1. Introduction
The bioavailability of drugs is influenced by their solubility and permeability. When per-oral administered drugs display good water solubility, permeability is a critical issue [
1,
2]. In the Biopharmaceutical Classification System (BCS) high water solubility is defined as: (a) 85% dissolution of the dose within 30 min at all pH values from 1 to 7.5 and (b) dose/solubility ≤250 mL. The difference between a high-solubility and a low-solubility compound can be one million-fold (0.1 µg/mL–100 mg/mL). A drug substance is considered highly intestinal permeable if >90% of the administered drug dose is absorbed in comparison with intravenous administration. The difference between a high-permeability and a low-permeability compound can be 50-fold (0.001–0.05 min
−1) [
3]. The permeability of orally dosed drugs depends on several factors such as intestinal permeability, solubility in gastrointestinal system, drug release from the dosage form, liability to efflux and metabolism. Generally strategies/structural modifications to improve permeability are based on a few fundamental concepts: reduction of ionizability, increase of lipophilicity, reduction of polarity or reduction of hydrogen bond donors or acceptors. Formulation is other strategy for improving permeability and bioavailability; for example, permeability enhancers, surfactants or pharmaceutical complexing agents can be used in the oral dosage form [
3]. The problem of poor permeability can be also solved by preparation of nanoparticles. In general, nanoparticles for systemic applications should range from 10 to 100 nm in size and have minimum surface charge [
4]. A lot of nano-based permeability enhancing techniques is known, e.g., preparation of nano-emulsions using excipients with solubilizing or permeation enhancing properties [
5,
6,
7]. Nanosize allows effective systemic circulation and enables many pharmacological agents to reach sites of action that are not available to larger particles [
8,
9,
10,
11].
Bisphosphonates (BPs) represent highly hydrophilic compounds with low oral bioavailability (in most cases less than 3%) [
12]. These pyrophosphate analogues are the most effective bone resorption inhibitors [
13]. BPs contain a P-C-P backbone with two covalently bound side chains by which BPs differ from each other. The mechanism of action of BPs is induction of apoptosis in osteoclasts [
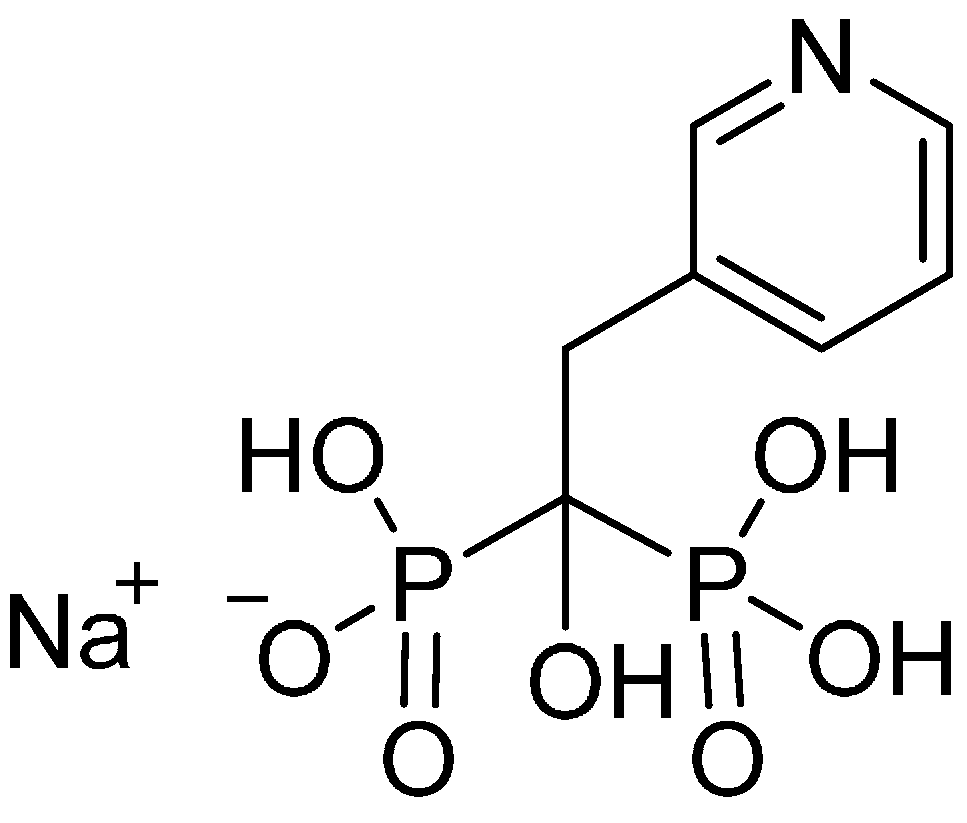
14]. Risedronate (risedronate monosodium salt, systematic name: sodium 1-hydroxy-1-phosphono-2-(pyridin-3-yl-ethyl)phosphonate,
Figure 1), is a member of Class III of the BCS and belongs to the third-generation BPs. It has a chemically unique component as compared with other BPs, which is believed to reduce the likelihood of gastro-intestinal side effects. Risedronate is more potent in blocking the dissolution of bone than other BPs [
15,
16]. It has good solubility in water, but its absolute bioavailability after oral administration is less than 1% [
17,
18]. Due to these facts risedronate was chosen as a model drug in this study.
BPs are used as per-oral or injection forms, nevertheless new formulations of BPs are developed with a view to increase their oral bioavailability and reduce side effects. For example, the use of ethylenediaminetetraacetic acid in BP formulations enhanced their absorption in intestine [
19]. Other approaches to the modification of BP properties are the preparation of co-crystals [
20,
21] or the preparation of chitosan-coated mucoadhesive liposomes or peptide prodrugs that can be recognized by the intestine carrier system and subsequently transported. Also, an adduct of risedronate with titanium dioxide particles was proposed as a controlled-release system [
22].
Figure 1.
Structure of sodium salt of risedronate.
Figure 1.
Structure of sodium salt of risedronate.
This contribution is a follow-up paper to our previous studies [
20,
21,
23,
24,
25] and deals with preparation of nanoparticles of risedronate monosodium salt by means of the solvent evaporation method. A solution of excipient is mixed under continuous stirring with a solution of the drug substance and then the second solvent is evaporated. An excellent review dealing with this technique was published by Thorat
et al. [
26]. Nanoparticles are stabilized by various excipients selected based on the Generally Recognized as Safe (GRAS) list [
27], which means that they are not toxic to the human body. The excipients were applied in various concentrations, because the optimal concentration of surfactant is important for optimal particles wetting. If the concentration is too low, particles float on the surface. If the concentration is too high, bubbles appear [
28]. The particle size of all the prepared samples was analysed by means of dynamic light scattering. The samples were also characterized by means of scanning electron microscopy (SEM), and the composition was verified by Fourier transform mid infrared (FT-MIR) spectroscopy.
2. Results and Discussion
Based on pilot screening [
23,
24,
25], five different excipients and one mixture of excipients were used in this investigation. The used excipients represent various classes of low-molecular or polymeric pharmaceutical adjuvants (non-ionic or anionic surfactants, emulsifiers/viscosity modifiers/thickeners) that can be utilized as solubility modifying compounds/nanoparticle stabilizers. They included sodium dodecyl sulfate (SDS, Sample series
1), polysorbate 80 (PLS, Sample series
2), macrogol 6000 (PEG, Sample series
3), sodium carboxymethyl cellulose (SCMC, Sample series
4), sodium carboxymethyl dextran (SCMD, Sample series
5) and sodium dodecyl sulfate/macrogol 6000 (DSP, Sample series
6). Three water solutions were prepared from each excipient with mass concentration of 1% (Samples
a), 3% (Samples
b) and 5% (Samples
c). As risedronate sodium salt is soluble in water, this medium was chosen as a solvent. Risedronate sodium was dissolved in water and added to the solution of excipient under continuous stirring. Then an ultrasonic bath was used for destruction of possible agglomeration, and finally the solvent was evaporated. Combination of all excipients with risedronate provided eighteen samples, see
Table 1.
Table 1.
Composition of samples, concentration (%) of individual excipients in water samples relative to risedronate, particle size (nm) and polydispersity index (PDI) of risedronate samples expressed as mean ± SD (n = 5 independent measurements). (SDS = sodium dodecyl sulfate, PLS = polysorbate 80, PEG = macrogol 6000, SCMC = sodium carboxymethyl cellulose, SCMD = sodium carboxymethyl dextran, DSP = sodium dodecyl sulfate/macrogol 6000).
Table 1.
Composition of samples, concentration (%) of individual excipients in water samples relative to risedronate, particle size (nm) and polydispersity index (PDI) of risedronate samples expressed as mean ± SD (n = 5 independent measurements). (SDS = sodium dodecyl sulfate, PLS = polysorbate 80, PEG = macrogol 6000, SCMC = sodium carboxymethyl cellulose, SCMD = sodium carboxymethyl dextran, DSP = sodium dodecyl sulfate/macrogol 6000).
| Sample | Excipient/Concentration (%) | Particle Size (nm) | PDI |
|---|
| 1a | SDS/1 | 95.6 ± 11.2 | 0.263 ± 0.030 |
| 1b | SDS/3 | 194.8 ± 34.6 | 0.377 ± 0.056 |
| 1c | SDS/5 | 75.4 ± 3.4 | 0.285 ± 0.022 |
| 2a | PLS/1 | 10.5 ± 0.3 | 0.173 ± 0.048 |
| 2b | PLS/3 | 10.1 ± 0.3 | 0.086 ± 0.043 |
| 2c | PLS/5 | 9.9 ± 0.1 | 0.139 ± 0.017 |
| 3a | PEG/1 | 9.1 ± 1.9 | 0.532 ± 0.095 |
| 3b | PEG/3 | 8.2 ± 1.1 | 0.401 ± 0.066 |
| 3c | PEG/5 | 7.7 ± 2.5 | 0.315 ± 0.028 |
| 4a | SCMC/1 | 12.9 ± 2.4 | 0.685 ± 0.101 |
| 4b | SCMC/3 | 2946.7 ± 367.6 | 0.560 ± 0.048 |
| 4c | SCMC/5 | 7287.6 ± 1352.5 | 0.651 ± 0.151 |
| 5a | SCMD/1 | 3.5 ± 1.1 | 0.473 ± 0.156 |
| 5b | SCMD/3 | 2.8 ± 0.4 | 0.287 ± 0.050 |
| 5c | SCMD/5 | 3.8 ± 1.2 | 0.244 ± 0.135 |
| 6a | DSP/1 | 110.1 ± 7.4 | 0.578 ± 0.029 |
| 6b | DSP/3 | 122.7 ± 7.4 | 0.524 ± 0.037 |
| 6c | DSP/5 | 4.00 ± 0.8 | 0.310 ± 0.033 |
The composition of the samples was verified by FT-MIR spectroscopy, see
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6. The individual figures illustrate spectra of the starting materials and the product. Also differential spectra (product-excipient) are illustrated to confirm the presence of risedronate and to verify possible interactions between risedronate and the excipient in the sample. No interactions were found,
i.e., all the samples were simple mixtures, in which risedronate particles were embedded in the excipient.
Figure 2.
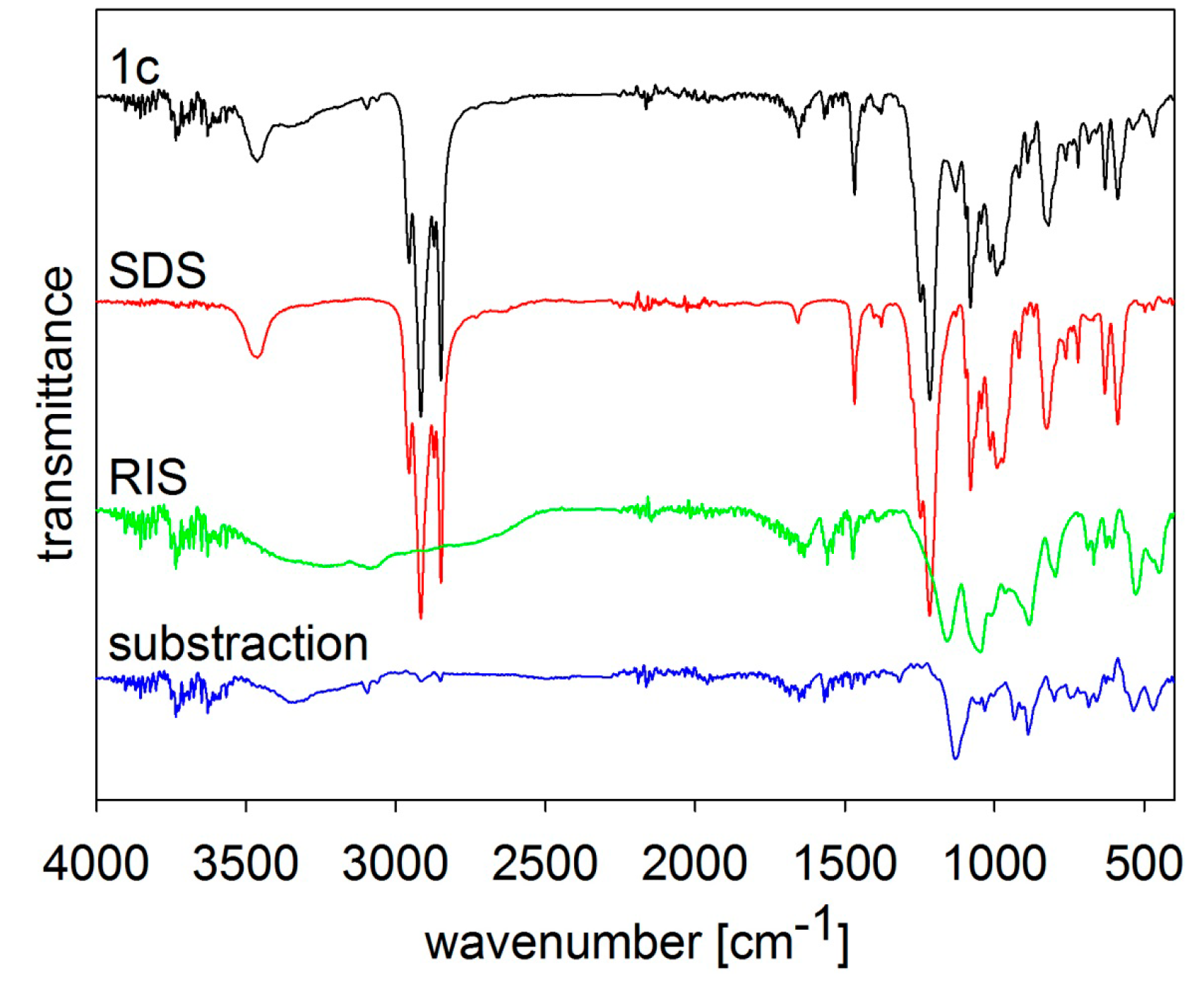
MIR spectra of risedronate (RIS), sodium dodecyl sulfate (SDS) and risedronate nanoparticles stabilized with 5% concentration of sodium dodecyl sulfate (Sample 1c) and differential spectrum.
Figure 2.
MIR spectra of risedronate (RIS), sodium dodecyl sulfate (SDS) and risedronate nanoparticles stabilized with 5% concentration of sodium dodecyl sulfate (Sample 1c) and differential spectrum.
Figure 3.
MIR spectra of risedronate (RIS), polysorbate 80 (PLS) and risedronate nanoparticles stabilized with 5% concentration of polysorbate 80 (Sample 2c) and differential spectrum.
Figure 3.
MIR spectra of risedronate (RIS), polysorbate 80 (PLS) and risedronate nanoparticles stabilized with 5% concentration of polysorbate 80 (Sample 2c) and differential spectrum.
Figure 4.
MIR spectra of risedronate (RIS), macrogol 6000 (PEG) and risedronate nanoparticles stabilized with 5% concentration of macrogol 6000 (Sample 3c) and differential spectrum.
Figure 4.
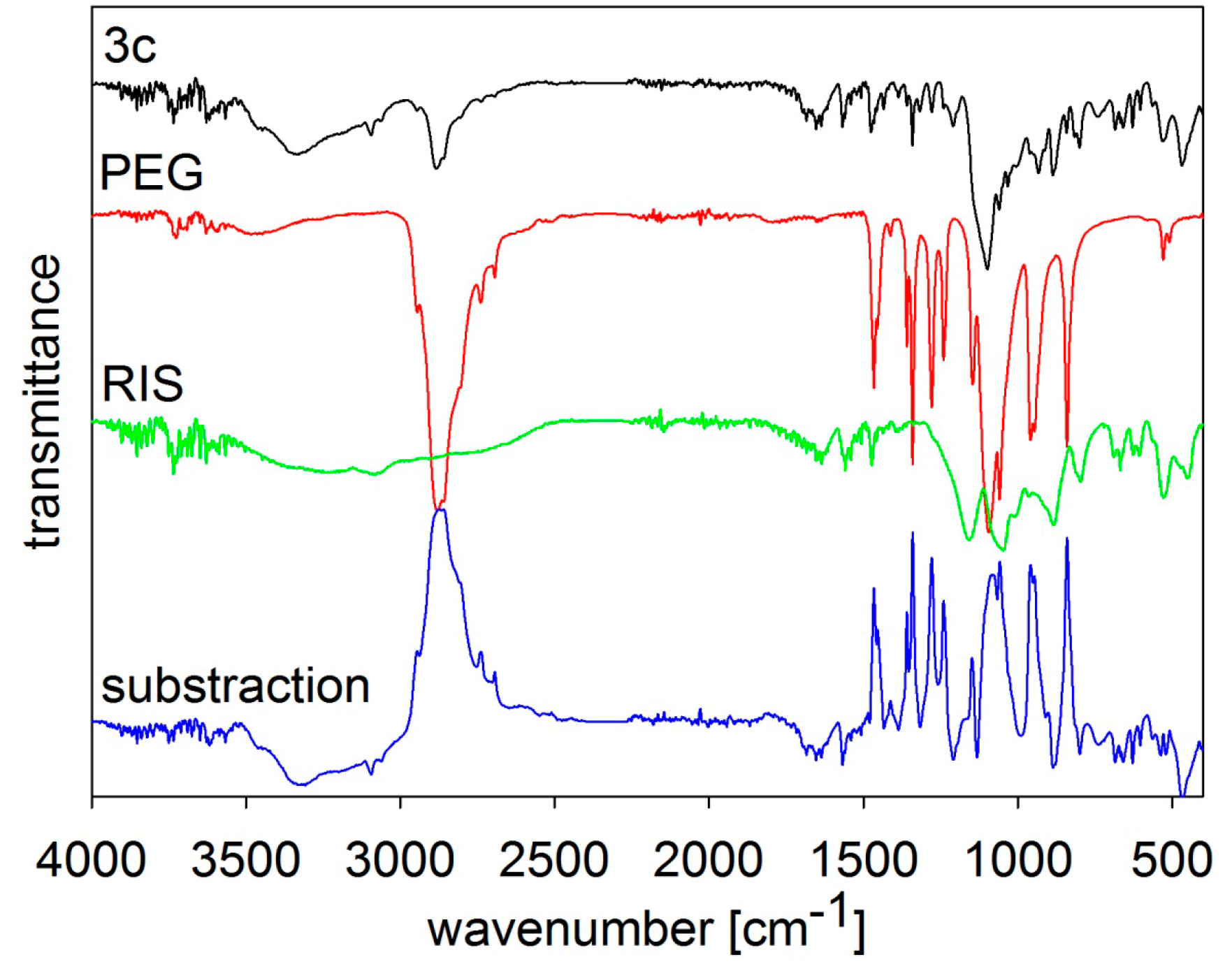
MIR spectra of risedronate (RIS), macrogol 6000 (PEG) and risedronate nanoparticles stabilized with 5% concentration of macrogol 6000 (Sample 3c) and differential spectrum.
Figure 5.
MIR spectra of risedronate (RIS), sodium carboxymethyl cellulose (SCMC) and risedronate nanoparticles stabilized with 5% concentration of sodium carboxymethyl cellulose (Sample 4c) and differential spectrum.
Figure 5.
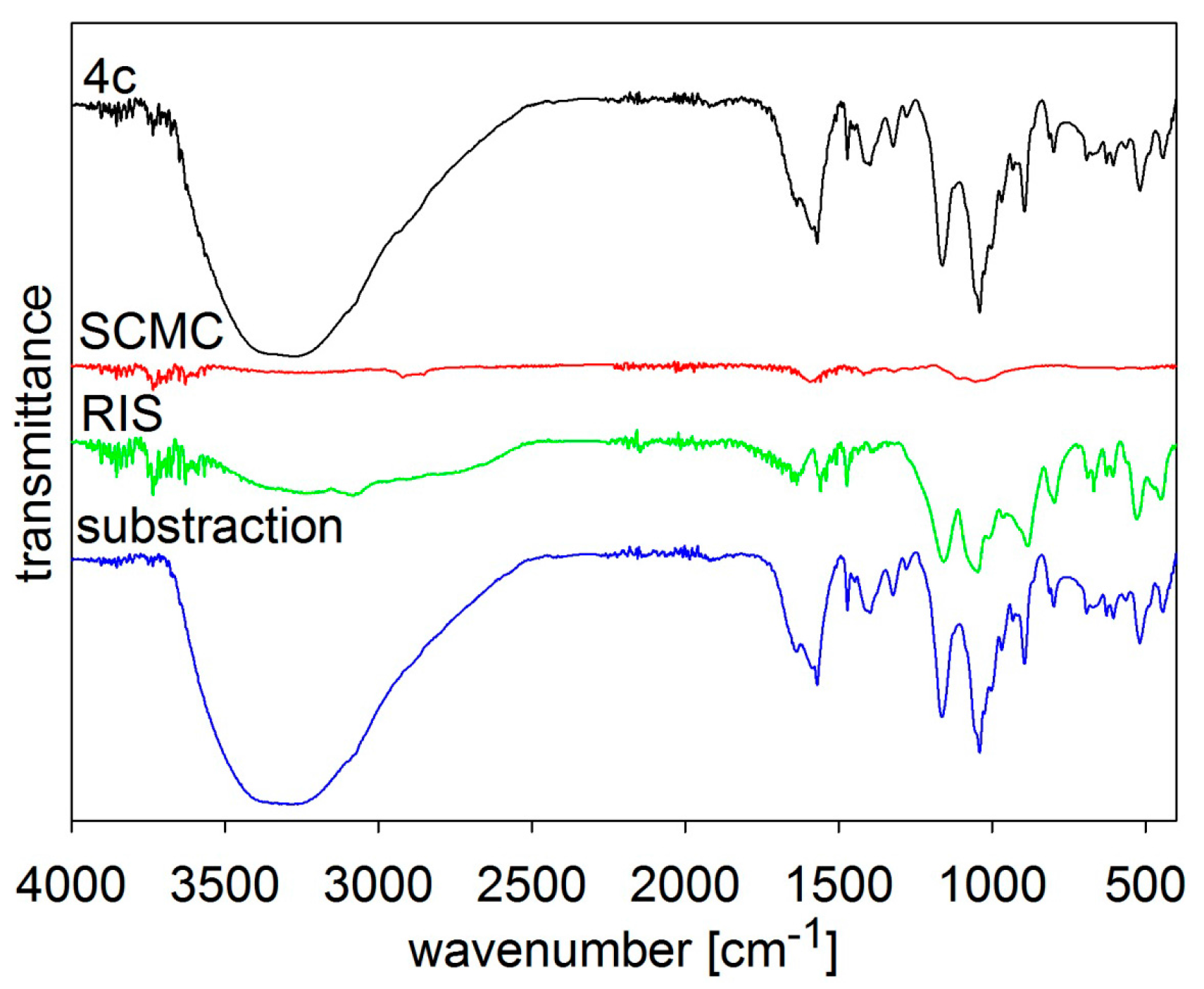
MIR spectra of risedronate (RIS), sodium carboxymethyl cellulose (SCMC) and risedronate nanoparticles stabilized with 5% concentration of sodium carboxymethyl cellulose (Sample 4c) and differential spectrum.
Figure 6.
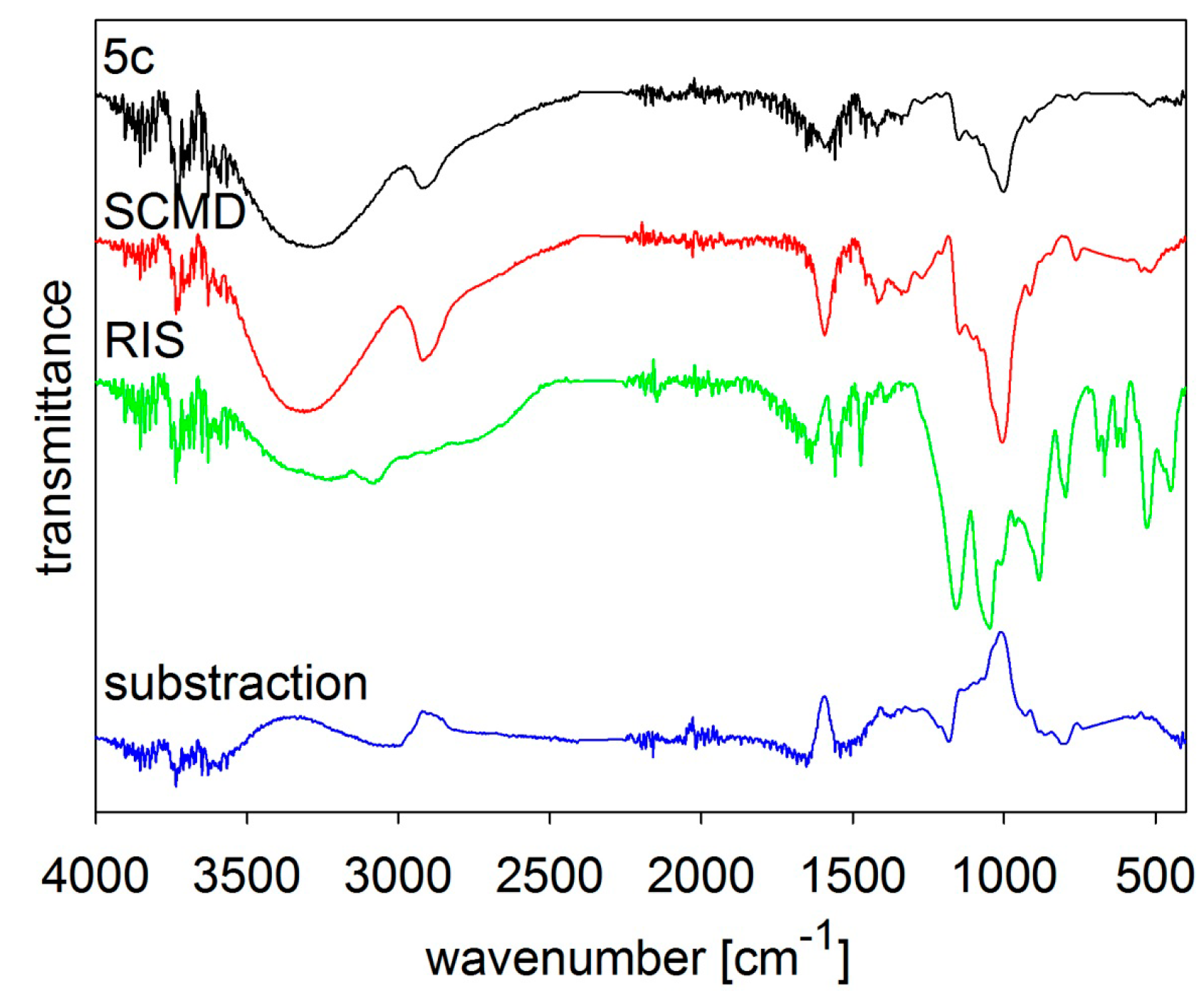
MIR spectra of risedronate (RIS), sodium carboxymethyl dextran (SCMD) and risedronate nanoparticles stabilized with 5% concentration of sodium carboxymethyl dextran (Sample 5c) and differential spectrum.
Figure 6.
MIR spectra of risedronate (RIS), sodium carboxymethyl dextran (SCMD) and risedronate nanoparticles stabilized with 5% concentration of sodium carboxymethyl dextran (Sample 5c) and differential spectrum.
Figure 2 shows the MIR spectra of risedronate, sodium dodecyl sulfate and risedronate nanoparticles stabilized with sodium dodecyl sulfate (Sample
1c) and the differential spectrum of sodium dodecyl sulfate and Sample
1c. The spectrum of Sample
1c contains all typical absorption bands of risedronate and sodium dodecyl sulfate in the region of 450–1700 cm
−1 and 2800–3800 cm
−1. The intensity of the absorption bands of sodium dodecyl sulfate overlapped the spectrum of risedronate. The differential spectrum does not vary significantly; there are no changes in these two structures after preparation process.
The MIR spectra of risedronate, polysorbate 80, risedronate nanoparticles stabilized with polysorbate 80 (Sample
2c) and the differential spectrum of polysorbate 80 and Sample
2c are shown in
Figure 3. The MIR spectrum of Sample
2c is composed of absorption bands characterizing both original substances in the region of 450–1750 cm
−1 and 2800–3800 cm
−1. There are no significant changes in the differential spectrum and the spectrum of risedronate.
The MIR spectra of risedronate, macrogol 6000 and their mixture (Sample
3c) and the differential spectrum of macrogol 6000 and Sample
3c are presented in
Figure 4. The spectrum of Sample
3c contains characteristic absorption bands of both substances as in previous figures.
Figure 5 and
Figure 6 illustrate spectra of risedronate and sodium carboxymethyl cellulose and risedronate and sodium carboxymethyl dextran respectively, including their mixtures (Samples
4c and
5c), and the differential spectra. There are no interactions between risedronate and excipient in Sample
4c as observed in the differential spectra in
Figure 5. In case of Sample
5c the spectrum of carboxymethyl dextran overlapped that of risedronate, see
Figure 6. All the MIR spectra (
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6) confirmed that Samples
1c–
5c contained both risedronate and the excipient and that there were no reactions between these two components.
All prepared samples were measured by dynamic light scattering [
29],
i.e., particle size and values of polydispersity index were determined. Moreover, samples were characterized by means of SEM, see below. The particle size distribution is presented in
Table 1. The investigated particles showed good particle size stability throughout the light scattering measurements. Within the period of measurements, no significant deviations from the mean values of particle size, which could be a result of possible sample ageing, were observed. Also, a regular visual check of the samples proved no changes in the sample structure, which was confirmed by the reproducible data obtained by the light scattering method.
Nanoparticles of size under 200 nm were prepared in the case of sixteen samples. Sodium carboxymethyl cellulose provided micro-size (Samples
4b,
4c). Sodium dodecyl sulfate in 3% concentration (Sample
1b) provided the largest nanoparticles or, more precisely, submicroparticles (
ca. 195 nm) and in 1% and 5% concentrations (Samples
1a,
1c) together with the combination of SDS with PEG in 1% and 3% concentrations (Samples
6a,
6b) provided particle size about 100 nm. A significant effect of the applied concentration of the excipient was observed for sodium dodecyl sulfate and the combination of SDS with PEG. The smallest particles were found at 5% concentration of the excipient (Samples
1c,
6c). In the case of sodium carboxymethyl cellulose its poor solubility in water plays an important role, and probably, therefore, the most significant effect of SCMC was observed at 1% concentration (Sample
4a). It is evident that carboxymethyl dextran sodium salt provided slightly smaller nanoparticles (range 2.8–3.5 nm) in comparison with macrogol (7.7–9.1 nm) or with polysorbate (9.9–10.5 nm). According to the results of SCMD, PEG and PLS application (see
Table 1), no influence of the concentration of the excipients on the particle size was observed.
The dispersity is a measure/degree of the homogeneity/heterogeneity of sizes of particles in a mixture/system. The uniformity of dispersed systems is expressed as polydispersity index (PDI), see
Table 1. Low PDI values demonstrate narrow size distribution and uniformity of samples contrary to PDI ≈ 1 that indicates that samples have a very broad size distribution and may contain large particles or aggregates and are not suitable for measurements [
29,
30]. In the prepared nanoparticles of risedronate PDI values ranged from 0.086 ± 0.043 to 0.578 ± 0.029, when the samples stabilised by sodium carboxymethyl cellulose (Samples
4a–
c) were eliminated. From the results it is evident that the mentioned excipient is not suitable as a nanoparticle stabilizing agent, either for high degree of heterogeneity (Sample
4a) or for generation of micro-size (Samples
4b,
4c). The highest uniformity can be seen for polysorbate, the lowest for sodium carboxymethyl cellulose. The rest of the used excipients showed approximately similar effect on particle size homogeneity.
The dependence of the type and the concentration of the excipient on the particle size and the polydispersity index of individual systems was discussed above.
Figure 7 shows the relationship between the polydispersity index values and the particle size of risedronate sodium in relation to the applied excipients. It can be stated that for generation of nanoparticles it is favourable when prepared particles are small, and polydispersity index values are sufficiently low (around 0.5 and less). Based on the results the samples placed in the bottom left quadrant of the graph in
Figure 7 meet these requirements. Based on this expectation polysorbate 80 (Samples
2a–
c) in all used concentrations seems to be preferable excipient followed by sodium carboxymethyl dextran and macrogol 6000 at 3% and 5% concentrations (Samples
5b,
5c and
3b,
3c). It can be concluded that the applied method can be used as an effective and affordable technique for preparation of nanoparticles. The selected conditions are convenient for formation of nanoparticles, and the used excipients are principally applicable as nanoparticle stabilizers.
Figure 7.
Relationships between polydispersity index values and particle size of risedronate sodium in relation to applied excipients. (SDS = sodium dodecyl sulfate, PLS = polysorbate 80, PEG = macrogol 6000, SCMC = sodium carboxymethyl cellulose, SCMD = sodium carboxymethyl dextran, DSP = sodium dodecyl sulfate/macrogol 6000; fill colour of individual symbols depends on concentration of excipient: 1% = blue, 3% = red, 5% = green).
Figure 7.
Relationships between polydispersity index values and particle size of risedronate sodium in relation to applied excipients. (SDS = sodium dodecyl sulfate, PLS = polysorbate 80, PEG = macrogol 6000, SCMC = sodium carboxymethyl cellulose, SCMD = sodium carboxymethyl dextran, DSP = sodium dodecyl sulfate/macrogol 6000; fill colour of individual symbols depends on concentration of excipient: 1% = blue, 3% = red, 5% = green).
Figure 8,
Figure 9,
Figure 10 and
Figure 11 represent SEM images showing surface structure and morphology of the selected samples at 5% mass concentration of the excipient, in which the occurrence of risedronate nanoparticles was confirmed by dynamic light scattering. The microscopy results confirmed previous results. The occurrence of nanoparticles clusters is clearly visible on
Figure 8 representing Sample
2c. The clusters of risedronate nanoparticles are hidden under the excipient polysorbate 80, which is a viscous excipient that creates coating.
Figure 8.
SEM image of risedronate with polysorbate 80 (Sample 2c) at magnification 500× (A) and 2000× (B).
Figure 8.
SEM image of risedronate with polysorbate 80 (Sample 2c) at magnification 500× (A) and 2000× (B).
Figure 9.
SEM image of risedronate with macrogol 6000 (Sample 3c) at magnification 2400× (A) and 5000× (B).
Figure 9.
SEM image of risedronate with macrogol 6000 (Sample 3c) at magnification 2400× (A) and 5000× (B).
Figure 10.
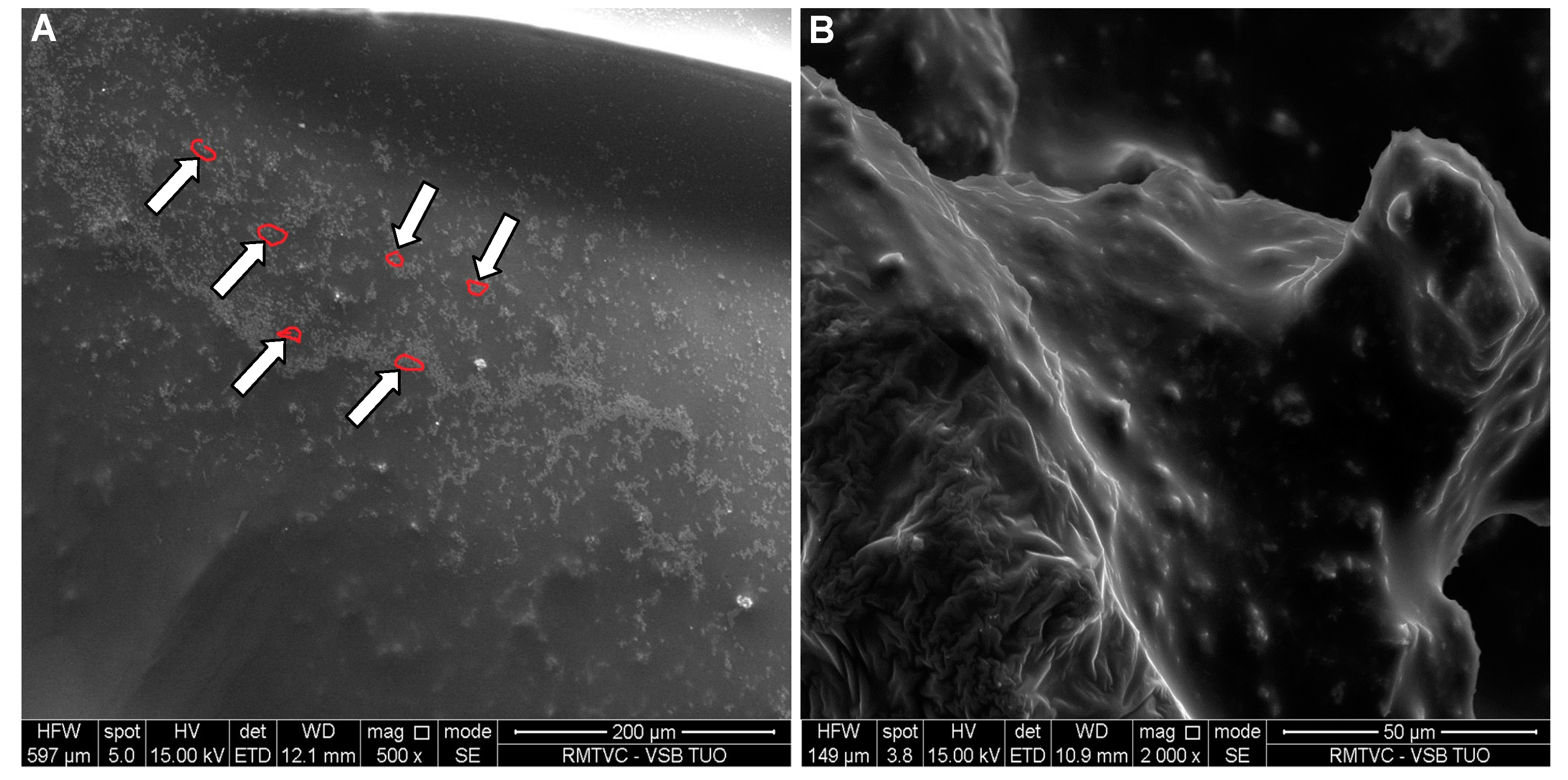
SEM image of risedronate with sodium carboxymethyl dextran (Sample 5c) at magnification 2000×.
Figure 10.
SEM image of risedronate with sodium carboxymethyl dextran (Sample 5c) at magnification 2000×.
Figure 11.
SEM image of risedronate with sodium dodecyl sulphate/macrogol 6000 (Sample 6c) at magnification 800× (A) and 1600× (B).
Figure 11.
SEM image of risedronate with sodium dodecyl sulphate/macrogol 6000 (Sample 6c) at magnification 800× (A) and 1600× (B).
Figure 9A shows spherical particles of risedronate with different sizes anchored in macrogol 6000 matrix; the structure of risedronate with macrogol is shown in
Figure 9B in detail. The risedronate nanoparticles placed in polymer matrices are illustrated in
Figure 10 and
Figure 11. In case of
Figure 10 sodium carboxylmethyl dextran forms a layered structure containing spherical nanoparticles of risedronate. In
Figure 11 micelles of sodium dodecyl sulphate containing risedronate nanoparticles and risedronate nanoparticles embedded in macrogel 6000 matrix are visible.
The particle size data listed in
Table 1 indicate the following relationships of chemical structure and particle size: obviously, the smallest size of particles was observed for hydrophilic non-ionic surfactants–polysorbate 80 (which is sorbitan monooleate with 20 oxyethylene units) and macrogol 6000 (polyethylene glycol). Also, hydrophilic surfactant/viscosity modifier sodium carboxymethyl dextran (which is branched polymeric α-
d-glucopyranose substituted by carboxymethyl moieties that are protonated by sodium ions) provides risedronate nanoparticles of the size of few nanometers. All three excipients have ability to generate films and thus coat different small molecules in comparison with sodium dodecyl sulfate, which is an anionic surfactant generating micelles. These results are supported by the fact that stable nanoparticles relatively independent on the excipient concentration were formed by the most effective polymeric “coated” excipients. It is important to note that polysorbate 80, macrogol 6000 and sodium carboxymethyl dextran are soluble in water in contrast to polymeric but poorly water soluble sodium carboxymethyl cellulose (nanoparticles generated only at mass concentration of 1%) that proved to be, due to its aqueous solubility, the least convenient stabilizer of nanoparticles.
Risedronate sodium is a small extremely hydrophilic molecule with a low p
Ka value that gives smaller and more homogenous nanoparticles with polymeric non-ionic stabilizers or with polymeric ionic excipients. Probably due to the character of risedronate this polymeric type of viscous excipients coats and better stabilizes individual particles of risedronate in comparison with the anionic micellar surfactant sodium dodecyl sulfate. This hypothesis is supported by the fact that the particle size of risedronate nanoparticles obtained by combination with sodium dodecyl sulfate and macrogol (3% and 5%) was smaller than that of risedronate nanoparticles with dodecyl sulfate only, see
Table 1. Moreover polysorbate 80, macrogol 6000 and sodium carboxymethyl dextran were also found as effective stabilizers for preparation of nanoparticles of steroid compounds, candesartan cilexetil or atorvastatin calcium [
23,
24].