Anti-Inflammatory Potential of Newly Synthesized 4-[(Butylsulfinyl)methyl]-1,2-benzenediol in Lipopolysaccharide-Stimulated BV2 Microglia
Abstract
:1. Introduction
2. Results and Discussion
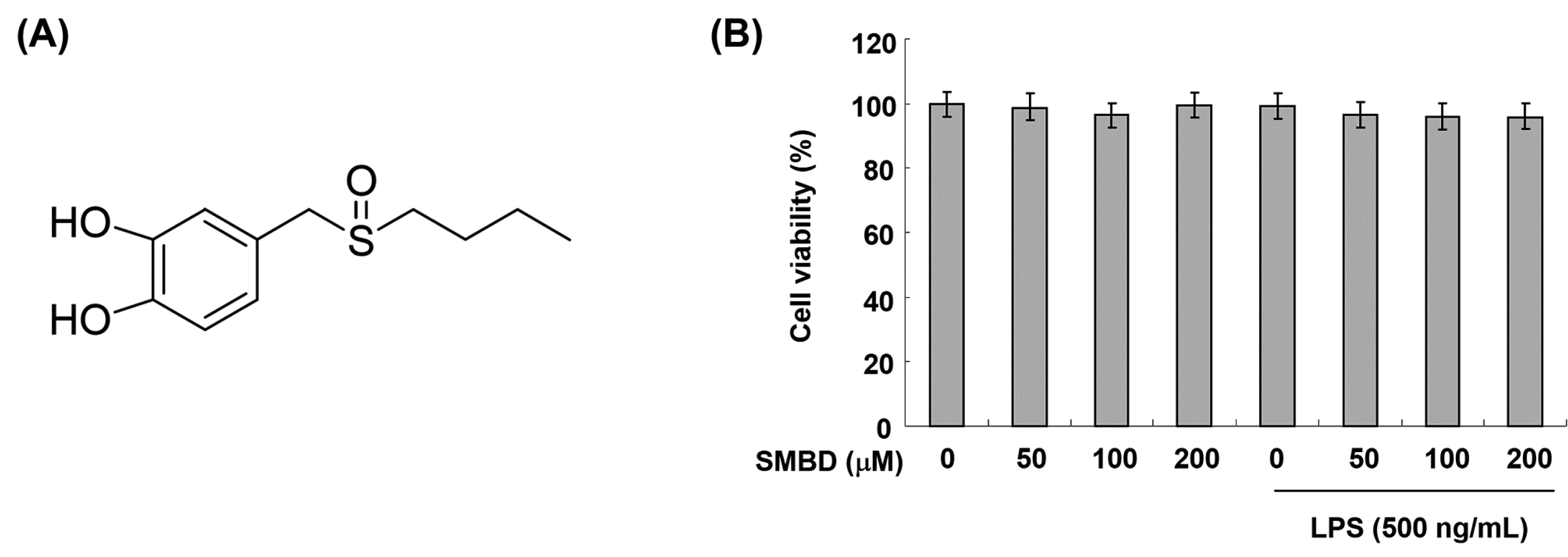
2.1. Effects of SMBD on the BV2 Cell Viability
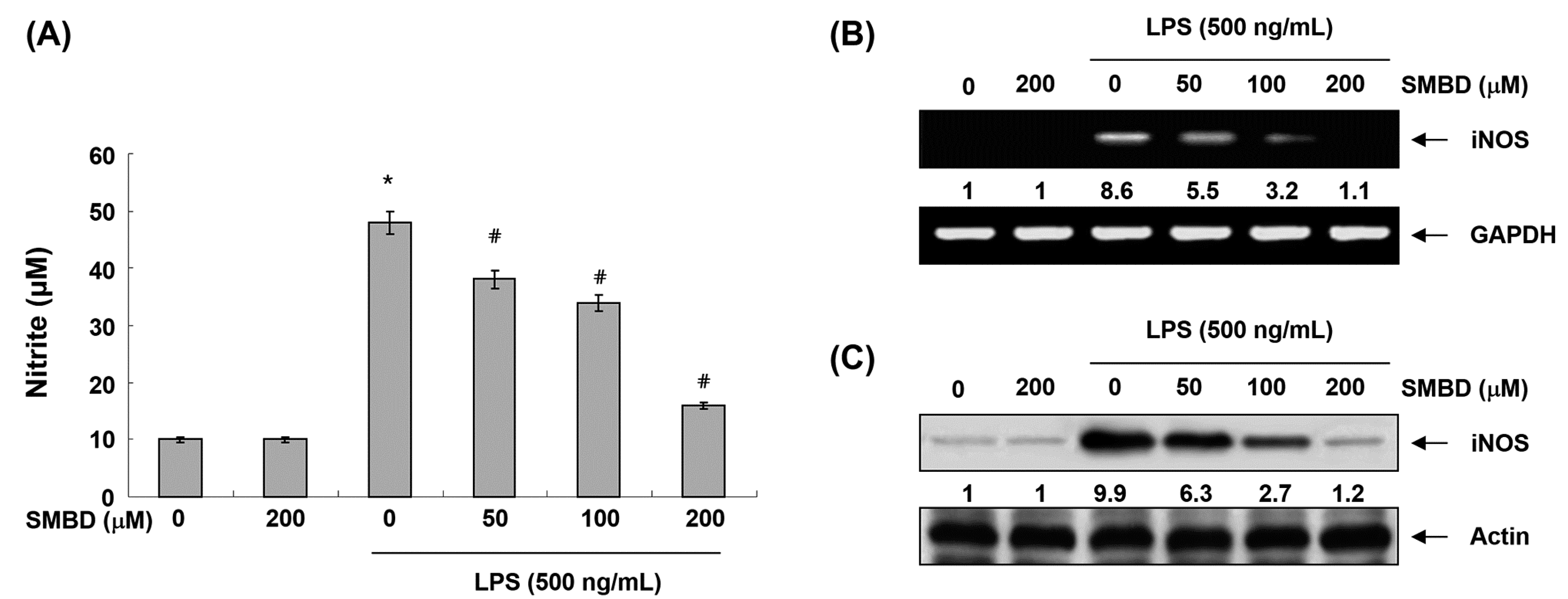
2.2. SMBD Inhibits LPS-Induced NO Production and iNOS Expression in BV2 Cells
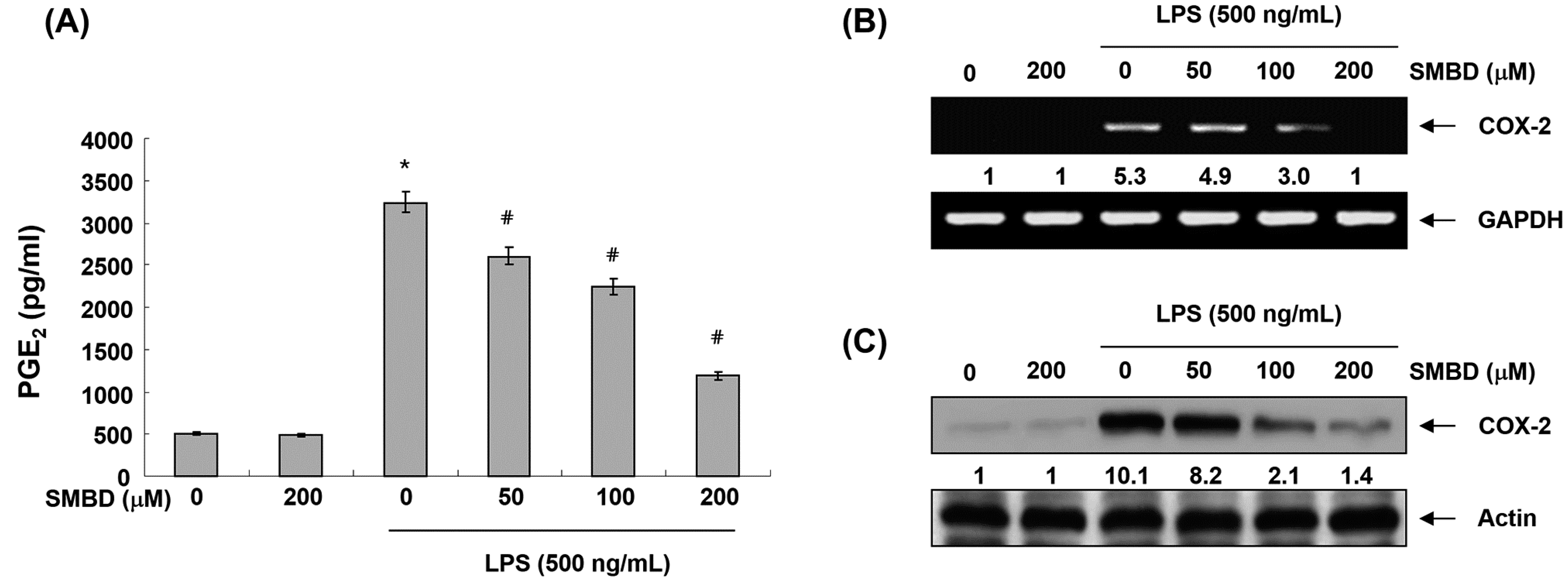
2.3. SMBD Inhibits LPS-Induced PGE2 Production and COX-2 Expression in BV2 Cells
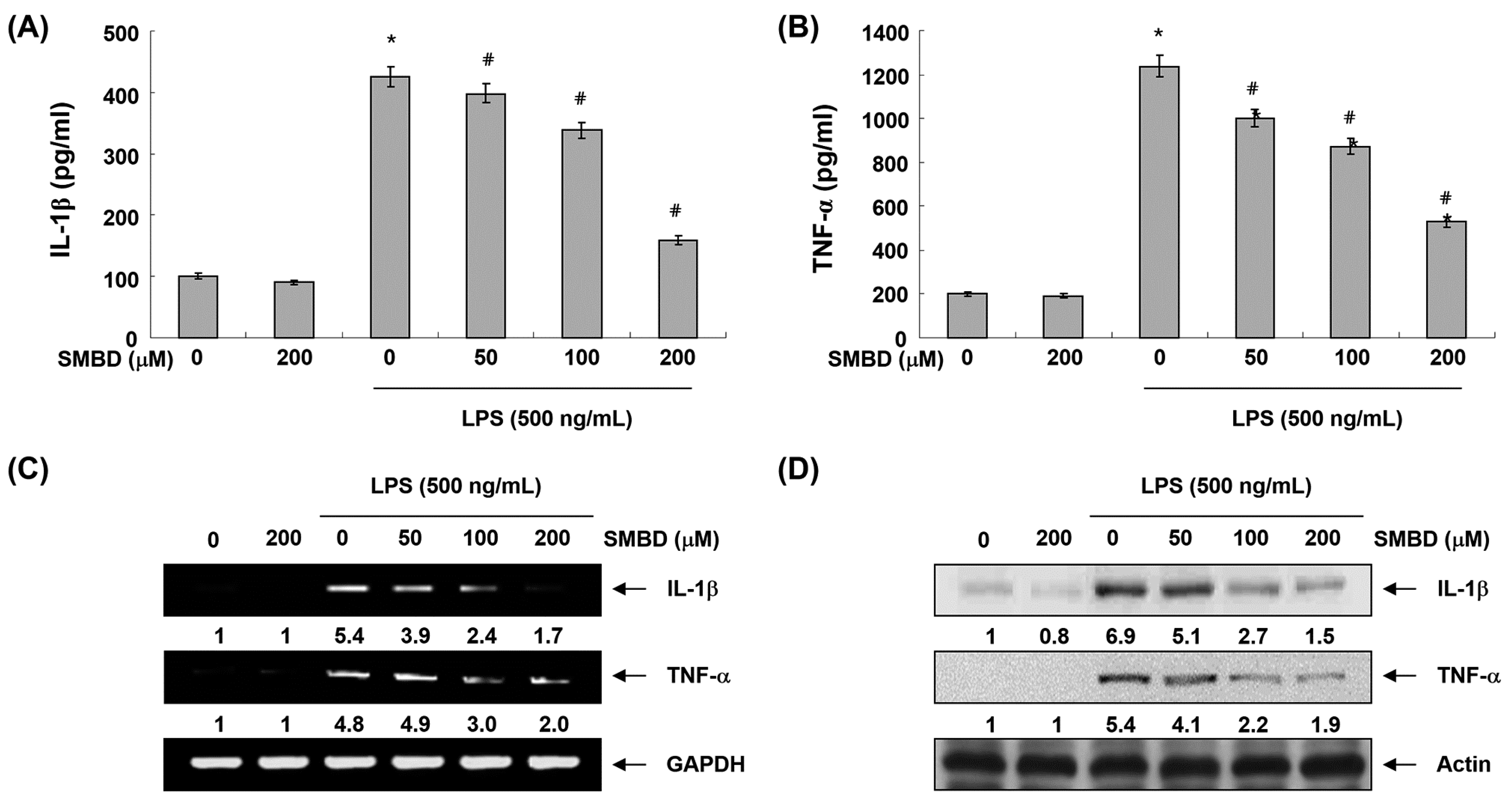
2.4. SMBD Reduces LPS-Induced Production and Expression of Pro-Inflammatory Cytokines in BV2 Cells
2.5. SMBD Prevents the LPS-Induced Nuclear Translocation of NF-κB and Degradation of IκB-α in BV2 Cells
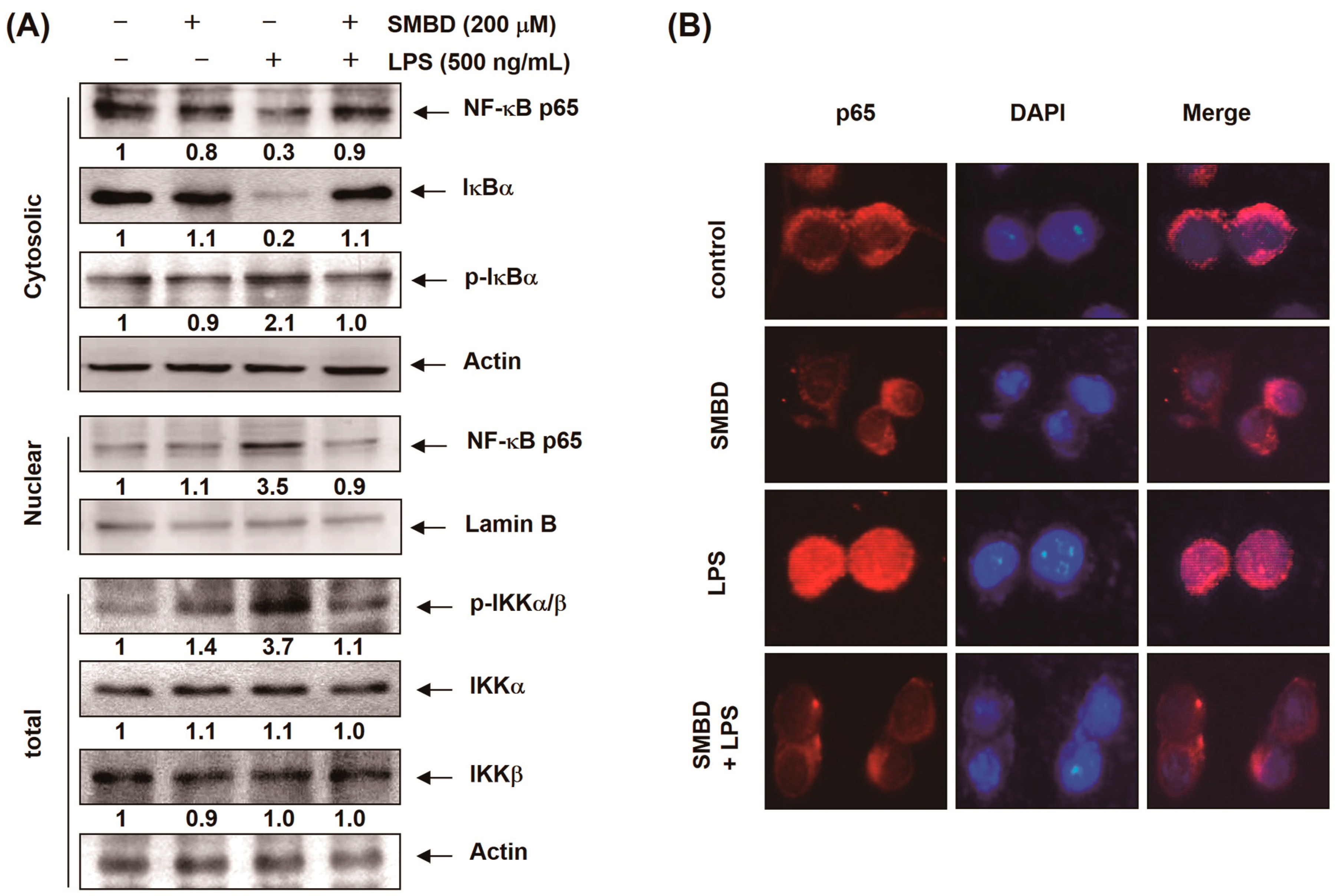
2.6. SMBD Reduces the LPS-Induced Activation of MAPKs and PI3K/Akt in BV2 Cells
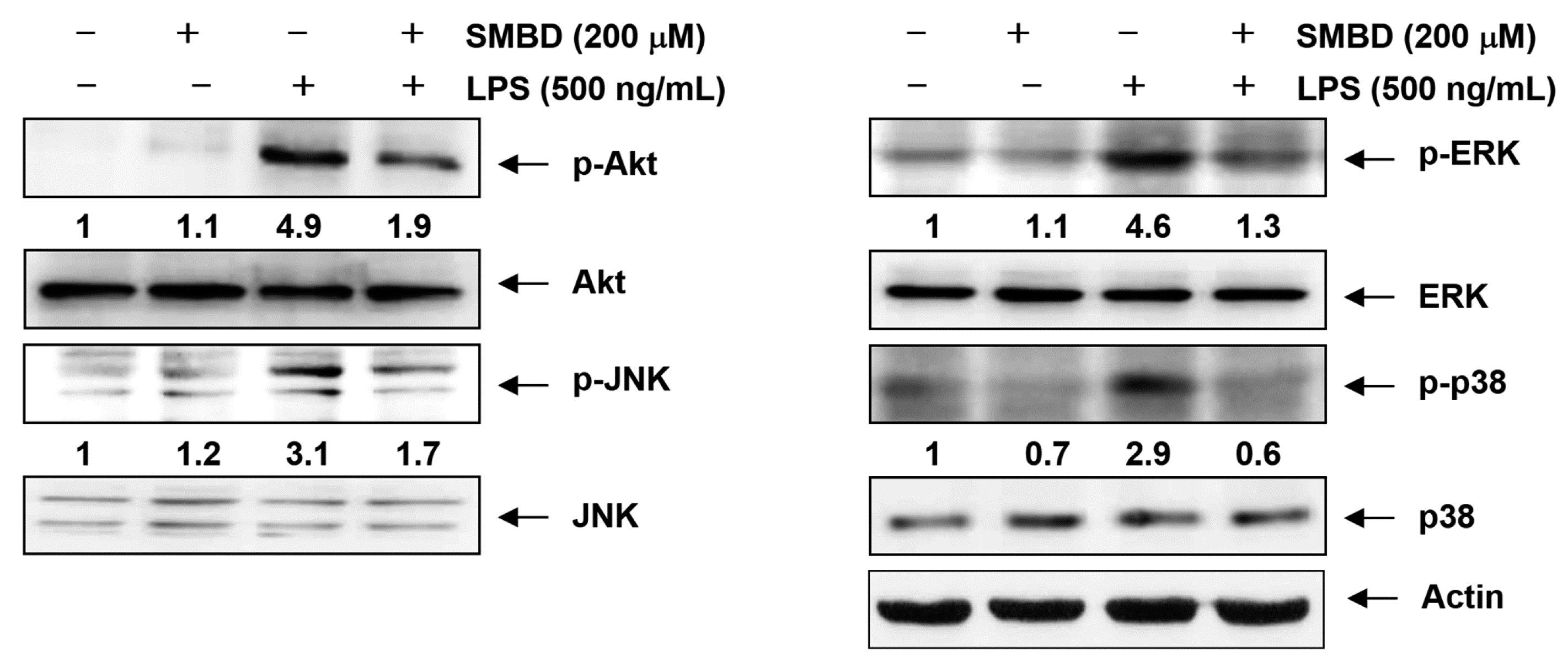
2.7. Discussion
3. Experimental Section
3.1. Cell Culture and SMBD Treatment
3.2. Cell Viability Assay
3.3. Measurement of NO Concentration
3.4. Determination of PGE2, TNF-α, and IL-1β Production
3.5. RNA Isolation and RT-PCR
3.6. Protein Extraction and Western Blot Analysis
| Antibody | Dilution | Product No. | Species of Origin and Supplier |
|---|---|---|---|
| iNOS | 0.3888889 | SC-509 | rabbit polyclonal, BD Transduction Laboratories |
| COX-2 | 0.7361111 | 160126 | rabbit polyclonal, Cayman Chemical |
| IL-1β | 0.3888889 | SC-7884 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| TNF-α | 0.3888889 | #3707 | rabbit polyclonal, Cell Signaling Technology, Inc. |
| NF-κB p65 | 0.3888889 | SC-109 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| IκB-α | 0.3888889 | SC-847 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| Lamin B | 1.4305556 | SC-6216 | goat polyclonal, Santa Cruz Biotechnology, Inc. |
| p-IKKα/β | 0.3888889 | SC-23470(R) | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| IKK-α | 0.7361111 | SC-7606 | mouse monoclonal, Santa Cruz Biotechnology, Inc. |
| IKK-β | 0.3888889 | SC-34673 | goat polyclonal, Santa Cruz Biotechnology, Inc. |
| Akt | 0.3888889 | SC-8312 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| p-Akt | 0.3888889 | SC-101629 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| JNK | 0.7361111 | #9252S | rabbit polyclonal, Cell Signaling Technology, Inc. |
| p-JNK | 0.3888889 | #9255 | mouse monoclonal, Cell Signaling Technology, Inc. |
| ERK | 1:1,000 | SC-154 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| p-ERK | 0.3888889 | #9106S | mouse monoclonal, Cell Signaling Technology, Inc. |
| p38 | 1:1,000 | SC-535 | rabbit polyclonal, Santa Cruz Biotechnology, Inc. |
| p-p38 | 0.3888889 | #9211S | rabbit polyclonal, Cell Signaling Technology, Inc. |
| Actin | 1:1,000 | SC-1615 | goat polyclonal, Santa Cruz Biotechnology, Inc. |
3.7. Immunofluorescence Staining for NF-κB p65
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lind, L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis 2003, 169, 203–214. [Google Scholar]
- Bertolini, A.; Ottani, A.; Sandrini, M. Dual acting anti-inflammatory drugs: A reappraisal. Pharmacol. Res. 2001, 44, 437–450. [Google Scholar]
- Napoli, I.; Neumann, H. Microglial clearance function in health and disease. Neuroscience 2009, 158, 1030–1038. [Google Scholar]
- Loane, D.J.; Byrnes, K.R. Role of microglia in neurotrauma. Neurotherapeutics 2010, 7, 366–377. [Google Scholar]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar]
- Dandona, P.; Chaudhuri, A.; Dhindsa, S. Proinflammatory and prothrombotic effects of hypoglycemia. Diabetes Care 2010, 33, 1686–1687. [Google Scholar]
- Salvemini, D.; Marino, M.H. Inducible nitric oxide synthase and inflammation. Expert. Opin. Investig. Drugs 1998, 7, 65–75. [Google Scholar]
- Harris, R.E.; Beebe-Donk, J.; Schuller, H.M. Chemoprevention of lung cancer by non-steroidal anti-inflammatory drugs among cigarette smokers. Oncol. Rep. 2002, 9, 693–695. [Google Scholar]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar]
- Morris, P.E.; Olmstead, L.E.; Howard-Carroll, A.E.; Dickens, G.R.; Goltz, M.L.; Courtney-Shapiro, C.; Fanti, P. In vitro and in vivo effects of HS-1605 on murine alveolar macrophage TNF alpha production. Inflammation 1999, 23, 231–239. [Google Scholar]
- Aggarwal, B.B.; Natarajan, K. Tumor necrosis factors: Developments during the last decade. Eur. Cytokine Netw. 1996, 7, 93–124. [Google Scholar]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar]
- Pasare, C.; Medzhitov, R. Toll-like receptors: Linking innate and adaptive immunity. Microbes Infect. 2004, 6, 1382–1387. [Google Scholar]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar]
- Anwar, M.A.; Basith, S.; Choi, S. Negative regulatory approaches to the attenuation of Toll-like receptor signaling. Exp. Mol. Med. 2013, 45. [Google Scholar] [CrossRef]
- Lehnardt, S.; Lachance, C.; Patrizi, S.; Lefebvre, S.; Follett, P.L.; Jensen, F.E.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002, 22, 2478–2486. [Google Scholar]
- Moreillon, P.; Majcherczyk, P.A. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 2003, 35, 632–641. [Google Scholar]
- Marshall, J.C. Lipopolysaccharide: An endotoxin or an exogenous hormone? Clin. Infect. Dis. 2005, 41, S470–S480. [Google Scholar]
- Ishii, Y.; Okamura, T.; Inoue, T.; Tasaki, M.; Umemura, T.; Nishikawa, A. Dietary catechol causes increased oxidative DNA damage in the livers of mice treated with acetaminophen. Toxicology 2009, 263, 93–99. [Google Scholar]
- Sarkar, D.; Saha, P.; Gamre, S.; Bhattacharjee, S.; Hariharan, C.; Ganguly, S.; Sen, R.; Mandal, G.; Chattopadhyay, S.; Majumdar, S.; et al. Anti-inflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-kappaB pathway. Int. Immunopharmacol. 2008, 8, 1264–1271. [Google Scholar]
- Majtan, J.; Majtan, V. Dimethyl sulfoxide attenuates TNF-α-induced production of MMP-9 in human keratinocytes. J. Toxicol. Environ. Health A 2011, 74, 1319–1322. [Google Scholar]
- Xing, L.; Remick, D.G. Mechanisms of dimethyl sulfoxide augmentation of IL-1 beta production. J. Immunol. 2005, 174, 6195–6202. [Google Scholar]
- Ramoutar, R.R.; Brumaghim, J.L. Antioxidant and anticancer properties and mechanisms of inorganic selenium, oxo-sulfur, and oxo-selenium compounds. Cell Biochem. Biophys. 2010, 58, 1–23. [Google Scholar]
- Kim, H.J.; Noh, J.S.; Kwon, M.J.; Kwon, M.J.; Song, S.; Suh, H.; Kim, M.J.; Song, Y.O. Lipid lowering and antioxidant effects of newly synthesized 4-[(Butylsulfinyl)methyl]-1,2-benzenediol (SMBD) in diet-induced hypercholesterolemic rabbits. Bull. Korean Chem. Soc. 2010, 31, 3327–333. [Google Scholar]
- Uto, T.; Fujii, M.; Hou, D.X. 6-(Methylsulfinyl)hexyl isothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem. Pharmacol. 2005, 70, 1211–1221. [Google Scholar]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.; Baldwin, A.S. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar]
- Kanno, S.; Shouji, A.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Ujibe, M.; Obara, Y.; Nakahata, N.; Ishikawa, M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006, 78, 673–681. [Google Scholar]
- Lin, W.J.; Yeh, W.C. Implication of Toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock 2005, 24, 206–209. [Google Scholar]
- Blatteis, C.M.; Li, S.; Li, Z.; Perlik, V.; Feleder, C. Signaling the brain in systemic inflammation: The role of complement. Front. Biosci. 2004, 9, 915–931. [Google Scholar]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar]
- Park, C.M.; Song, Y.S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, G.-H.; Choi, I.-W.; Jeong, J.-W.; Kim, G.-Y.; Kim, J.; Suh, H.; Ryu, C.-H.; Kim, W.-J.; Choi, Y.H. Anti-Inflammatory Potential of Newly Synthesized 4-[(Butylsulfinyl)methyl]-1,2-benzenediol in Lipopolysaccharide-Stimulated BV2 Microglia. Molecules 2014, 19, 16609-16623. https://doi.org/10.3390/molecules191016609
Jo G-H, Choi I-W, Jeong J-W, Kim G-Y, Kim J, Suh H, Ryu C-H, Kim W-J, Choi YH. Anti-Inflammatory Potential of Newly Synthesized 4-[(Butylsulfinyl)methyl]-1,2-benzenediol in Lipopolysaccharide-Stimulated BV2 Microglia. Molecules. 2014; 19(10):16609-16623. https://doi.org/10.3390/molecules191016609
Chicago/Turabian StyleJo, Guk-Heui, Il-Whan Choi, Jin-Woo Jeong, Gi-Young Kim, Jinwoo Kim, Hongsuk Suh, Chung-Ho Ryu, Wun-Jae Kim, and Yung Hyun Choi. 2014. "Anti-Inflammatory Potential of Newly Synthesized 4-[(Butylsulfinyl)methyl]-1,2-benzenediol in Lipopolysaccharide-Stimulated BV2 Microglia" Molecules 19, no. 10: 16609-16623. https://doi.org/10.3390/molecules191016609





