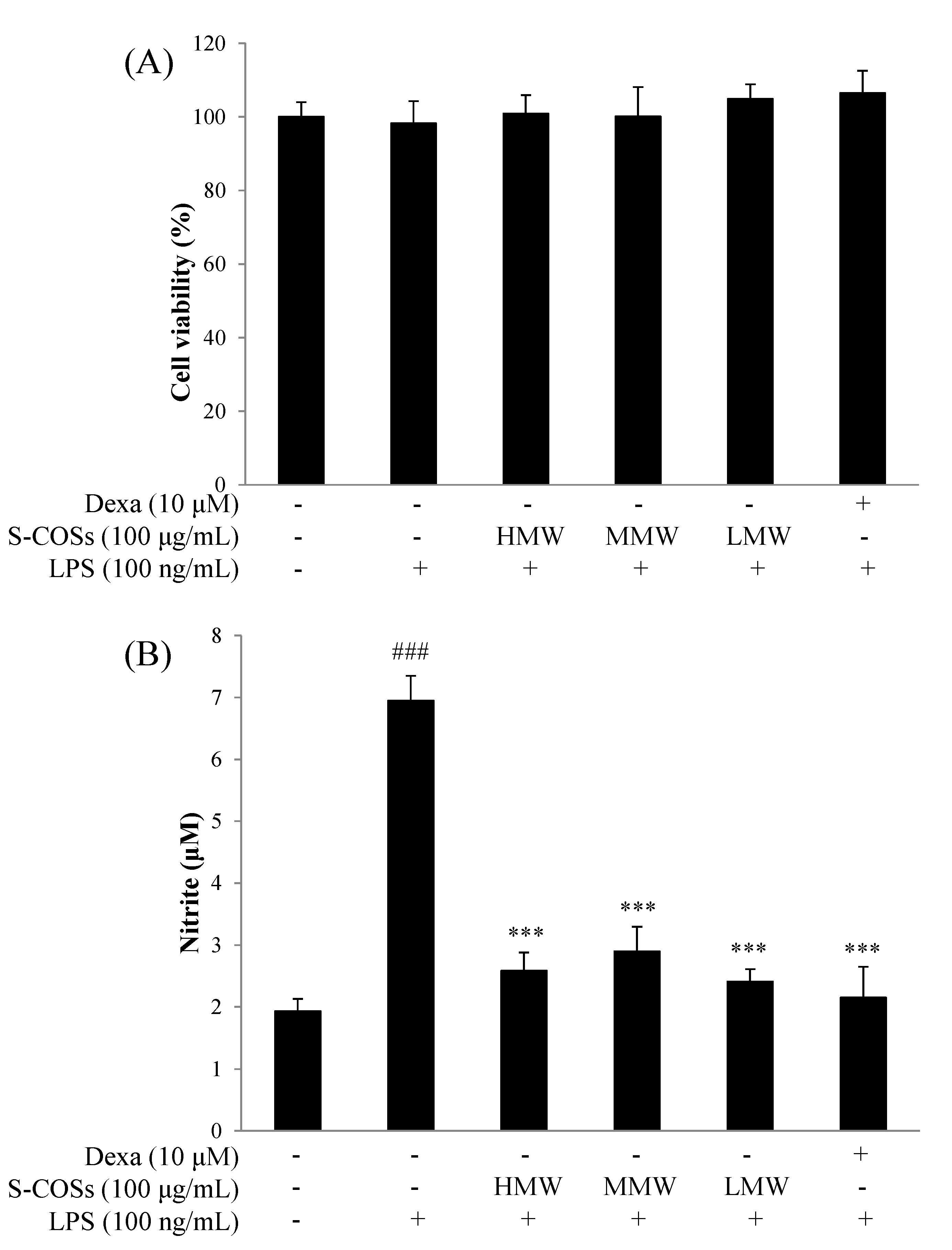
2.3. Effects of Sulfated Chitosan Oligosaccharides (S-COSs) on LPS-Induced Production of NO in Macrophages
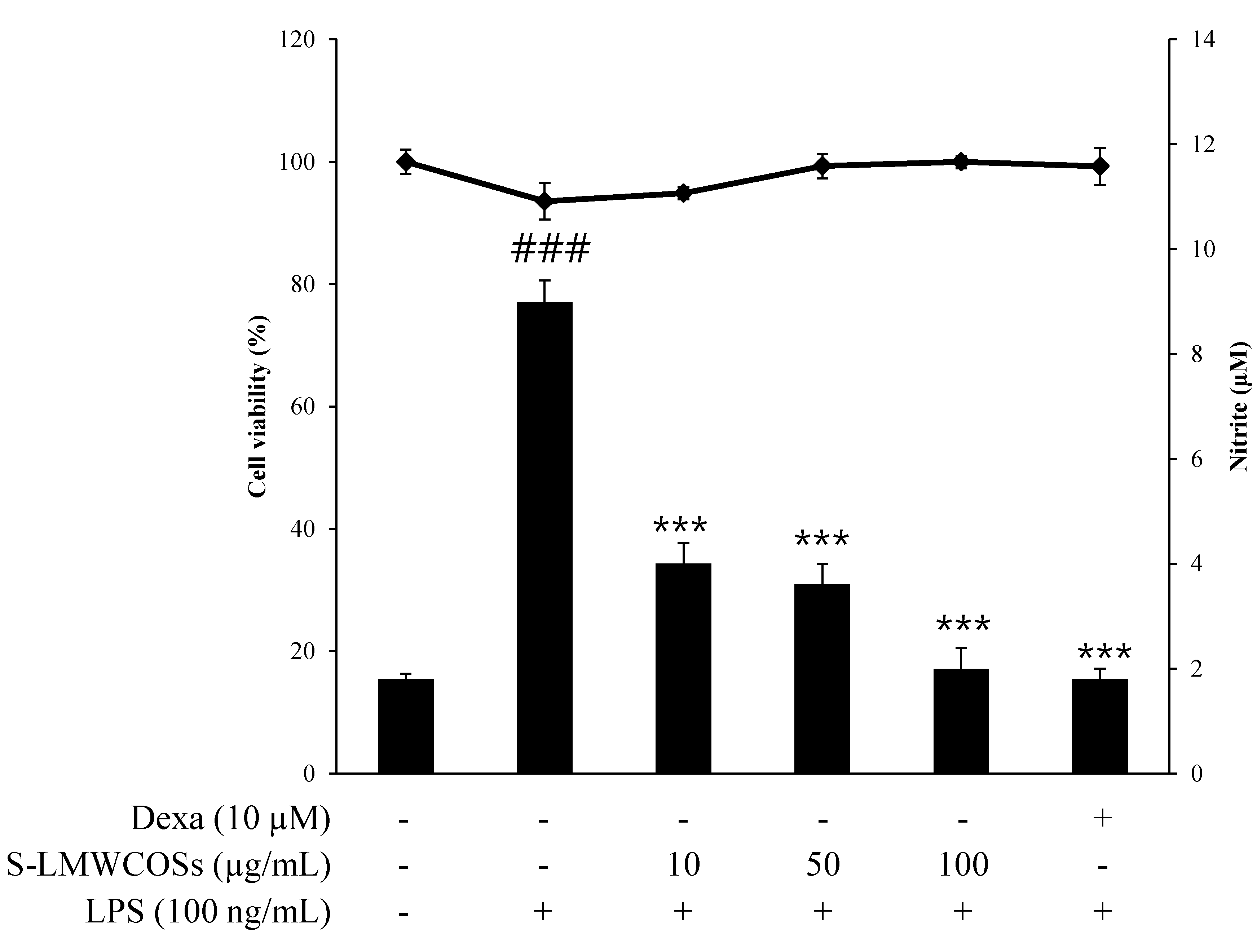
2.4. Inhibitory Effects of S-LMWCOSs Pretreatment on LPS-Induced ROS in Macrophages

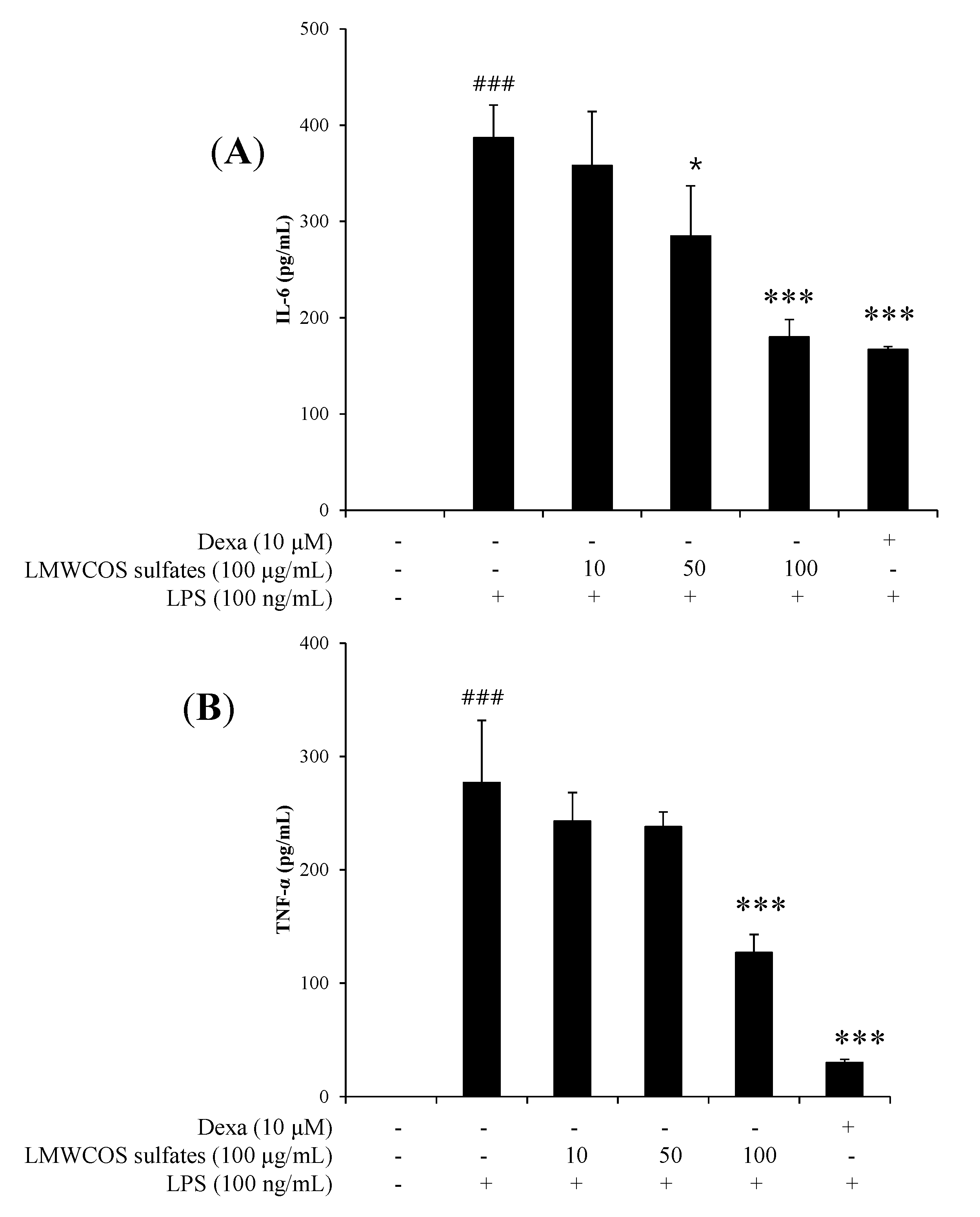
2.5. Inhibitory Effects of S-LMWCOS Pretreatment on LPS-Induced Pro-Inflammatory Cytokines in Macrophages
2.6. Inhibitory Effects of S-LMWCOSs Pretreatment on LPS-Induced iNOS and COX-2 in Macrophages

2.7. Inhibitory Effects of S-LMWCOS Pretreatment on LPS-Induced Phosphorylation of MAP Kinase and Activation of NF-κB in Macrophages
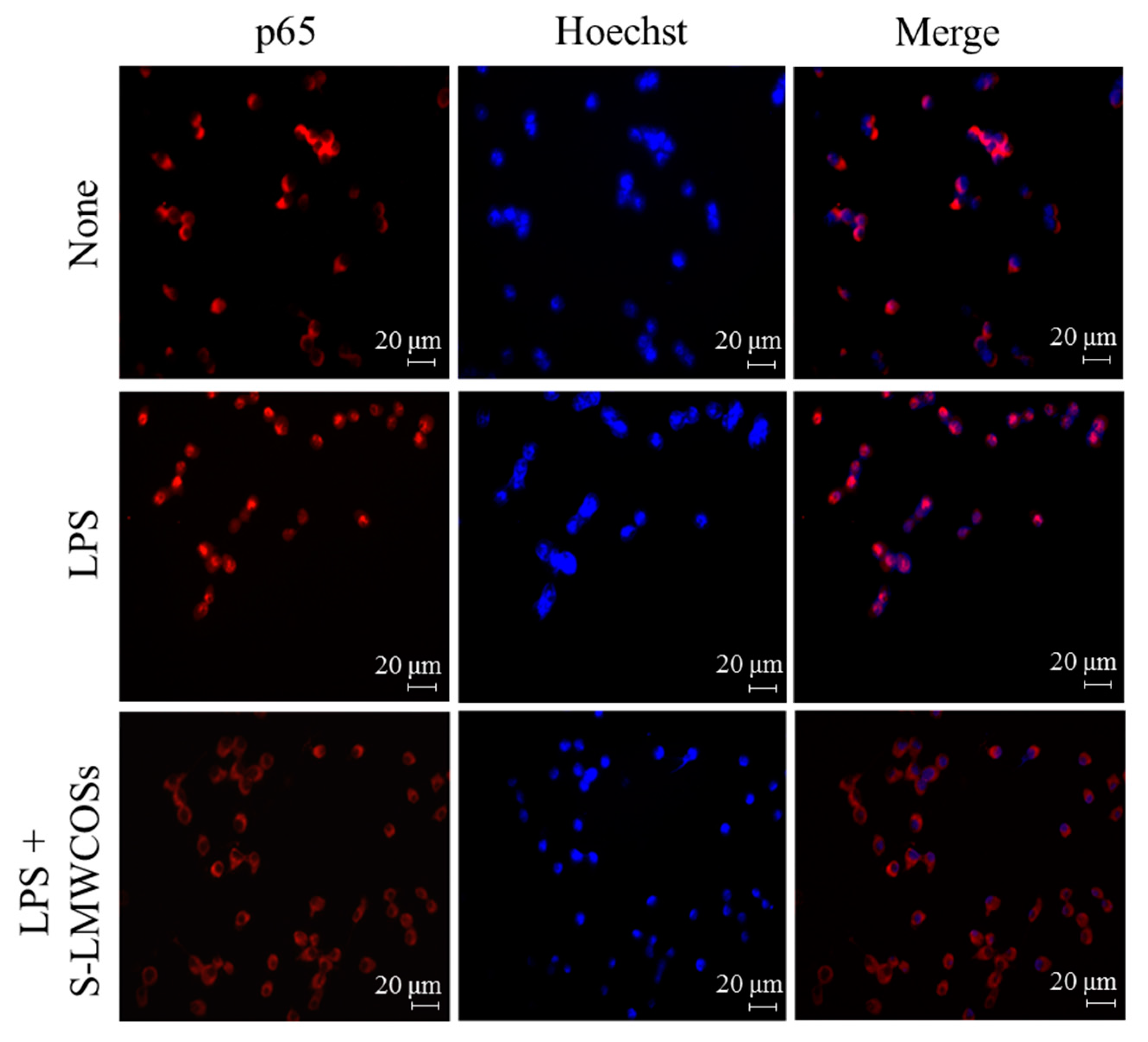
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Preparation of Chitooligosaccharides (COSs)
4.3. Preparation of Sulfated Chitooligosaccharides (S-COSs)
4.4. Cell Culture
4.5. Determination of Cell Viability Assay
4.6. Determination of Nitric Oxide (NO) Production
4.7. Reactive Oxygen Species (ROS) Assay
4.8. Measurement of Cytokine (IL-6 and TNF-α)
4.9. Western Blot Analysis
4.10. Immunofluorescence Assay
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-Inflammatory Activity of Chitooligosaccharides in Vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar]
- Sun, T.; Yao, Q.; Zhou, D.; Mao, F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorg. Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar]
- Sun, T.; Zhou, D.X.; Xie, J.L.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar]
- Jung, C.H.; Jung, H.; Shin, Y.C.; Park, J.H.; Jun, C.Y.; Kim, H.M.; Yim, H.S.; Shin, M.G.; Bae, H.S.; Kim, S.H.; et al. Eleutherococcus senticosus extract attenuates LPS-induced iNOS expression through the inhibition of Akt and JNK pathways in murine macrophage. J. Ethnopharmacol. 2007, 113, 183–187. [Google Scholar]
- Byun, H.G.; Kim, Y.T.; Park, P.J.; Lin, X.; Kim, S.K. Chitooligosaccharides as a novel β-secretase inhibitor. Carbohydr. Polym. 2005, 61, 198–202. [Google Scholar]
- Wijesinghe, W.A.J.P.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar]
- Adams, D.O.; Hamilton, T.A. The cell biology of macrophage activation. Annu. Rev. Immunol. 1984, 2, 283–318. [Google Scholar]
- Duffield, J.S. The inflammatory macrophage: A story of Jekyll and Hyde. Clin. Sci. 2003, 104, 27–38. [Google Scholar]
- MacMicking, J.; Xie, Q.; Nathan, W.C. Nitric oxide and macrophage function. Ann. Rev. Immunol. 1997, 15, 323–350. [Google Scholar]
- Moncada, S.; Palmer, R.M.; Higgs, D.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Hobbs, A.J.; Higgs, A.; Moncada, S. Inhibition of nitric oxide synthase as a potential therapeutic target. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 191–220. [Google Scholar]
- Tsai, S.Y.; Chang, Y.; Chen, T.L.; Chen, R.M. Therapeutic concentrations of propofol protects mouse macrophages from nitric oxide-induced cell death and apoptosis. Can. J. Anesth. 2002, 49, 477–480. [Google Scholar]
- Chung, H.T.; Pae, H.O.; Choi, B.M.; Billiar, T.R.; Kim, Y.M. Nitric oxide as a bioregulator of apoptosis. Biochem. Biophys. Res. Commun. 2001, 282, 1075–1079. [Google Scholar]
- Xie, Q.W.; Cho, H.J.; Calaycay, J.; Mumford, R.A.; Swiderek, K.M.; Lee, T.D. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992, 256, 225–228. [Google Scholar]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar]
- Ohmori, Y.; Hamilton, T.A. Requirement for STAT1 in LPS-induced gene expression in macrophages. J. Leukoc. Biol. 2001, 69, 598–604. [Google Scholar]
- Park, P.J.; Lee, H.K.; Kim, S.K. Preparation of Hetero-Chitooligosaccharides and Their Antimicrobial Activity on Vibrio parahaemolyticus. J. Microbiol. Biotechnol. 2004, 14, 41–47. [Google Scholar]
- Warner, D.T.; Coleman, L.L. Selective sulfonation of amino groups in amino alcohols. J. Org. Chem. 1958, 23, 1133–1135. [Google Scholar]
- Lloyd, A.G.; Embery, G.; Fowler, L.J. Studies on heparin degradation. I. Preparation of (35S) sulphamate derivatives for studies on heparin degrading enzymes of mammalian origin. Biochem. Pharmacol. 1971, 20, 637–648. [Google Scholar]
- Wolfrom, M.L.; Shen Han, T.M. The Sulfation of Chitosan. J. Am. Chem. Soc. 1959, 81, 1764–1766. [Google Scholar]
- Vongchan, P.; Sajomasang, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activity of a sulfated chitosan. Carbohydr. Res. 2002, 337, 1239–1242. [Google Scholar]
- Aruoma, O.I.; Grootveld, M.; Bahorun, T. Free Radicals in biology and medicine: From inflammation to biotechnology. Biofactors 2006, 27, 1–3. [Google Scholar]
- Wang, J.X.; Hou, L.F.; Yang, Y.; Tang, W.; Li, Y.; Zuo, J.P. SM905, an artemisinin derivative, inhibited NO and pro-inflammatory cytokine production by suppressing MAPK and NF-κB pathways in RAW 264.7 macrophages. Acta Pharmacol. Sin. 2009, 10, 1428–1435. [Google Scholar]
- Liu, H.T.; Li, W.M.; Huang, P.; Chen, W.J.; Liu, Q.S.; Bai, X.F.; Du, Y.G.; Yu, C. Chitosan oligosaccharides inhibit TNF-induced VCAM-1 and ICAM-1 expression in human umbilical vein endothelial cells by blocking p38 and ERK1/2 signaling pathways. Carbohydr. Polym. 2010, 81, 49–56. [Google Scholar]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF- κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Ann. Rev. Immunol. 1998, 16, 225–260. [Google Scholar]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar]
- Detmers, P.A.; Hernandez, M.; Mudgett, M.; Hassing, H.; Burton, C.; Mundt, S. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 2000, 165, 3430–3435. [Google Scholar]
- Bosca, L.; Zeini, M.; Traves, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar]
- Akira, S.; Takeda, K. Toll-like receptor signaling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar]
- Chen, T.H.; Kao, Y.C.; Chen, B.C.; Chen, C.H.; Chan, P.; Lee, H.M. Dipyridamole activation of mitogen-activated protein kinase phosphatase-1 mediates inhibition of lipopolysaccharide-induced cyclooxygenase-2 expression in RAW 264.7 cells. Eur. J. Pharmacol. 2006, 541, 138–146. [Google Scholar]
- Kang, S.R.; Park, K.I.; Park, H.S.; Lee, D.H.; Kim, J.A.; Nagappana, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar]
- Schoonbroodt, S. Piette, Oxidative stress interference with the nuclear factor-κB activation pathways. J. Biochem. Pharmacol. 2000, 60, 1075–1083. [Google Scholar]
- Ben, P.; Liu, J.; Lu, C.; Xu, Y.; Xin, Y.; Fu, J.; Huang, H.; Zhang, Z.; Gao, Y.; Luo, L.; et al. Curcumin promotes degradation of inducible nitric oxide synthase and suppresses its enzyme activity in RAW 264.7 cells. Int. Immunopharmacol. 2011, 11, 179–186. [Google Scholar]
- Kwon, O.K.; Lee, M.Y.; Yuk, J.E.; Oh, S.R.; Chin, Y.W.; Lee, H.K.; Ahn, K.S. Anti-inflammatory effects of methanol extracts of the root of Lilium lancifolium on LPS-stimulated Raw264.7 cells. J. Ethnopharmacol. 2010, 130, 28–34. [Google Scholar]
- Su, Y.W.; Chiou, W.F.; Chao, S.H.; Lee, M.H.; Chen, C.C.; Tsai, Y.C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int. Immunopharmacol. 2011, 11, 1166–1172. [Google Scholar]
- Lin, Q.Y.; Jin, L.J.; Cao, Z.H.; Xu, Y.P. Inhibition of inducible nitric oxide synthase by Acanthopanax senticosus extract in RAW264.7 macrophages. J. Ethnopharmacol. 2008, 118, 231–236. [Google Scholar]
- Yoon, W.J.; Ham, Y.M.; Kim, S.S.; Yoo, B.S.; Moon, J.Y.; Baik, J.S.; Lee, N.H.; Hyun, C.G. Suppression of proinflammatory cytokines, iNOS, and COX-2 expression by brown algae Sargassum micracanthum in RAW264.7 macrophages. Eurasian J. BioSci. 2009, 3, 130–143. [Google Scholar]
- Bass, D.A.; Parce, W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.C.; Thomas, M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J. Immunol. 1983, 30, 1910–1917. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Kim, Y.-S.; Hwang, J.-W.; Han, Y.-K.; Lee, J.-S.; Kim, S.-K.; Jeon, Y.-J.; Moon, S.-H.; Jeon, B.-T.; Bahk, Y.Y.; et al. Sulfated Chitosan Oligosaccharides Suppress LPS-Induced NO Production via JNK and NF-κB Inactivation. Molecules 2014, 19, 18232-18247. https://doi.org/10.3390/molecules191118232
Kim J-H, Kim Y-S, Hwang J-W, Han Y-K, Lee J-S, Kim S-K, Jeon Y-J, Moon S-H, Jeon B-T, Bahk YY, et al. Sulfated Chitosan Oligosaccharides Suppress LPS-Induced NO Production via JNK and NF-κB Inactivation. Molecules. 2014; 19(11):18232-18247. https://doi.org/10.3390/molecules191118232
Chicago/Turabian StyleKim, Jung-Hyun, Yon-Suk Kim, Jin-Woo Hwang, Young-Ki Han, Jung-Suck Lee, Se-Kwon Kim, You-Jin Jeon, Sang-Ho Moon, Byong-Tae Jeon, Young Yil Bahk, and et al. 2014. "Sulfated Chitosan Oligosaccharides Suppress LPS-Induced NO Production via JNK and NF-κB Inactivation" Molecules 19, no. 11: 18232-18247. https://doi.org/10.3390/molecules191118232





