Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula lutea Flowers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Determination
| Compound | Name | Structure |
|---|---|---|
| 1 | (E)-5-Ethylidenefuran-2(5H)-one-5-O-β-d-glucopyranoside |  |
| 2 | 4-Hydroxy-3-methylbut-2-enoic acid |  |
| 3 | Verbenone-5-O-β-d-glucopyranoside |  |
| 4 | 5-O-Caffeoylquinic acid |  |
| 5 | Methyl caffeate |  |
| 6 | Methyl 3,5-O-Dicaffeoylquinate |  |
| 7 | 3,5-O-Dicaffeoylquinic acid |  |
| 8 | Isorhamnetin-3-O-α-l-rhamnopyranosyl(1→6)-β-d-glucopyranoside,narcissin |  |
| 9 | (−)-Marmesin |  |
| 10 | Isoimperatorin |  |
| 11 | 2,3,6-Trimethylbenzaldehyde |  |
| Position | 1 (in CD3OD) | 2 (in DMSO-d6) | ||
|---|---|---|---|---|
| 1H | 13C | 1H | 13C | |
| 1 | - | 175.8 | - | 167.5 |
| 2 | 6.49, d (5.7) | 115.9 | 5.84, m | 113.0 |
| 3 | 8.04, d (5.7) | 155.7 | - | 158.2 |
| 4 | - | 163.2 | 3.91, br s | 65.1 |
| 5 | - | 142.2 | 1.95, d (1.5) | 15.1 |
| 6 | 2.44, s | 14.3 | ||
| 1′ | 4.85, d (7.5) | 104.0 | ||
| 2′ | 3.20-3.39, m | 74.6 | ||
| 3′ | 77.1 | |||
| 4′ | 69.7 | |||
| 5′ | 76.7 | |||
| 6′a | 3.83, dd (12; 2.4) | 61.1 | ||
| 6′b | 3.67, dd ( 12; 5.4) | |||
| OH | 5.16, s | |||
| OH (acid) | 11.86, s | |||
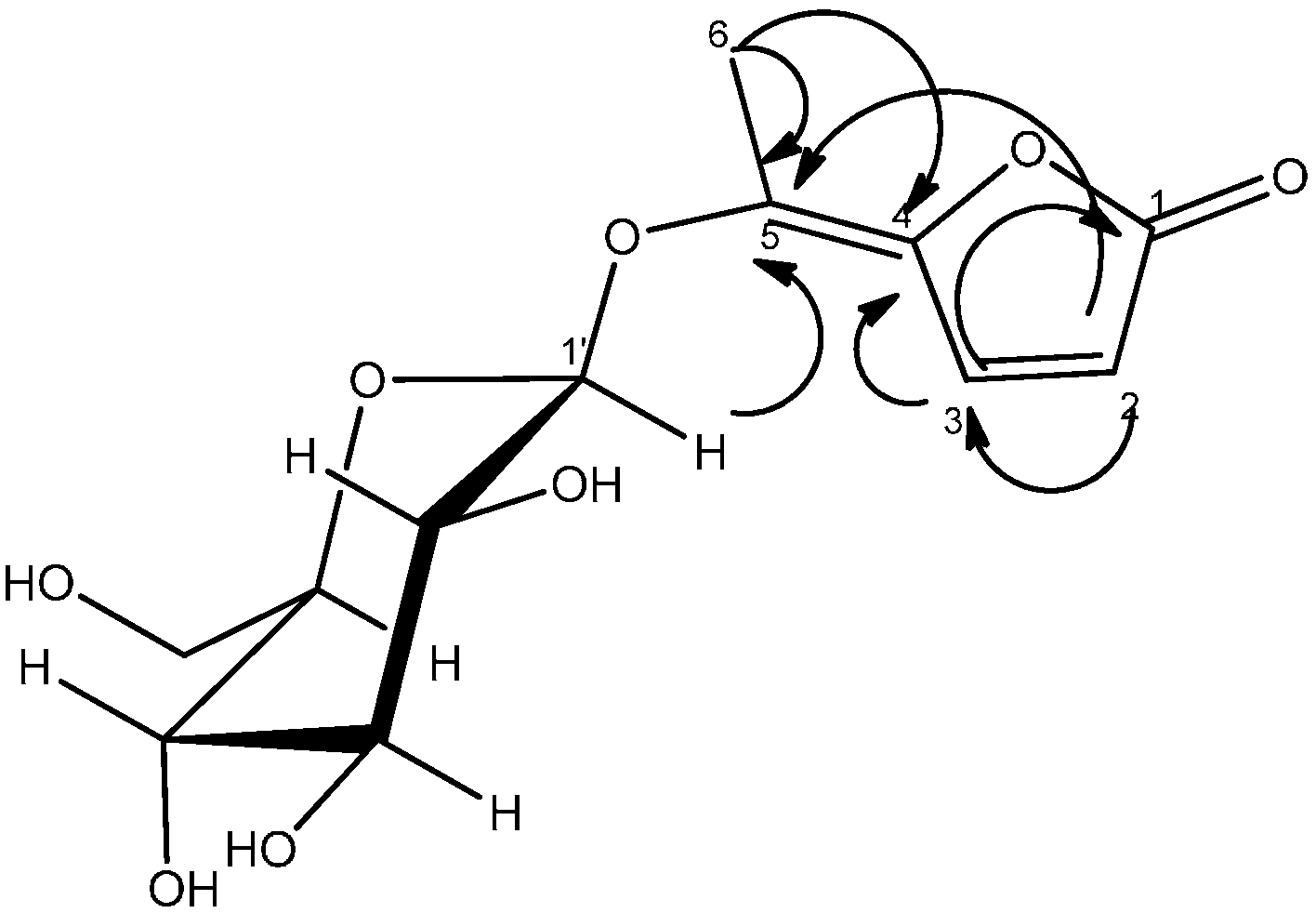
2.2. Antioxidant Activity
| Compound | IC50 (µmol/L) DPPH Assay | IC50 (µmol/L) ABTS+ Assay | 5-Lipoxygenase Inhibitory | Cytotoxic Activity (IC50 µmol/L) | |||
|---|---|---|---|---|---|---|---|
| (% at 80 µmol/L) | IC50 (µmol/L) | HCT-116 | IGROV-1 | OVCAR-3 | |||
| 1 | nt | nt | 16.9 ± 1.2 | >100 | >100 | >100 | |
| 2 | nt | nt | 0 | >100 | >100 | >100 | |
| 3 | nt | nt | 12.4 ± 0.8 | >100 | >100 | >100 | |
| 4 | 127.4 ± 1.2 | 132.2 ± 1.5 | 17.1 ± 1.1 | >100 | >100 | >100 | |
| 5 | 39.2 ± 0.5 | 40.8 ± 0.4 | 0 | 22.5 ± 2.4 | 17.8 ± 1.1 | 25 ± 1.1 | |
| 6 | 26.6 ± 1.3 | 20.0 ± 0.3 | 5.28 ± 0.1 | >100 | >100 | >100 | |
| 7 | 18.0 ± 0.5 | 19.7 ± 0.7 | 10 ± 0.2 | >100 | >100 | >100 | |
| 8 | >300 | >300 | 0 | >100 | >100 | >100 | |
| 9 | nt | nt | 0 | 50.0 ± 4.9 | >100 | >100 | |
| 10 | nt | nt | 0 | >100 | >100 | >100 | |
| 11 | nt | nt | 0 | >100 | >100 | >100 | |
| Vitamin C | 25.0 ± 1.1 | 23.3 ± 0.1 | - | ||||
| Doxorubicin | 0.18 ± 0.01 | ||||||
| Tamoxifen | 2.0 ± 0.1 | 1.3 ± 0.1 | |||||
| NDGA | - | - | 98.0 ± 0.1 | 6.2 ± 0.5 | |||
2.3. 5-Lipoxygenase Inhibitory
2.4. Cytotoxicity Evaluation
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection of Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic and Physical Data of the Isolated Compounds
3.5. Biological Activity
3.5.1. Free Radical Scavenging Activity DPPH Test
3.5.2. ABTS Radical-Scavenging Test
3.5.3. 5-Lipoxygenase Inhibitory
3.5.4. Cytotoxicity Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kibagendi Osoro, E.; Zhi Hea, Y.; Ndagijimanab, A.; Sivoko Imbenzi, P. A review on phenolic compounds in Illicium species and their pharmacological effects. Pelagia Res. Libr. 2013, 4, 17–30. [Google Scholar]
- Baxter, H.; Harborne, J.B.; Moss, G.P. (Eds.) Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants, 2nd ed.; Taylor & Francis: London, UK, 1999.
- Downie, S.R.; Watson, M.F.; Spalik, K.; Katz-Downie, D.S. Molecular systematics of Old World 2. Apioideae (Apiaceae): Relationships among some members of tribe Peucedaneaesensulato, the placement of several islandendemic species, and resolution within the apioidsuperclade. Can. J. Bot. 2000, 78, 506–528. [Google Scholar]
- Alapetite, G.P. Flora of Tunisia, Angiospermes-Dicotyledones, Apetales-Dialypetales; Apetales-Dialypetales: Tunis, Tunisia, 1979; pp. 607–609. [Google Scholar]
- Gárdenas-Rodríguez, N.; González-Trujano, M.E.; Aguirre-Hernández, E.; Ruíz-García, M.; Sampieri, A., III; Coballase-Urrutia, E.; Garmona-Aparicio, L. Anticonvulsant and Antioxidant Effects of Tilia americana var. mexicana and Flavonoids Constituents in the Pentylenetetrazole-Induced Seizures. Oxid. Med. Cell. Longev. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Ben Salem, S.; Jabrane, A.; Harzallah-Skhiri, F.; Ben Jannet, H. New bioactive dihydrofuranocoumarins from the roots of the Tunisian Ferula lutea (Poir.) Maire. Bioorg. Med. Chem. Lett. 2013, 23, 4248–4252. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Razek, M.H.; Ohta, S.; Hirata, T. Terpenoid coumarins from the genus Ferula. Heterocycles 2003, 60, 689–716. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E.; Loi, M.; Sollai, F.; Ballero, M.; Pintus, M.; Rescigno, A. Isolation and characterization of polyphenol oxidase from Sardinian poisonous and non-poisonous chemotypes of Ferula communis (L.). Phytochemistry 2013, 90, 16–24. [Google Scholar] [CrossRef]
- Ahmed, A.A. Sesquiterpene coumarins and sesquiterpenes from Ferula sinaica. Phytochemistry 1999, 50, 109–112. [Google Scholar] [CrossRef]
- Iranshahi, M.; Kalategi, F.; Rezaee, R.; Shahverdi, A.R.; Ito, C.; Furukawa, H.; Tokuda, H.; Itoigawa, M. Cancer Chemopreventive Activity of Terpenoid Coumarins from Ferula Species. Planta Med. 2008, 74, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Teranishi, R.; Kawazoe, K.; Takaishi, Y.; Honda, G.; Itoh, M.; Takeda, Y.; Kodzhimatov, O.K. Sesquiterpenoids from Ferula kuhistanica. Phytochemistry 2000, 54, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Jabrane, A.; Ben Jannet, H.; Mighri, Z.; Mirjoletd, J.F.; Duchamp, O.; Harzallah-Skhiri, F.; Lacaille-Dubois, M.A. Two New Sesquiterpene Derivatives from the Tunisian Endemic Ferula tunetana Pom. Chem. Biodivers. 2010, 7, 392–399. [Google Scholar] [CrossRef]
- Iranshahi, M.; Amanolahi, F.; Schneider, B. New sesquiterpene coumarin from the roots of Ferula latisecta. Avicenna J. Phytomed. 2012, 2, 133–138. [Google Scholar] [PubMed]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative Constituents from Umbelliferae Plants. V. A New Furanocoumarin and Falcarindiol Furanocoumarin Ethers from the Root of Angelica japonica. Chem. Pharm. Bull. 1999, 47, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Structure Elucidation by NMR in Organic Chemistry a Practicle Guide, 2nd ed.; John Wiley and Sons Ltd.: West Sussex, UK, 1995. [Google Scholar]
- Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O. Terpenoids and γ-pyrone derivatives from Prangos tschimganica. Phytochemistry 2001, 57, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, C.; Wang, Z. Water-soluble chemical constituents of Angelica sinensis (Oliv.) Diels. Chin. Pharmaceut. J. 2010, 45, 101–103. [Google Scholar]
- Lin, Y.L.; Wang, W.Y.; Kuo, Y.H.; Chen, C.F. Nonsteroidal Constituents from Solanum incanum L. J. Chin. Chem. Soc. 2000, 47, 247–251. [Google Scholar]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Huang, C.; Wang, M. Chemical composition, nutritional value, and antioxidant constituents of Kalopanax pictus leaves. Food Chem. 2012, 131, 449–455. [Google Scholar] [CrossRef]
- Taira, J.; Uehara, M.; Tsuchida, E.; Ohmine, W. Inhibition of the β-Catenin/Tcf Signaling by Caffeoylquinic Acids in Sweet Potato Leaf through down Regulation of the Tcf-4 Transcription. J. Agric. Food Chem. 2014, 67, 167–172. [Google Scholar] [CrossRef]
- Saerom, P.; Seokwon, Y.; Dalrea, A.; Jae, H.Y.; Dae, K.K. Antioxidative Phenolic Compounds from the Whole Plant of Juncus diastrophanthus. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 685–692. [Google Scholar] [CrossRef]
- Kouassi, A.; Ioset, M.; Ransijn, J.R.; Mauel, A.; Mavi, J.; Kurt, S.H. Antileishmanial and antifungal acridone derivatives from the roots of Thamnosma rhodesica. Phytochemistry 2004, 65, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyuki, K.; Maho, S.; Masahiro, S.; Masahiko, T.; Kimiye, B. In vitro and In vivo antiproliferative effect of a combination of ultraviolet-A and alkoxy furocoumarins Isolated from Umbelliferae Medicinal Plants, in Melanoma Cells. Photochem. Photobiol. 2013, 89, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Znati, M.; Jabrane, A.; Hajlaoui, H.; Bouajila, J.; Casanova, J.; Ben jannet, H. Chemical Composition and in vitro Evaluation of Antimicrobial and Antiacetylcholinesterase of the Flower Oil of Ferula lutea L. Nat. Prod. Comm. 2012, 7, 947–950. [Google Scholar]
- Li, W.N.; Xiao, Y.; Chen, Y.; Liu, Y.H. Comparative study on the anti-oxidative ability of chlorogenic acid extract in leaves of Eucommia ulmoides, chlorogenic acid and vitamin C. Sci. Technol. Food Ind. 2012, 33, 137–140. [Google Scholar]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Kyung, S.S.D.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J.H.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Viola, G.; Piacente, S.; Mariella, C.E.; Innocenti, G. Cytotoxic Constituents of Roots of Chaerophyllum hirsutum. J. Nat. Prod. 2004, 67, 1588–1590. [Google Scholar] [CrossRef]
- Ren-Bo, A.; Dong-Hwan, S.; Gil-Saeng, J.; Youn-Chul, K. In Vitro hepatoprotective compounds from Suaeda glauca. Arch. Pharm. Res. 2008, 31, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, J.; Ben Sghaier, M.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Isorhamnetin 3-O-robinobioside from Nitraria retusa leaves enhance antioxidant and antigenotoxic activity in human chronic myelogenous leukemia cell line K562. BMC Complement. Altern. Med. 2012, 12, 135–143. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013, 55, 202–208. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Znati, M.; Ben Jannet, H.; Cazaux, S.; Souchard, J.P.; Harzallah Skhiri, F.; Bouajila, J. Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula lutea Flowers. Molecules 2014, 19, 16959-16975. https://doi.org/10.3390/molecules191016959
Znati M, Ben Jannet H, Cazaux S, Souchard JP, Harzallah Skhiri F, Bouajila J. Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula lutea Flowers. Molecules. 2014; 19(10):16959-16975. https://doi.org/10.3390/molecules191016959
Chicago/Turabian StyleZnati, Mansour, Hichem Ben Jannet, Sylvie Cazaux, Jean Pierre Souchard, Féthia Harzallah Skhiri, and Jalloul Bouajila. 2014. "Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula lutea Flowers" Molecules 19, no. 10: 16959-16975. https://doi.org/10.3390/molecules191016959




