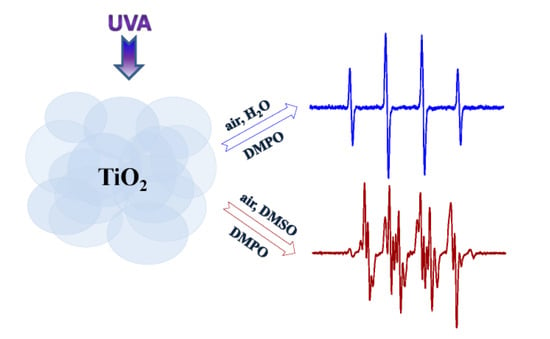
Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study)
Abstract
:1. Introduction
2. Results and Discussion
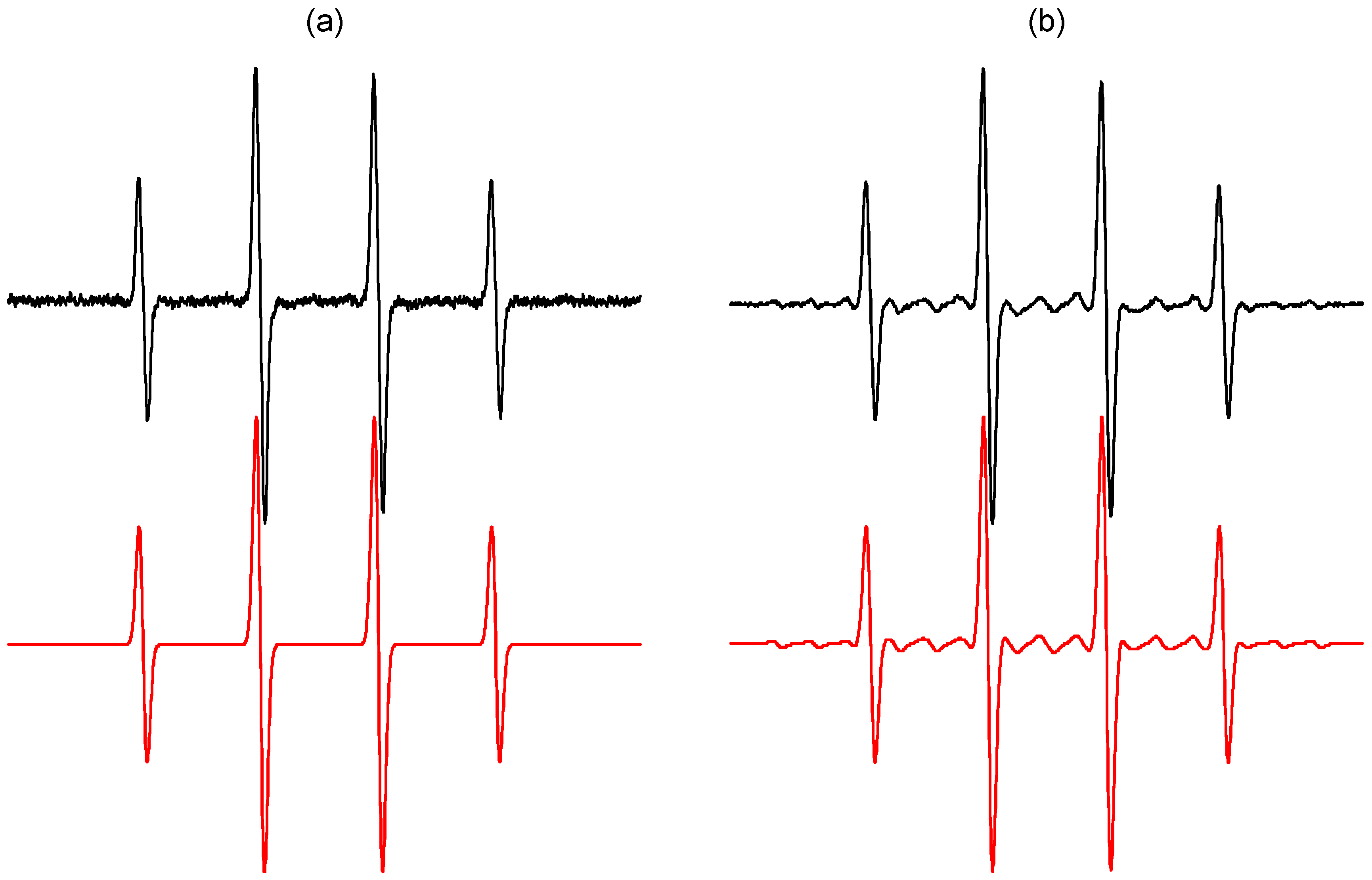
2.1. Spin Trapping in the Aqueous TiO2 Suspensions

| Solvent | , M−1∙s−1 | , s−1 | τ (1/), μs |
|---|---|---|---|
| Water | – | 2.4 × 105 | 4.2 |
| Dimethylsulfoxide # | 7.0 × 109 | 5.2 × 104 | 19 |
| Acetonitrile # | 2.2 × 107 | 1.4 × 104 | 71 |
| Methanol # | 8.3 × 108 | 1.1 × 105 | 9 |
| Ethanol # | 2.2 × 109 | 7.9 × 104 | 13 |
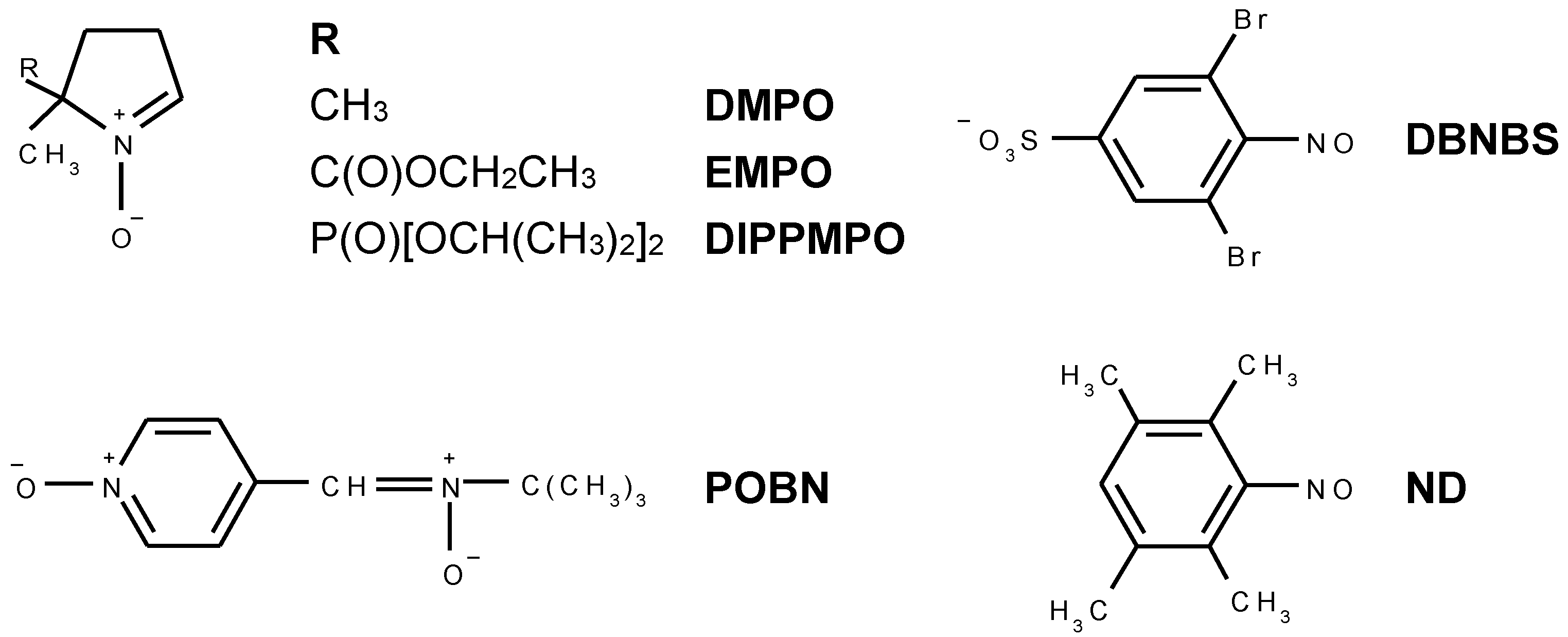
| Spin-Adduct | Hyperfine Coupling Constants(mT) | g-Value | Reference | |
|---|---|---|---|---|
| aNO | ai | |||
| Water | ||||
| •DMPO–OH | 1.497 | aHβ = 1.477 | 2.0057 | [50] |
| •DMPO–17OH | 1.494 | aHβ = 1.480; = 0.469 | 2.0057 | [51,57] |
| •DMPO–N3 | 1.481 | aHβ = 1.426; aN = 0.314 | 2.0057 | [50] |
| trans-•EMPO–OH | 1.410 | aHβ = 1.278; aHγ = 0.066; aHγ = 0.043 | 2.0056 | [58,59] |
| cis-•EMPO–OH | 1.410 | aHβ = 1.542 | 2.0056 | [58,59] |
| •EMPOdegr | 1.514 | aHβ = 2.187 | 2.0056 | – |
| trans-•DIPPMPO–OH | 1.410 | aHβ = 1.319; aP = 4.692 | 2.0055 | [59,60] |
| cis-•DIPPMPO–OH | 1.646 | aHβ = 1.236; aP = 3.572 | 2.0055 | [59,60] |
| •DIPPMPOdegr | 1.469 | aHβ = 2.147; aP = 4.830 | 2.0055 | – |
| •POBN–OH | 1.508 | aHβ = 0.169 | 2.0057 | [44] |
| •POBNdegr | 1.461 | aHβ = 1.413 | 2.0055 | [61] |
| Water/DMSO (5:1 v:v) | ||||
| •DMPO–OH | 1.469 | aHβ = 1.358; aHγ = 0.067 | 2.0057 | [56] |
| •DMPO–CH3 | 1.588 | aHβ = 2.250 | 2.0055 | [50] |
| •DBNBS–CH3 | 1.434 | aH(3H) = 1.331; aH(2Hm) = 0.069; = 0.929 | 2.0063 | [50,62] |
| •DBNBS–CD3 | 1.434 | aD(3D) = 0.201; aH(2Hm) = 0.070 | 2.0063 | [50] |
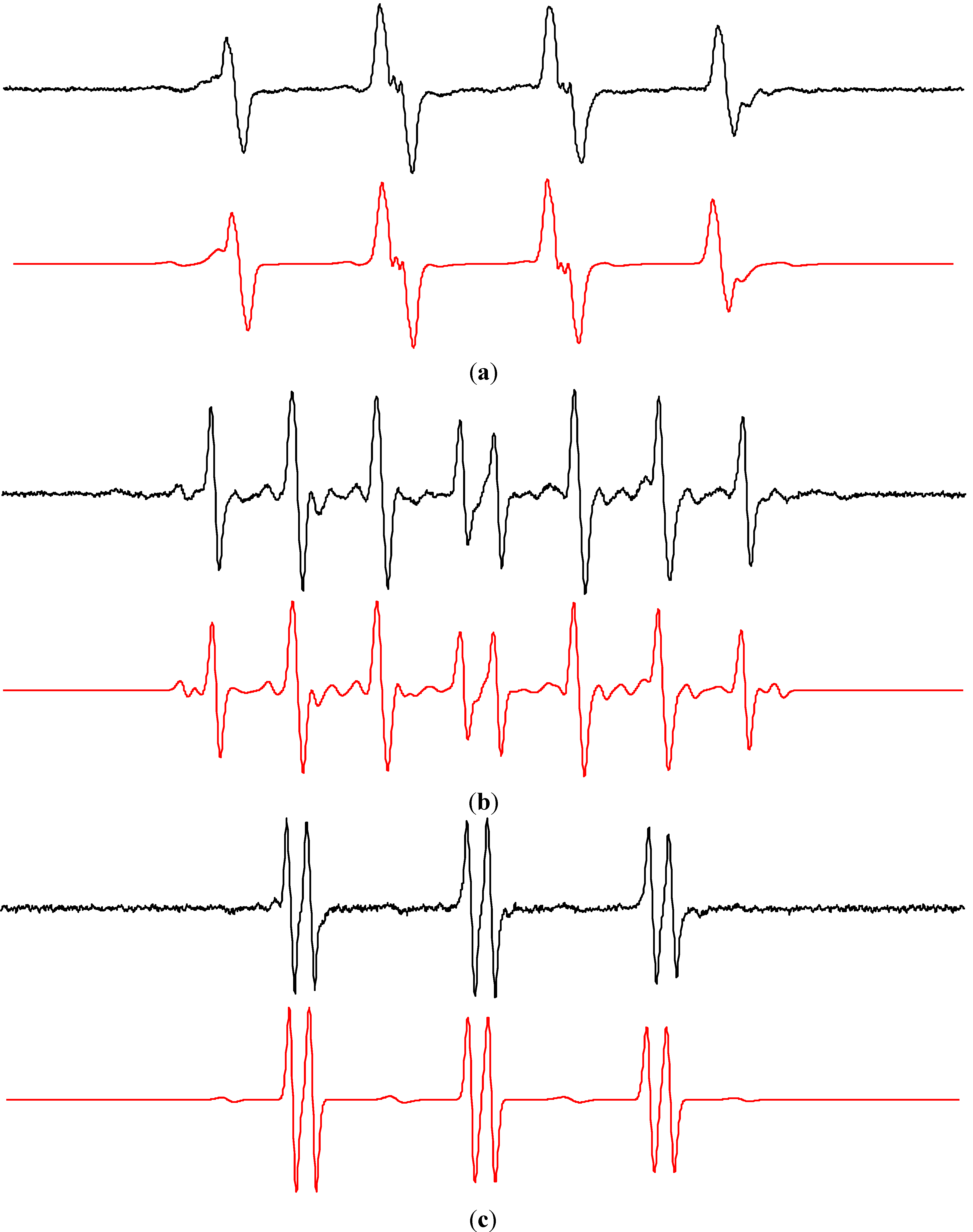
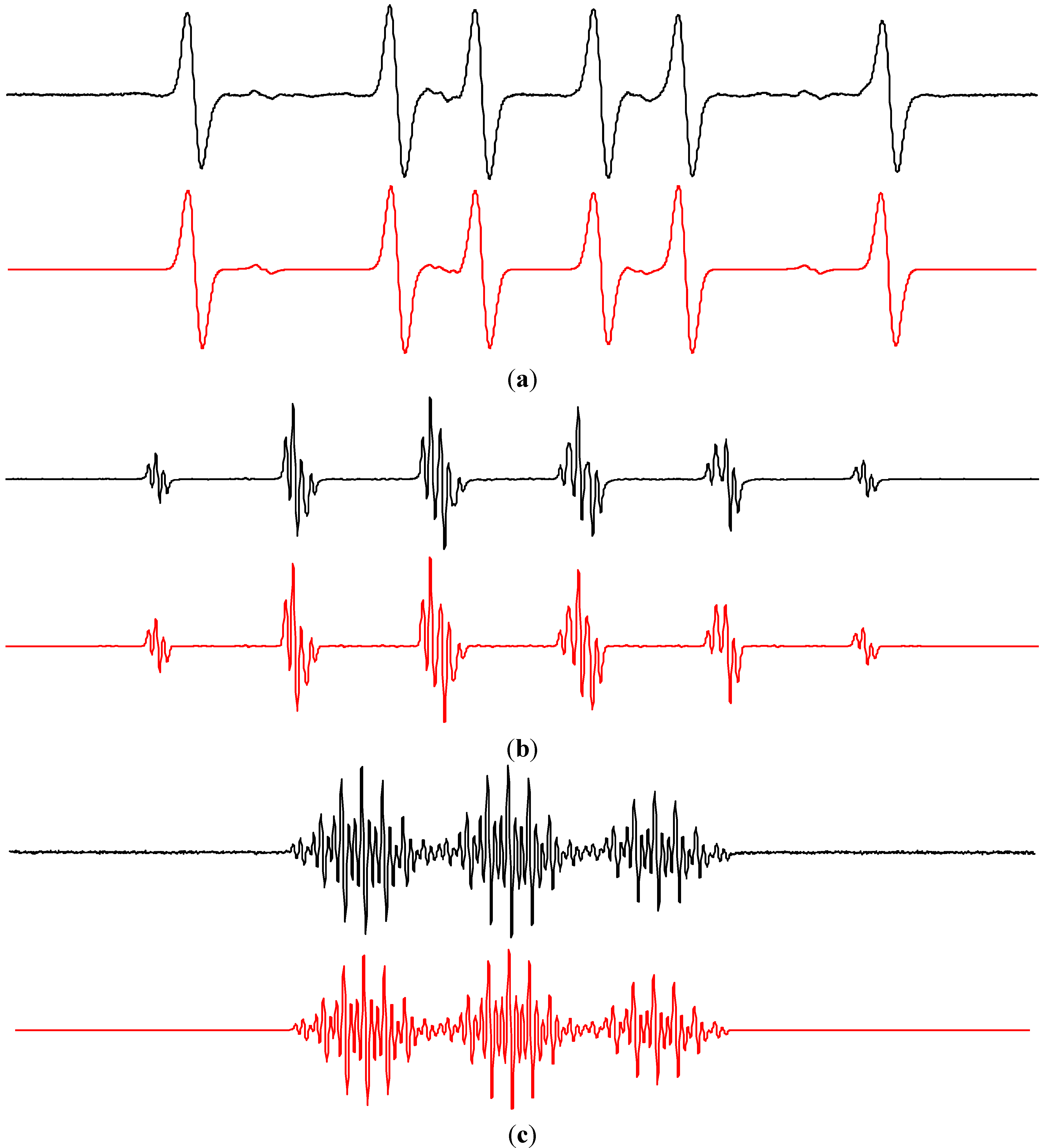
2.2. Spin Trapping in Non-Aqueous TiO2 Suspensions
| Solvent | , mM | Reference |
|---|---|---|
| Water | 1.0 | [77] |
| Dimethylsulfoxide | 2.1 | [78] |
| Acetonitrile | 8.1 | [78] |
| Methanol | 9.4–10.3 | [79] |
| Ethanol | 7.5–11.6 | [79] |
2.2.1. Dimethylsulfoxide
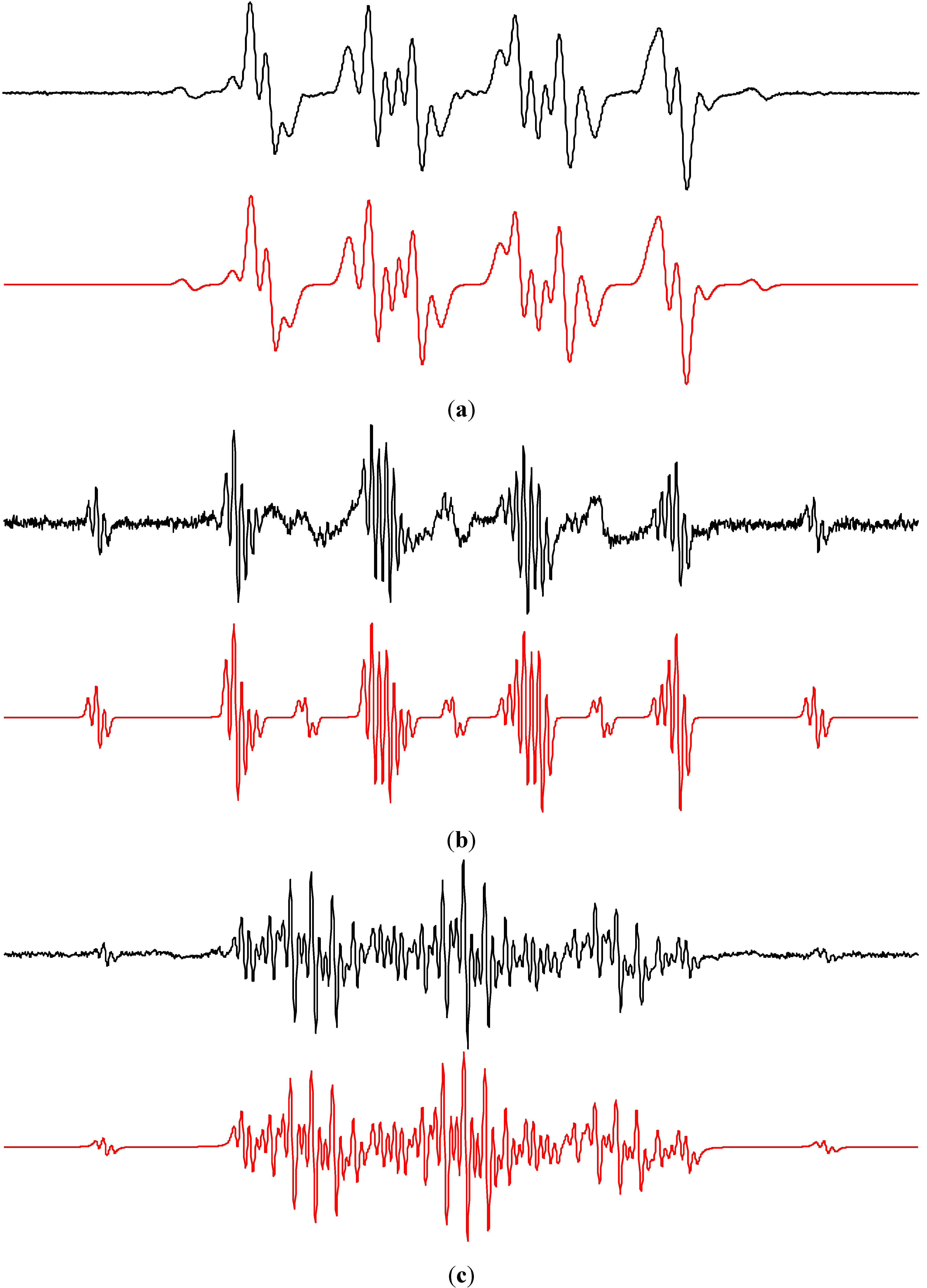
2.2.2. Acetonitrile
2.2.3. Methanol and Ethanol
| Spin-Adduct | Hyperfine Coupling Constants (mT) | g-Value | Reference | |
|---|---|---|---|---|
| aNO | ai | |||
| DMSO | ||||
| •DMPO–O2− | 1.287 | aHβ = 1.041; aHγ = 0.139 | 2.0057 | [25,50,83] |
| •DMPO–OCH3 | 1.329 | aHβ = 0.808; aHγ = 0.164 | 2.0057 | [50,84] |
| •DMPO–OR | 1.301 | aHβ = 1.464 | 2.0057 | [84] |
| •DMPO–CH3 | 1.462 | aHβ = 2.093 | 2.0056 | [50] |
| •DBNBS–CH3 | 1.337 | aH(3H) = 1.211; aH(2Hm) = 0.067 | 2.0064 | [50] |
| •DBNBS–CD3 | 1.334 | aD(3D) = 0.183; aH(2Hm) = 0.067 | 2.0064 | [50] |
| •DBNBS–SO3− | 1.295 | aH(2Hm) = 0.054 | 2.0064 | [85] |
| ACN | ||||
| •DMPO–O2− # | 1.296 | aHβ = 1.044; aHγ = 0.133 | 2.0057 | [50,74] |
| •DMPO–OH # | 1.382 | aHβ = 1.200; aHγ = 0.080 | 2.0057 | [74] |
| •DMPO–OCH3# | 1.312 | aHβ = 0.796; aHγ = 0.179 | 2.0057 | [74] |
| •DMPOdegr# | 1.479 | 2.0056 | – | |
| •ND–CH2CN | 1.342 | aH(2H) = 0.977 | 2.0057 | [86,87] |
| ND•+ | 2.608 | 2.0057 | [50] | |
| Methanol | ||||
| •DMPO–O2− # | 1.376 | aHβ = 0.963; aHγ = 0.132 | 2.0057 | [50] |
| •DMPO–OCH3# | 1.363 | aHβ = 0.775; aHγ = 0.167 | 2.0057 | [50] |
| •DMPO–OCH2OH # | 1.414 | aHβ = 1.266; aHγ = 0.075 | 2.0057 | [50] |
| •DMPO–CH2OH # | 1.506 | aHβ = 2.116 | 2.0056 | [50] |
| •DMPOdegr# | 1.523 | 2.0056 | – | |
| •ND–CH2OH | 1.387 | aH(2H) = 0.771 | 2.0057 | [62,86,87] |
| Ethanol | ||||
| •DMPO–O2− # | 1.322 | aHβ = 1.050; aHγ = 0.133 | 2.0057 | [50] |
| •DMPO–OCH2CH3# | 1.356 | aHβ = 0.761; aHγ = 0.174 | 2.0057 | [50] |
| •DMPO–OR # | 1.470 | aHβ = 1.094; aHγ = 0.090 | 2.0057 | [50] |
| •DMPO–CR1# | 1.481 | aHβ = 2.195 | 2.0056 | [50] |
| •DMPO–CR2# | 1.534 | aHβ = 2.215 | 2.0056 | [50] |
| •ND–CH(CH3)OH | 1.398 | aH = 0.702 | 2.0057 | [62,86,87] |
| •ND–CH3 | 1.452 | aH(3H) = 1.345 | 2.0057 | [62,86,87] |

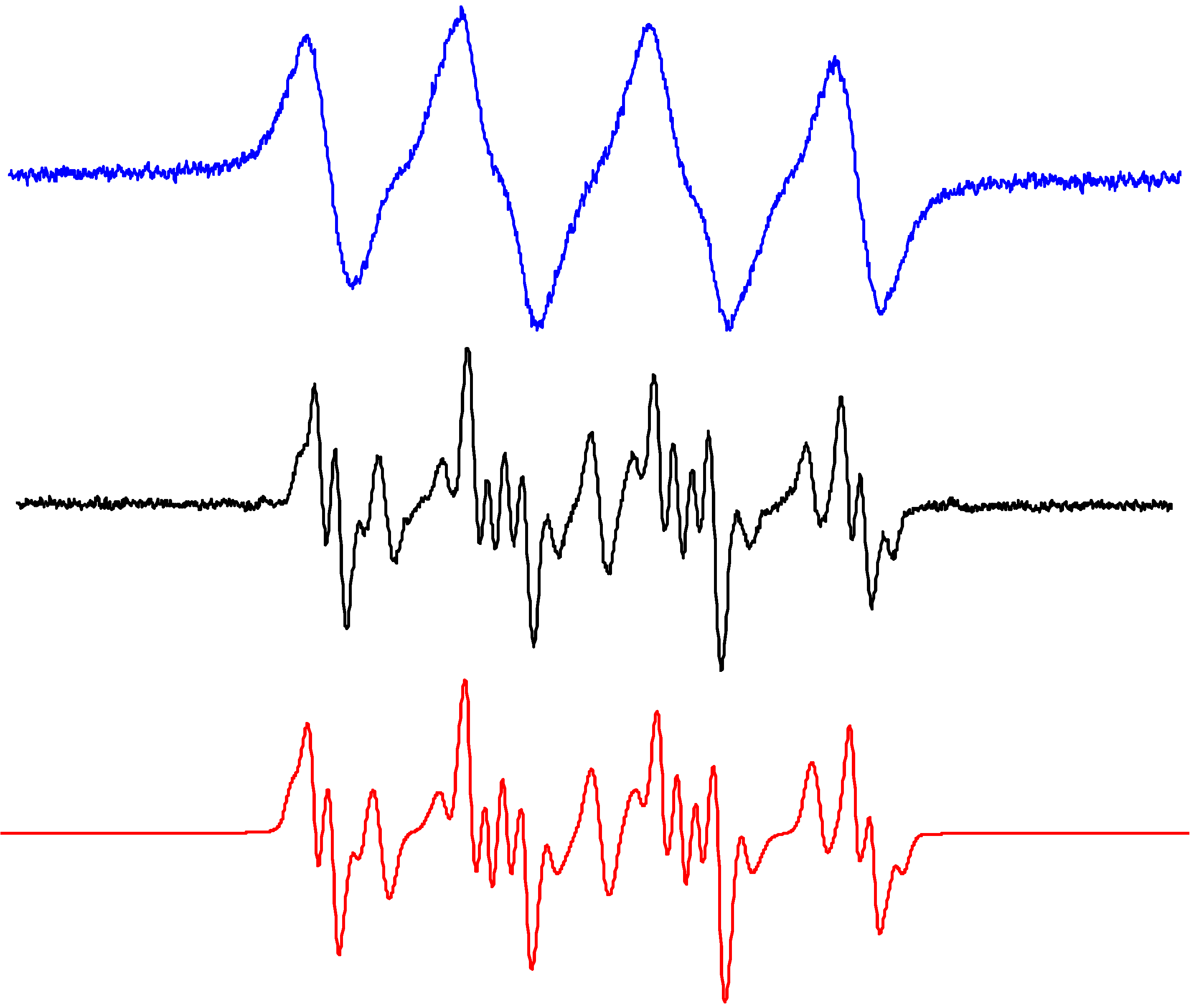
2.3. Oxidation of Sterically Hindered Amine in TiO2 Suspensions
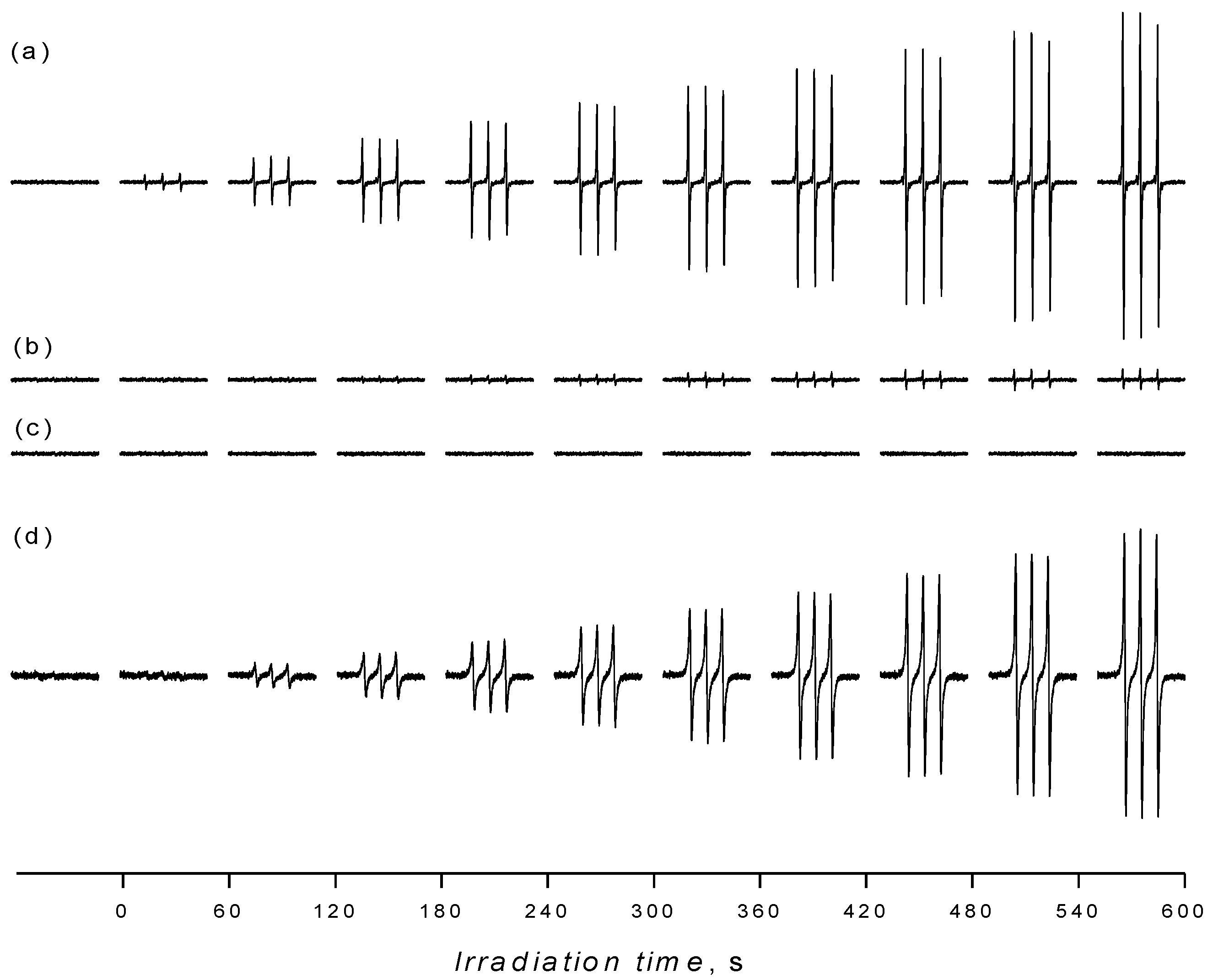
3. Experimental Section
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Minero, C.; Maurino, V.; Vione, D. Photocatalytic mechanisms and reaction pathways drawn from kinetic and probe molecules. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications, 1st ed.; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 53–72. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar]
- McCullagh, C.; Robertson, J.; Bahnemann, D.; Robertson, P. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar]
- Agrios, A.; Pichat, P. State of the art and perspectives on materials and applications of photocatalysis over TiO2. J. Appl. Electrochem. 2005, 35, 655–663. [Google Scholar]
- Carp, O.; Huisman, C.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar]
- Kanakaraju, D.; Glass, B.; Oelgemoller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar]
- Lang, X.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Selective aerobic oxidation mediated by TiO2 photocatalysis. Acc. Chem. Res. 2014, 47, 355–363. [Google Scholar]
- Ahmed, S.; Rasul, M.; Martens, W.; Brown, R.; Hashib, M. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar]
- Gaya, U.; Abdullah, A. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Rev. 2008, 9, 1–12. [Google Scholar] [Green Version]
- McCullagh, C.; Skillen, N.; Adams, M.; Robertson, P. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar]
- Amadelli, R.; Samiolo, L.; Maldotti, A.; Molinari, A.; Gazzoli, D. Selective photooxidation and photoreduction processes at TiO2 surface-modified by grafted vanadyl. Int. J. Photoenergy 2011, 2011. [Google Scholar] [CrossRef]
- Molinari, A.; Montoncello, M.; Rezala, H.; Maldotti, A. Partial oxidation of allylic and primary alcohols with O2 by photoexcited TiO2. Photochem. Photobiol. Sci. 2009, 8, 613–619. [Google Scholar]
- Henderson, M. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar]
- Diebold, U. Structure and properties of TiO2 surfaces: A brief review. Appl. Phys. A: Mater. Sci. Process. 2003, 76, 681–687. [Google Scholar]
- Pelaez, M.; Nolan, N.; Pillai, S.; Seery, M.; Falaras, P.; Kontos, A.; Dunlop, P.; Hamilton, J.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar]
- Zhang, J.; Nosaka, Y. Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar]
- Montoya, J.; Ivanova, I.; Dillert, R.; Bahnemann, D.; Salvador, P.; Peral, J. Catalytic role of surface oxygens in TiO2 photooxidation reactions: Aqueous benzene photooxidation with Ti18O2 under anaerobic conditions. J. Phys. Chem. Lett. 2013, 4, 1415–1422. [Google Scholar]
- Salvador, P. On the nature of photogenerated radical species active in the oxidative degradation of dissolved pollutants with TiO2 aqueous suspensions: A revision in the light of the electronic structure of adsorbed water. J. Phys. Chem. C 2007, 111, 17038–17043. [Google Scholar]
- Green, J.; Carter, E.; Murphy, D. An EPR investigation of acetonitrile reactivity with superoxide radicals on polycrystalline TiO2. Res. Chem. Intermed. 2009, 35, 145–154. [Google Scholar]
- Carter, E.; Carley, A.; Murphy, D. Evidence for O2− radical stabilization at surface oxygen vacancies on polycrystalline TiO2. J. Phys. Chem. C 2007, 111, 10630–10638. [Google Scholar]
- Berger, T.; Sterrer, M.; Diwald, O.; Knozinger, E.; Panayotov, D.; Thompson, T.; Yates, J. Light-induced charge separation in anatase TiO2 particles. J. Phys. Chem. B 2005, 109, 6061–6068. [Google Scholar]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc Chem. Res. 1981, 14, 393–400. [Google Scholar]
- Harbour, J.R.; Hair, M.L. Detection of superoxide ions in nonaqueous media. Generation by photolysis of pigment dispersions. J. Phys. Chem. 1978, 82, 1397–1399. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Identification and roles of the active species generated on various photocatalysts. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications, 1st ed.; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 3–24. [Google Scholar]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2•− and •OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A 2007, 325, 105–111. [Google Scholar]
- Hirakawa, T.; Daimon, T.; Kitazawa, M.; Ohguri, N.; Koga, C.; Negishi, N.; Matsuzawa, S.; Nosaka, Y. An approach to estimating photocatalytic activity of TiO2 suspension by monitoring dissolved oxygen and superoxide ion on decomposing organic compounds. J. Photochem. Photobiol. A Chem. 2007, 190, 58–68. [Google Scholar]
- Wang, Z.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Probing paramagnetic species in titania-based heterogeneous photocatalysis by electron spin resonance (ESR) spectroscopy—A mini review. Chem. Eng. J. 2011, 170, 353–362. [Google Scholar]
- Micic, O.; Zhang, Y.; Cromack, K.; Trifunac, A.; Thurnauer, M. Trapped holes on TiO2 colloids studied by electron-paramagnetic-resonance. J. Phys. Chem. 1993, 97, 7277–7283. [Google Scholar]
- Nakaoka, Y.; Nosaka, Y. ESR investigation into the effects of heat treatment and crystal structure on radicals produced over irradiated TiO2 powder. J. Photochem. Photobiol. A Chem. 1997, 110, 299–305. [Google Scholar]
- Coronado, J.; Maira, A.; Conesa, J.; Yeung, K.; Augugliaro, V.; Soria, J. EPR study of the surface characteristics of nanostructured TiO2 under UV irradiation. Langmuir 2001, 17, 5368–5374. [Google Scholar]
- Dimitrijevic, N.; Saponjic, Z.; Rabatic, B.; Poluektov, O.; Rajh, T. Effect of size and shape of nanocrystalline TiO2 on photogenerated charges. An EPR study. J. Phys. Chem. C 2007, 111, 14597–14601. [Google Scholar]
- Kokorin, A.I. Electron Spin Resonance of nanostructured oxide semiconductors. In Chemical Physics of Nanostructured Semiconductors; Kokorin, A.I., Bahnemann, D.W., Eds.; VSP BV: Utrecht, The Netherlands, 2003; pp. 203–263. [Google Scholar]
- Ghiazza, M.; Alloa, E.; Oliaro-Bosso, S.; Viola, F.; Livraghi, S.; Rembges, D.; Capomaccio, R.; Rossi, F.; Ponti, J.; Fenoglio, I. Inhibition of the ROS-mediated cytotoxicity and genotoxicity of nano-TiO2 toward human keratinocyte cells by iron doping. J. Nanopart. Res. 2014, 16, 2263. [Google Scholar]
- Chiesa, M.; Paganini, M.C.; Livraghi, S.; Giamello, E. Charge trapping in TiO2 polymorphs as seen by electron paramagnetic resonance spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 9435–9447. [Google Scholar]
- Li, M.; Yin, J.J.; Wamer, W.G.; Lo, Y.M. Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J. Food Drug Anal. 2014, 22, 76–86. [Google Scholar]
- Grela, M.A.; Coronel, M.E.J.; Colussi, A.J. Quantitative spin-trapping studies of weakly illuminated titanium dioxide sols. Implications for the mechanism of photocatalysis. J. Phys. Chem. 1996, 100, 16940–16946. [Google Scholar]
- Jaeger, C.D.; Bard, A.J. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at TiO2 particulate systems. J. Phys. Chem. 1979, 83, 3146–3152. [Google Scholar]
- Dvoranová, D.; Brezová, V.; Mazúr, M.; Malati, M.A. Investigations of metal-doped titanium dioxide photocatalysts. Appl. Catal. B 2002, 37, 91–105. [Google Scholar]
- Taborda, A.V.; Brusa, M.A.; Grela, M.A. Photocatalytic degradation of phthalic acid on TiO2 nanoparticles. Appl. Catal. A 2001, 208, 419–426. [Google Scholar]
- Brezová, V.; Staško, A.; Biskupič, S.; Blažková, A.; Havlínová, B. Kinetics of hydroxyl radical spin trapping in photoactivated homogeneous (H2O2) and heterogeneous (TiO2, O2) aqueous systems. J. Phys. Chem. 1994, 98, 8977–8984. [Google Scholar]
- Nosaka, Y.; Komori, S.; Yawata, K.; Hirakawa, T.; Nosaka, A. Photocatalytic •OH radical formation in TiO2 aqueous suspension studied by several detection methods. Phys. Chem. Chem. Phys. 2003, 5, 4731–4735. [Google Scholar]
- Brezová, V.; Dvoranová, D.; Staško, A. Characterization of titanium dioxide photoactivity following the formation of radicals by EPR spectroscopy. Res. Chem. Intermed. 2007, 33, 251–268. [Google Scholar]
- Brezová, V.; Billik, P.; Vrecková, Z.; Plesch, G. Photoinduced formation of reactive oxygen species in suspensions of titania mechanochemically synthesized from TiCl4. J. Mol. Catal. A Chem. 2010, 327, 101–109. [Google Scholar]
- Hawkins, C.L.; Davies, M.J. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 708–721. [Google Scholar]
- Spasojevic, I. Free radicals and antioxidants at a glance using EPR spectroscopy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 114–142. [Google Scholar]
- Alberti, A.; Macciantelli, D. Spin Trapping. In Electron Paramagnetic Resonance: A Practitioner’s Toolkit; Brustolon, M., Giamelo, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 287–323. [Google Scholar]
- Dodd, N.J.F.; Jha, A.N. Photoexcitation of aqueous suspensions of titanium dioxide nanoparticles: An electron spin resonance spin trapping study of potentially oxidative reactions. Photochem. Photobiol. 2011, 87, 632–640. [Google Scholar]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar]
- Brezová, V.; Barbieriková, Z.; Zukalová, M.; Dvoranová, D.; Kavan, L. EPR study of 17O-enriched titania nanopowders under UV irradiation. Catal. Today 2014, 230, 112–118. [Google Scholar]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol. Pharmacol. 1982, 21, 262–265. [Google Scholar]
- Buxton, G.; Greenstock, C.; Helman, W.; Ross, A. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (•OH/•O−) in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar]
- Wilkinson, F.; Helman, W.; Ross, A. Rate constants for the decay and reactions of the lowest electronically excited singlet-state of molecular-oxygen in solution—An expanded and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–1021. [Google Scholar]
- Zalibera, M.; Rapta, P.; Staško, A.; Brindzová, L.; Brezová, V. Thermal generation of stable SO4•− spin trap adducts with super-hyperfine structure in their EPR spectra: An alternative EPR spin trapping assay for radical scavenging capacity determination in dimethylsulphoxide. Free Radic. Res. 2009, 43, 457–469. [Google Scholar]
- Mottley, C.; Connor, H.D.; Mason, R.P. [17O]oxygen hyperfine structure for the hydroxyl and superoxide radical adducts of the spin traps DMPO, PBN and 4-POBN. Biochem. Biophys. Res. Commun. 1986, 141, 622–628. [Google Scholar]
- Stolze, K.; Rohr-Udilova, N.; Rosenau, T.; Hofinger, A.; Kolarich, D.; Nohl, H. Spin trapping of C- and O-centered radicals with methyl-, ethyl-, pentyl-, and phenyl-substituted EMPO derivatives. Bioorg. Med. Chem. 2006, 14, 3368–3376. [Google Scholar]
- Culcasi, M.; Rockenbauer, A.; Mercier, A.; Clément, J.L.; Pietri, S. The line asymmetry of electron spin resonance spectra as a tool to determine the cis:trans ratio for spin-trapping adducts of chiral pyrrolines N-oxides: The mechanism of formation of hydroxyl radical adducts of EMPO, DEPMPO, and DIPPMPO in the ischemic-reperfused rat liver. Free Radic. Biol. Med. 2006, 40, 1524–1538. [Google Scholar]
- Chalier, F.; Tordo, P. 5-Diisopropoxyphosphoryl-5-methyl-1-pyrroline N-oxide, DIPPMPO, a crystalline analog of the nitrone DEPMPO: Synthesis and spin trapping properties. J. Chem. Soc. Perkin Trans. 2 2002, 2110–2117. [Google Scholar] [CrossRef]
- Huling, S.G.; Arnold, R.G.; Sierka, R.A.; Miller, M.R. Measurement of hydroxyl radical activity in a soil slurry using the spin trap α-(4-pyridyl-1-oxide)-N-tert-butylnitrone. Environ. Sci. Technol. 1998, 32, 3436–3441. [Google Scholar]
- Brezová, V.; Tarábek, P.; Dvoranová, D.; Staško, A.; Biskupič, S. EPR study of photoinduced reduction of nitroso compounds in titanium dioxide suspensions. J. Photochem. Photobiol. A Chem. 2003, 155, 179–198. [Google Scholar]
- Clement, J.; Gilbert, B.; Ho, W.; Jackson, N.; Newton, M.; Silvester, S.; Timmins, G.; Tordo, P.; Whitwood, A. Use of a phosphorylated spin trap to discriminate between the hydroxyl radical and other oxidising species. J. Chem. Soc. Perkin Trans. 2 1998, 1715–1718. [Google Scholar] [CrossRef]
- Patel, A.; Rohr-Udilova, N.; Rosenau, T.; Stolze, K. Synthesis and characterization of 5-alkoxycarbonyl-4-hydroxymethyl-5-alkyl-pyrroline N-oxide derivatives. Bioorg. Med. Chem. 2011, 19, 7643–7652. [Google Scholar]
- Stolze, K.; Rohr-Udilova, N.; Hofinger, A.; Rosenau, T. Spin trapping properties of aminocarbonyl- and methylamino-carbonyl-substituted EMPO derivatives. Free Radic. Res. 2009, 43, 81–81. [Google Scholar]
- Abbas, K.; Hardy, M.; Poulhès, F.; Karoui, H.; Tordo, P.; Ouari, O.; Peyrot, F. Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 71, 281–290. [Google Scholar]
- Halliwel, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; p. 60. [Google Scholar]
- Hirakawa, T.; Nakaoka, Y.; Nishino, J.; Nosaka, Y. Primary passages for various TiO2 photocatalysts studied by means of luminol chemiluminescent probe. J. Phys. Chem. B 1999, 103, 4399–4403. [Google Scholar]
- Nosaka, Y.; Yamashita, Y.; Fukuyama, H. Application of chemiluminescent probe to monitoring superoxide radicals and hydrogen peroxide in TiO2 photocatalysis. J. Phys. Chem. B 1997, 101, 5822–5827. [Google Scholar]
- Nosaka, Y.; Fukuyama, H. Application of chemiluminescent probe to the characterization of TiO2 photocatalysts in aqueous suspension. Chem. Lett. 1997, 26, 383–384. [Google Scholar]
- Marino, T.; Molinari, R.; García, H. Selectivity of gold nanoparticles on the photocatalytic activity of TiO2 for the hydroxylation of benzene by water. Catal. Today 2013, 206, 40–45. [Google Scholar]
- Molinari, A.; Maldotti, A.; Amadelli, R. Probing the role of surface energetics of electrons and their accumulation in photoreduction processes on TiO2. Chem. Eur. J. 2014, 20, 7759–7765. [Google Scholar]
- Brezová, V.; Gabčová, S.; Dvoranová, D.; Staško, A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (An EPR study). J. Photochem. Photobiol. B Biol. 2005, 79, 121–134. [Google Scholar]
- Barbieriková, Z.; Mihalíková, M.; Brezová, V. Photoinduced oxidation of sterically hindered amines in acetonitrile solutions and titania suspensions (An EPR study). Photochem. Photobiol. 2012, 88, 1442–1454. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 86th ed.; CRC Press: Boca Raton, FL, USA, 2005.
- Mitroka, S.; Zimmeck, S.; Troya, D.; Tanko, J. How solvent modulates hydroxyl radical reactivity in hydrogen atom abstractions. J. Am. Chem. Soc. 2010, 132, 2907–2913. [Google Scholar]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, USA, 2010; p. 1008. [Google Scholar]
- Wadhawan, J.; Welford, P.; McPeak, H.; Hahn, C.; Compton, R. The simultaneous voltammetric determination and detection of oxygen and carbon dioxide—A study of the kinetics of the reaction between superoxide and carbon dioxide in non-aqueous media using membrane-free gold disc microelectrodes. Sens. Actuat. B 2003, 88, 40–52. [Google Scholar]
- Golovanov, I.; Zhenodarova, S. Quantitative structure-property relationship: XXIII. Solubility of oxygen in organic solvents. Russ. J. Gen. Chem. 2005, 75, 1795–1797. [Google Scholar]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem.Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar]
- Addamo, M.; Augugliaro, V.; Coluccia, S.; di Paola, A.; García-López, E.; Loddo, V.; Marcì, G.; Martra, G.; Palmisano, L. The role of water in the photocatalytic degradation of acetonitrile and toluene in gas-solid and liquid-solid regimes. Int. J. Photoenergy 2006, 2006. [Google Scholar] [CrossRef]
- Micic, O.; Zhang, Y.; Cromack, K.; Trifunac, A.; Thurnauer, M. Photoinduced hole transfer from TiO2 to methanol molecules in aqueous-solution studied by electron-paramagnetic-resonance. J. Phys. Chem. 1993, 97, 13284–13288. [Google Scholar]
- Pieta, P.; Petr, A.; Kutner, W.; Dunsch, L. In situ ESR spectroscopic evidence of the spin-trapped superoxide radical, O2•−, electrochemically generated in DMSO at room temperature. Electrochim. Acta 2008, 53, 3412–3415. [Google Scholar]
- Barbieriková, Z.; Bella, M.; Kučerák, J.; Milata, V.; Jantová, S.; Dvoranová, D.; Veselá, M.; Staško, A.; Brezová, V. Photoinduced superoxide radical anion and singlet oxygen generation in the presence of novel selenadiazoloquinolones (An EPR study). Photochem. Photobiol. 2011, 87, 32–44. [Google Scholar]
- Guo, R.; Davies, C.; Nielsen, B.; Hamilton, L.; Symons, M.; Winyard, P. Reaction of the spin trap 3,5-dibromo-4-nitrosobenzene sulfonate with human biofluids. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 133–142. [Google Scholar]
- Konaka, R.; Terabe, S.; Mizuta, T.; Sakata, S. Spin trapping by use of nitrosodurene and its derivatives. Can. J. Chem. 1982, 60, 1532–1542. [Google Scholar]
- Terabe, S.; Kuruma, K.; Konaka, R. Spin trapping by use of nitroso-compounds. Part VI. Nitrosodurene and other nitrosobenzene derivatives. J. Chem. Soc. 1973, 9, 1252–1258. [Google Scholar]
- Daimon, T.; Hirakawa, T.; Nosaka, Y. Monitoring the formation and decay of singlet molecular oxygen in TiO2 photocatalytic systems and the reaction with organic molecules. Electrochemistry 2008, 76, 136–139. [Google Scholar]
- Daimon, T.; Nosaka, Y. Formation and behavior of singlet molecular oxygen in TiO2 photocatalysis studied by detection of near-infrared phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar]
- Daimon, T.; Hirakawa, T.; Kitazawa, M.; Suetake, J.; Nosaka, Y. Formation of singlet molecular oxygen associated with the formation of superoxide radicals in aqueous suspensions of TiO2 photocatalysts. Appl. Catal. A 2008, 340, 169–175. [Google Scholar]
- Nakamura, K.; Ishiyama, K.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y.; Kohno, M. Reevaluation of analytical methods for photogenerated singlet oxygen. J. Clin. Biochem. Nutr. 2011, 49, 87–95. [Google Scholar]
- Wu, H.; Song, Q.; Ran, G.; Lu, X.; Xu, B. Recent developments in the detection of singlet oxygen with molecular spectroscopic methods. TrAC Trends Anal. Chem. 2011, 30, 133–141. [Google Scholar]
- Barbieriková, Z.; Bella, M.; Sekeráková, L.; Lietava, J.; Bobeničová, M.; Dvoranová, D.; Milata, V.; Sádecká, J.; Topoľská, D.; Heizer, T.; et al. Spectroscopic characterization, photoinduced processes and cytotoxic properties of substituted N-ethyl selenadiazoloquinolones. J. Phys. Org. Chem. 2013, 26, 565–574. [Google Scholar]
- Lion, Y.; Gandin, E.; van de Vorst, A. On the production of nitroxide radicals by singlet oxygen reaction: An EPR study. Photochem. Photobiol. 1980, 31, 305–309. [Google Scholar]
- Nosaka, Y.; Natsui, H.; Sasagawa, M.; Nosaka, A. Electron spin resonance studies on the oxidation mechanism of sterically hindered cyclic amines in TiO2 photocatalytic systems. J. Phys. Chem. B 2006, 110, 12993–12999. [Google Scholar]
- Maldotti, A.; Amadelli, R.; Carassiti, V. An electron spin resonance spin trapping investigation of azide oxidation on TiO2 powder suspensions. Can. J. Chem. 1988, 66, 76–80. [Google Scholar]
- Konovalova, T.A.; Lawrence, J.; Kispert, L.D. Generation of superoxide anion and most likely singlet oxygen in irradiated TiO2 nanoparticles modified by carotenoids. J. Photochem. Photobiol. A Chem. 2004, 162, 1–8. [Google Scholar]
- Jomová, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar]
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B. 1994, 104, pp. 105–110. Available online: http://www.niehs.nih.gov/research/resources/software/tox-pharm/tools/ (accessed on 22 October 2014).
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study). Molecules 2014, 19, 17279-17304. https://doi.org/10.3390/molecules191117279
Dvoranová D, Barbieriková Z, Brezová V. Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study). Molecules. 2014; 19(11):17279-17304. https://doi.org/10.3390/molecules191117279
Chicago/Turabian StyleDvoranová, Dana, Zuzana Barbieriková, and Vlasta Brezová. 2014. "Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study)" Molecules 19, no. 11: 17279-17304. https://doi.org/10.3390/molecules191117279