Identification of a New Peritrophic Membrane Protein from Larval Holotrichia parallela (Coleoptera: Motschulsky)
Abstract
:1. Introduction
2. Results and Discussion
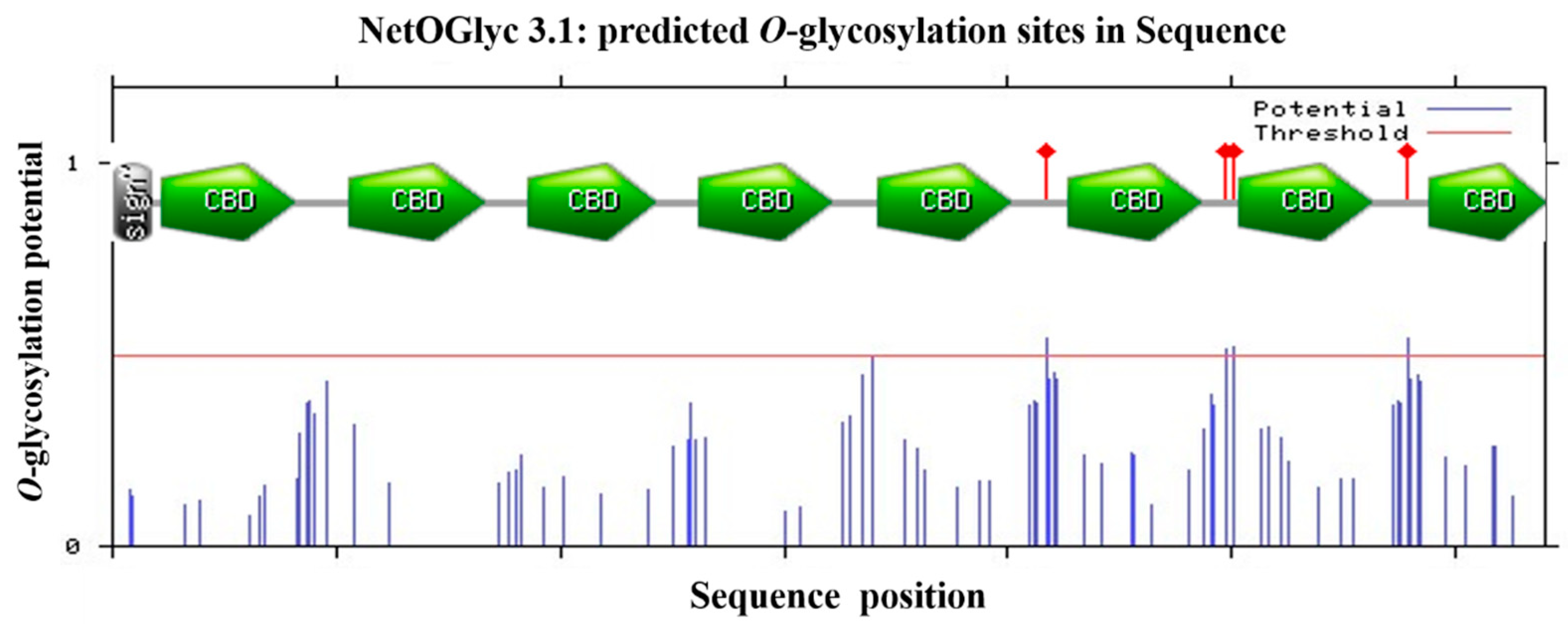
2.1. cDNA Cloning and Sequence Analysis of HpCBP45


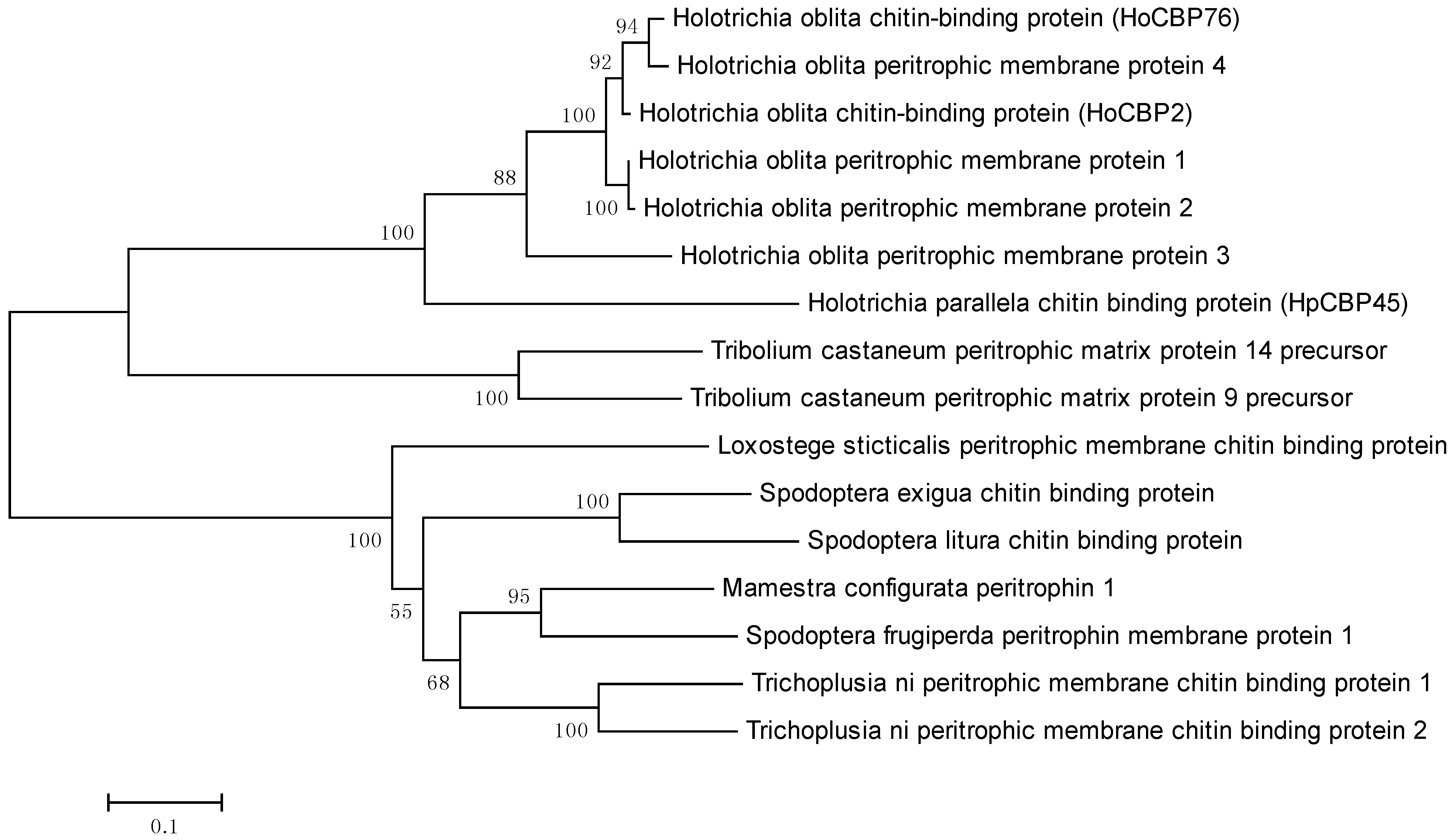
2.2. Phylogenetic Analysis of HpCBP45
2.3. Expression of Recombinant HpCBP45
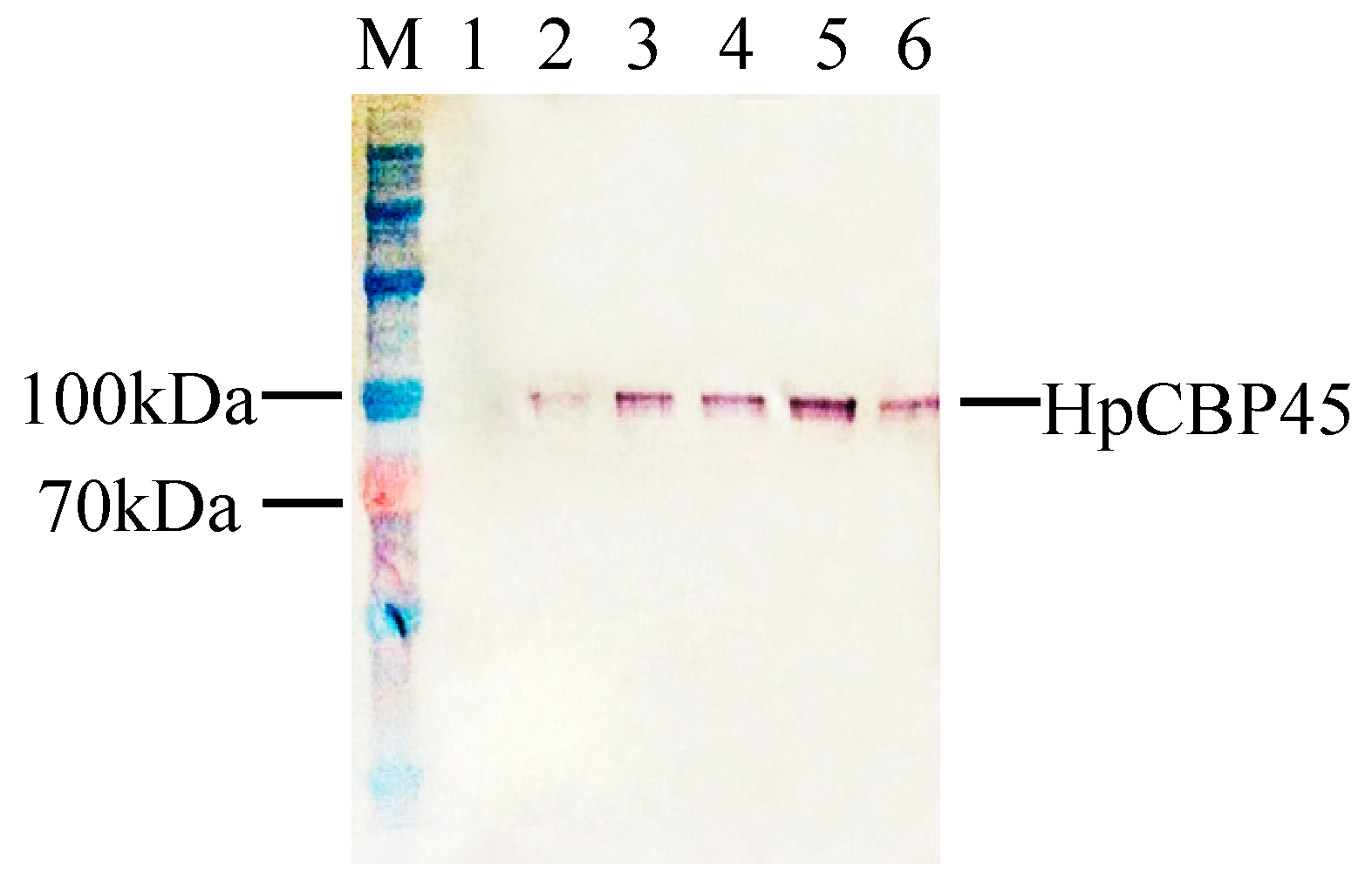
2.4. Chitin-Binding Activity of Recombinant HpCBP45

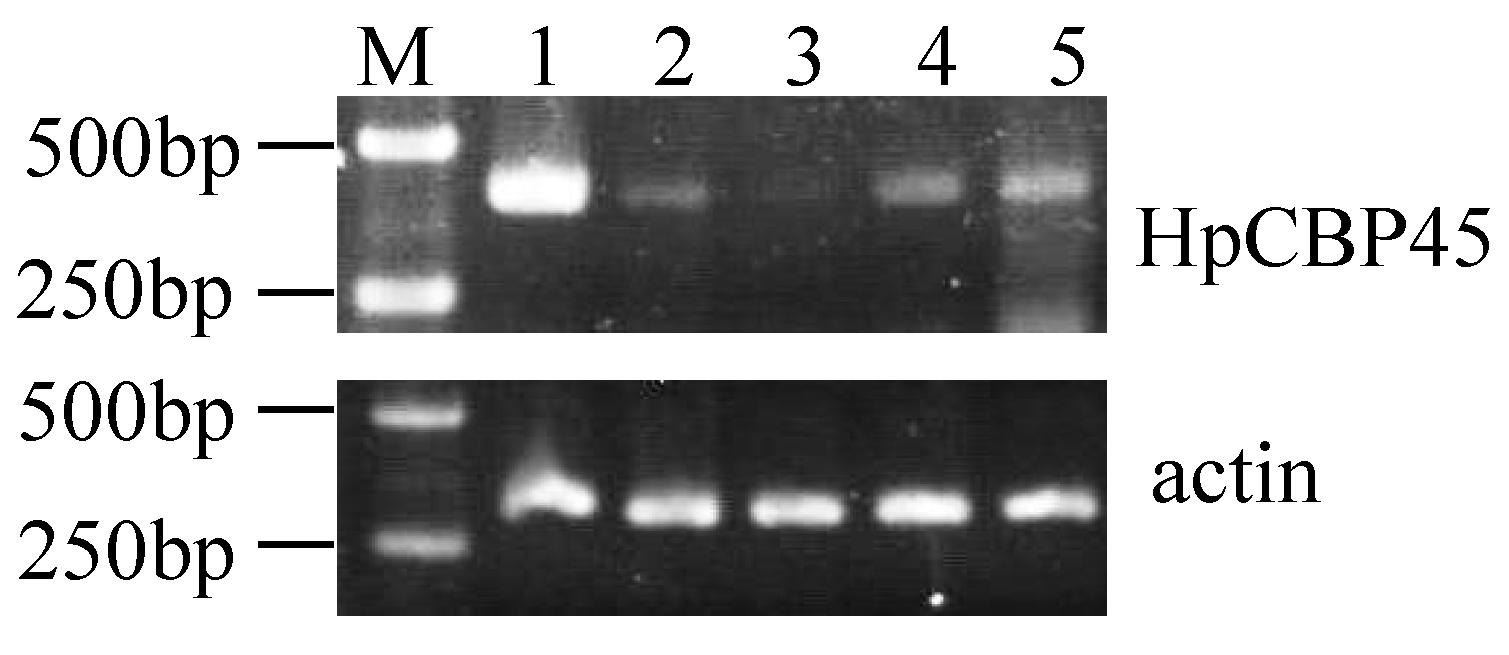
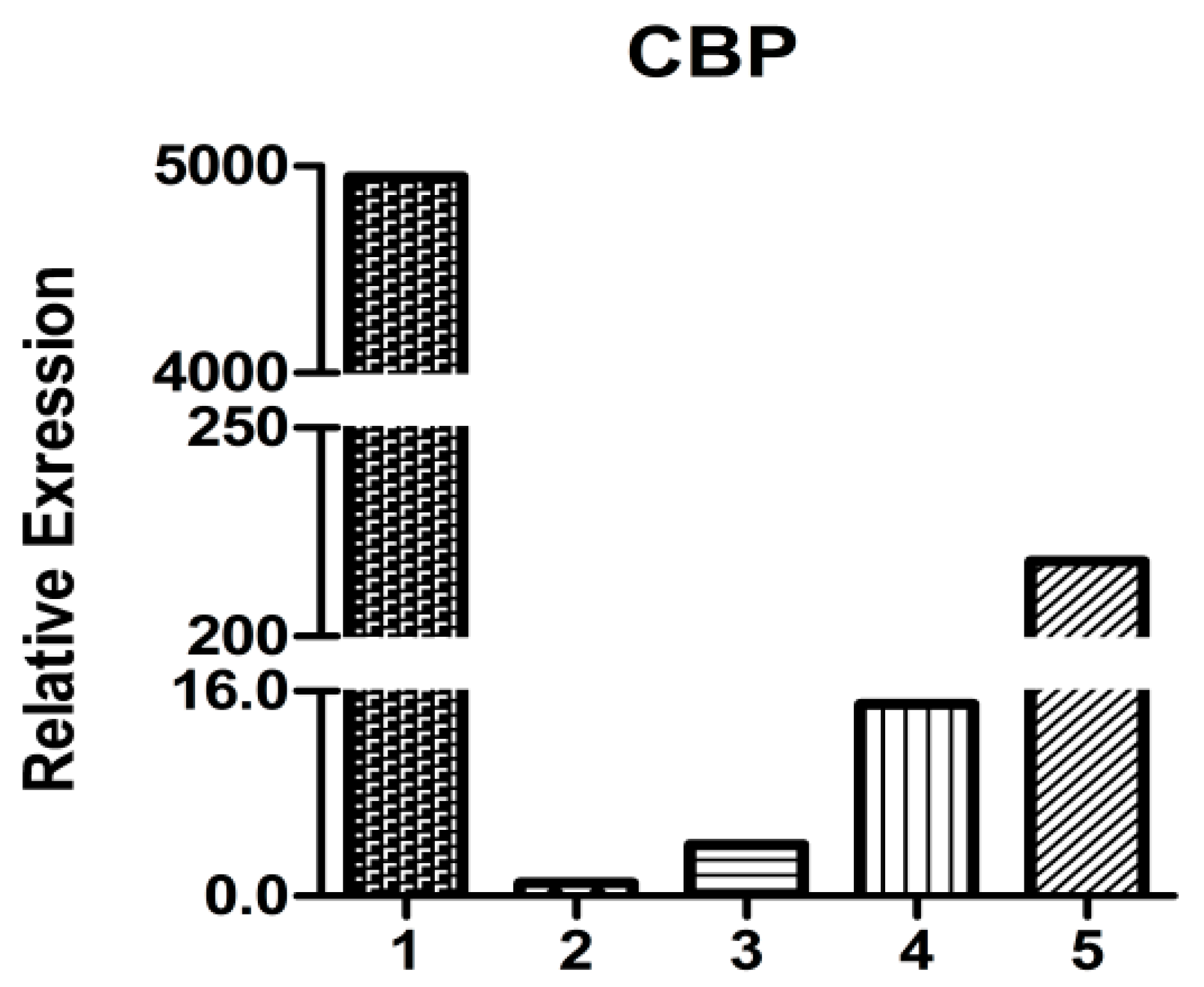
2.5. HpCBP45 Transcriptional Analysis in Different Tissues
3. Experimental Section
3.1. Insect Larvae and PM Preparation
3.2. Cloning of H. Parallela Chitin Binding Protein cDNA and Sequence Analysis
3.3. Preparation of Expression Construct for Recombinant HpCBP45
3.4. Expression of Recombinant Chitin Binding Protein HpCBP45
3.5. Chitin-Binding Assay
3.6. Semi-Quantitative RT-PCR and Quantitative Real-Time RT-PCR
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peters, W. Peritrophic Membranes; Springer-Verlag: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Barbehenn, R.V. Roles of peritrophic membranes in protecting herbivorous insects from ingested plant allelochemicals. Arch. Insect Biochem. 2001, 47, 86–99. [Google Scholar] [CrossRef]
- Hegedus, D.; Erlandson, M.; Gillott, C. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, R.; Ribeiro, A.F.; Terra, W.R. The peritrophic membrane of Spodoptera frugiperda: secretion of peritrophins and role in immobilization and recycling digestive enzymes. Arch. Insect Biochem. 2001, 47, 62–75. [Google Scholar] [CrossRef]
- Shao, L.; Devenport, M.; Jacobs-Lorena, M. The peritrophic matrix of hematophagous insects. Arch. Insect Biochem. 2001, 47, 119–125. [Google Scholar] [CrossRef]
- Shen, Z.; Jacobs-Lorena, M. A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin cloning, expression, and characterization. J. Biol. Chem. 1998, 273, 17665–17670. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, G.; Eisemann, C.; Riding, G. A novel family of chitin–binding proteins from insect type 2 peritrophic matrix. cDNA sequences, chitin binding activity, and cellular localization. J. Biol. Chem. 2001, 276, 15527–15536. [Google Scholar] [CrossRef] [PubMed]
- Tellam, R.L.; Wijffels, G.; Willadsen, P. Peritrophic matrix proteins. Insect Biochem. Mol. Biol. 1999, 29, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.K.; Triplett, A.; Christensen, A.C. A peritrophin–like protein expressed in the embryonic trachea of Drosophila melanogaster. Insect Biochem. Mol. Biol. 1999, 29, 319–327. [Google Scholar] [CrossRef]
- Ross, L.; Tellam, C.E.; Rosanne, C.; Roger, P. The intrinsic peritrophic matrix protein peritrophin-95 from larvae of Lucilia cuprinais synthesised in the cardia and regurgitated or excreted as a highly immunogenic protein. Insect Biochem. Mol. Biol. 2000, 30, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Granados, R.R. Molecular structure of the peritrophic membrane (PM): Identification of potential PM target sites for insect control. Arch. Insect Biochem. 2001, 47, 110–118. [Google Scholar] [CrossRef]
- Shen, Z.; Jacobs-Lorena, M. Evolution of chitin-binding proteins in invertebrates. J. Mol. Evol. 1999, 48, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sarauer, B.L.; Gillott, C.; Hegedus, D. Characterization of an intestinal mucin from the peritrophic matrix of the diamondback moth, Plutella xylostella. Insect Mol. Biol. Biol. 2003, 12, 333–334. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.X.; Granados, R.R. Identification of two new peritrophic membrane proteins from larval Trichoplusia ni: Structural characteristics and their functions in the protease rich insect gut. Insect Biochem. Mol. 2003, 34, 215–227. [Google Scholar]
- Guo, W.P.; Li, G.X.; Pang, Y.; Wang, P. A novel chitin binding protein identified from the peritrophic membrane of the cabbage looper, Trichoplusia ni. Insect Biochem. Mol. Biol. 2005, 35, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Tan, X.M.; Li, C.Y.; Wang, J.P.; Sun, X.G.; Guo, W.; Li, G.X. Molecular cloning and sequencing of cDNAs of two peritrophic membrane proteins from Holotrichia oblita (Cileoptera: Melolonthidae). Acta Entomol. Sin. 2009, 52, 10–16. [Google Scholar]
- Lehane, M.; Billingsley, P. The Biology of the Insect Midgut; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Wang, P.; Granados, R.R. Molecular cloning and sequencing of a novel invertebrate intestinal mucin cDNA. J. Biol. Chem. 1997, 272, 16663–16669. [Google Scholar] [CrossRef] [PubMed]
- Casu, R.E.; Eisemann, C.H.; Vuocolo, T.; Tellam, R.L. The major excretory/secretory protease from Lucilia cuprina larvae is also a gut digestive protease. Int. J. Parasitol. 1996, 26, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Tristram, J. Normal and cocoon–forming peritrophic membrane in larvae of the beetle Gibbium psylloides. J. Insect Physiol. 1977, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- BLAST. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 16 July 2014).
- PROSITE. Available online: http://prosite.expasy.org/prosite.html (accessed on 16 July 2014).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Guo, W.; Li, S.; Li, R.; Xu, D.; Lu, X. Identification of a New Peritrophic Membrane Protein from Larval Holotrichia parallela (Coleoptera: Motschulsky). Molecules 2014, 19, 17799-17809. https://doi.org/10.3390/molecules191117799
Zhao D, Guo W, Li S, Li R, Xu D, Lu X. Identification of a New Peritrophic Membrane Protein from Larval Holotrichia parallela (Coleoptera: Motschulsky). Molecules. 2014; 19(11):17799-17809. https://doi.org/10.3390/molecules191117799
Chicago/Turabian StyleZhao, Dan, Wei Guo, Shaoya Li, Ruijun Li, Daqing Xu, and Xiujun Lu. 2014. "Identification of a New Peritrophic Membrane Protein from Larval Holotrichia parallela (Coleoptera: Motschulsky)" Molecules 19, no. 11: 17799-17809. https://doi.org/10.3390/molecules191117799



