1. Introduction
Heterogeneous photocatalytic oxidation (HPO) based on TiO
2 as photocatalyst and solar light has emerged as a promising route for the degradation of persistent organic pollutants [
1,
2,
3]. During photocatalytic oxidation, illumination of TiO
2 with light energy larger than the band gap generates conduction band electrons (e
−cb) and valence band holes (h
+vb), which are the initial “reactive reagents” of TiO
2 photocatalysis. The h
+vb and e
−cb can react with the H
2O solvent and the dissolved molecular oxygen, respectively, to produce various reactive oxygen species, such as hydroxyl radicals (∙OH), hydrogen peroxide (H
2O
2), superoxide radicals (O
2∙
−) or hydroperoxyl radicals (∙OOH) [
4,
5]. However, the roles of H
2O and O
2, and the details of their photocatalytic reaction pathways are still elusive. Generally, the degradation reaction of organic pollutants is believed to be initiated by the ∙OH radical, which is formed through the oxidation of H
2O by h
+vb and can oxidize almost all organic compounds [
6]. O
2, the final oxidant of the whole photocatalytic oxidation process was exclusively considered as a scavenger of e
−cb to depress the recombination of photogenerated h
+vb/e
−cb pairs and regenerate the photocatalyst. According to this mechanism, the trapping of the e
−cb by O
2 will only determine the rate of the photocatalytic reaction, but not change the reaction pathway and mechanism. However, recent studies have indicated that the participation of O
2 in the photocatalytic reaction would greatly influence the degradation product distribution. For example, in the photoelectrochemical degradation of 4-chlorophenol, when the e
−cb is removed by using an appropriate bias, instead of by dioxygen [
7], the mineralization of 4-chlorophenol cannot occur any more. This observation suggests that molecular oxygen may play an important role in the degradation mechanism such as the opening of aromatic rings and the subsequent mineralization, and not just act as an electron acceptor. Nevertheless, detailed mechanisms for the roles of molecular oxygen in photocatalysis are not fully understood so far.
One important reason that hinders the understanding of the mechanisms is the complexity of the photocatalytic process. In the photocatalytic reaction, the h
+vb-induced oxidation half reaction and the e
−cb-induced reduction half reaction proceed on the surface of one photocatalyst particle (usually of a nano size) at the same time, which makes it difficult to distinguish them in space and time. Moreover, the photocatalytic reaction involves a series of active free radical species and processes. It is challenging to investigate these species and processes with steady-state techniques. The isotopic labeling method is one of the most powerful techniques to unravel complicated reaction mechanisms [
8]. Stable isotope marking, especially by
13C/
12C, H/D (D =
2H) and
18O/
16O, is a versatile analytical tool across many realms of science [
9]. In the TiO
2 photocatalytic system, the main reaction components O
2, H
2O and TiO
2 all contain oxygen atoms. Accordingly, oxygen atom isotopic labeling can be the most direct and reliable method to trace the O-atom origin of products and distinguish the role and pathways of these components in the different photocatalytic reactions. Another advantage of oxygen isotopic labeling technique is its flexibility,
i.e., each component such as
18O-labeled
18O
2 [
10,
11,
12,
13,
14], H
218O [
15,
16,
17], Ti
18O
2 [
18,
19,
20,
21,
22,
23] and
18O-labeled substrate [
24,
25,
26] can be labeled.
On the TiO
2 surface, the
18O-labeled method has been frequently used in oxygen isotopic exchange measurements to study the stability of surface oxygen in thermally activated catalytic reactions [
27,
28]. More often, this method was employed in gas phase TiO
2 photocatalytic systems to investigate the photoinduced oxygen isotopic exchange with the aim of understanding the evolution of the intermediate species on the TiO
2 surface [
29,
30,
31,
32,
33,
34,
35,
36]. However, the photoinduced oxygen isotopic exchange and oxygen isotopic exchange on TiO
2 surface hinder the application of the isotopic labeling method in aerated aqueous TiO
2 photocatalysis systems, because the isotope scrambling among reaction components can make the assignment of the origin of the intermediates and products uncertain. On the other hand, many researchers have also reported that, in the gas phase, the adsorption of water and organic species would inhibit the progress of the photoinduced oxygen isotopic exchange [
37,
38,
39,
40]. All this suggests that, by deliberate selection of the appropriate photocatalytic systems and conditions, the photoinduced oxygen isotopic exchange can be largely avoided, even if the reaction is carried out in aerated aqueous solutions. In fact, the oxygen isotopic exchange and photoinduced oxygen isotopic exchange between the O
2 and TiO
2 or between O
2 and H
2O were shown to be rather slow in the aqueous TiO
2 photocatalysis systems, compared to the photocatalytic oxidation reaction in the gas phase [
41,
42,
43,
44,
45]. Accordingly, in many situations,
18O-labeled methods can be employed to trace the pathway of oxygen-involved reaction in aqueous photocatalytic systems.
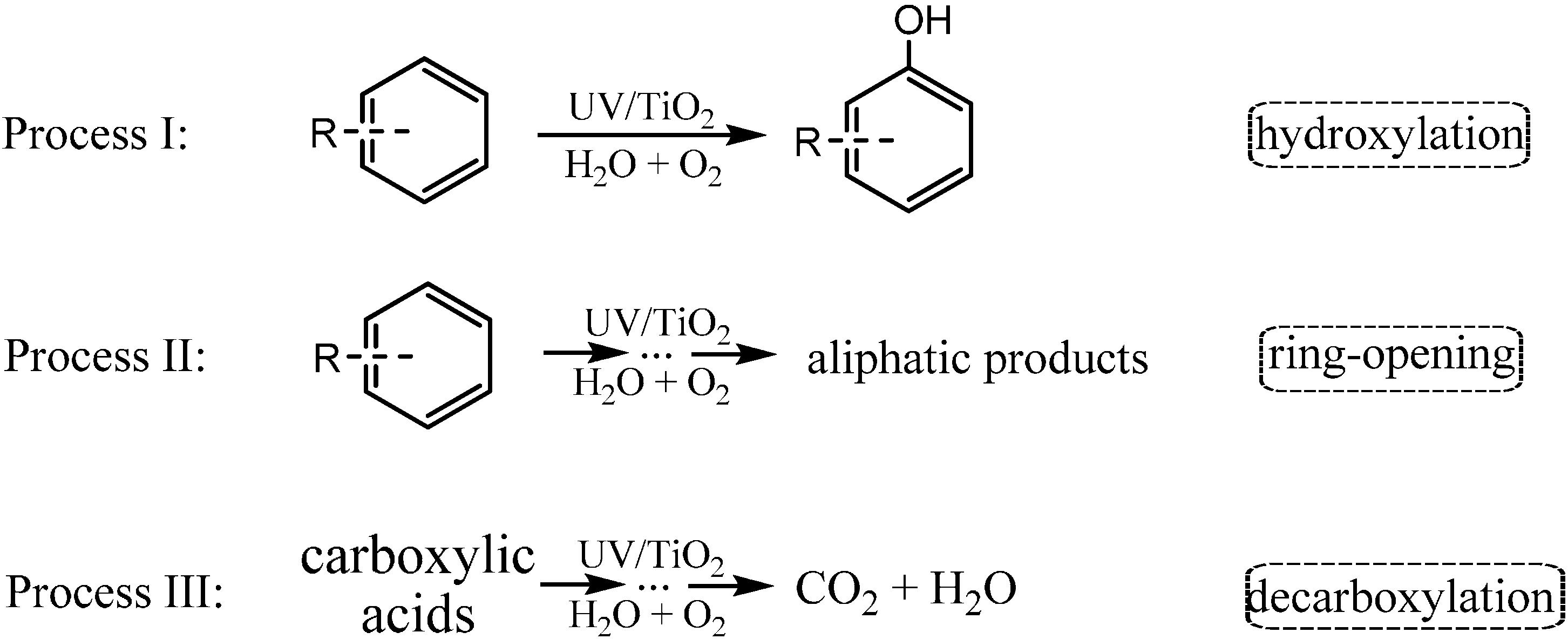
Aromatic rings are basic constituents of many kinds of organic pollutants, such as dyes, explosives, pesticides and pharmaceuticals. The release of these compounds could greatly affect the environment and human health [
46,
47]. Accordingly, aromatic compounds are the most frequently used model substrates to investigate photocatalytic mechanisms and to test the activity of photocatalysts. Before the complete mineralization of the aromatic compounds into CO
2 and H
2O, the photocatalytic degradation would proceed through many main intermediates with different functional groups. For example, hydroxylation, in which the hydrogen on the aromatics is replaced by the electron-donating hydroxyl (
Scheme 1 process I), is regarded as an important process in the degradation of aromatic contaminants, especially at the beginning stage of the reaction [
6,
48,
49,
50,
51]. On the way of mineralization, the cleavage of the aromatic ring, sometimes the hydroxylated one, to aliphatic compounds represents another critical process (
Scheme 1 process II). The most stable intermediates after cleavage of aromatic ring should be the aliphatic carboxylates. The oxidative decarboxylation of these intermediates would lead to the formation of CO
2 and H
2O (
Scheme 1 process III). Accordingly, this review is organized along these three processes: (1) hydroxylation of aromatics; (2) oxidative cleavage of aromatic rings; and (3) decarboxylation of saturated carboxylic acids, and tries to shed light on the application of
18O-labeling methods to the study of photocatalytic mechanisms. We will first introduce how to distinguish the O-source and the investigation of the O
2-incorporation mechanism in the photocatalytic hydroxylation of aromatics by
18O-labeling methods [
41,
52]. The application of
18O-labeling methods in the study of the TiO
2-photocatalytic aryl ring-opening mechanism is illustrated by photocatalytic degradation of 3,5-di-
tert-butylcatechol (DTBC) in aqueous solution [
53]. Finally, we will focus on
18O-labeling studies of the decarboxylation pathways of saturated mono- [
44] and dicarboxylic acids [
43]. These studies revealed that molecular oxygen can incorporate into the products to a different extent during all three of these processes, which means that O
2 is not just a conduction band electron scavenger, but also plays a crucial role in the degradation and mineralization of organic pollutants. Moreover, we also give a detailed picture of how molecular oxygen incorporates into the products. These studies also demonstrate that
18O-isotope labeling is a very reliable and powerful method to study aspects of the mechanism of photocataytic oxidations, such as the role and reaction pathway of molecular oxygen and the solvent (water) in the photocatalytic reaction.
Scheme 1.
The three main processes of the TiO2 photocatalytic oxidation of aromatic compounds.
Scheme 1.
The three main processes of the TiO2 photocatalytic oxidation of aromatic compounds.
2. TiO2 Photocatalytic Hydroxylation of Aromatics
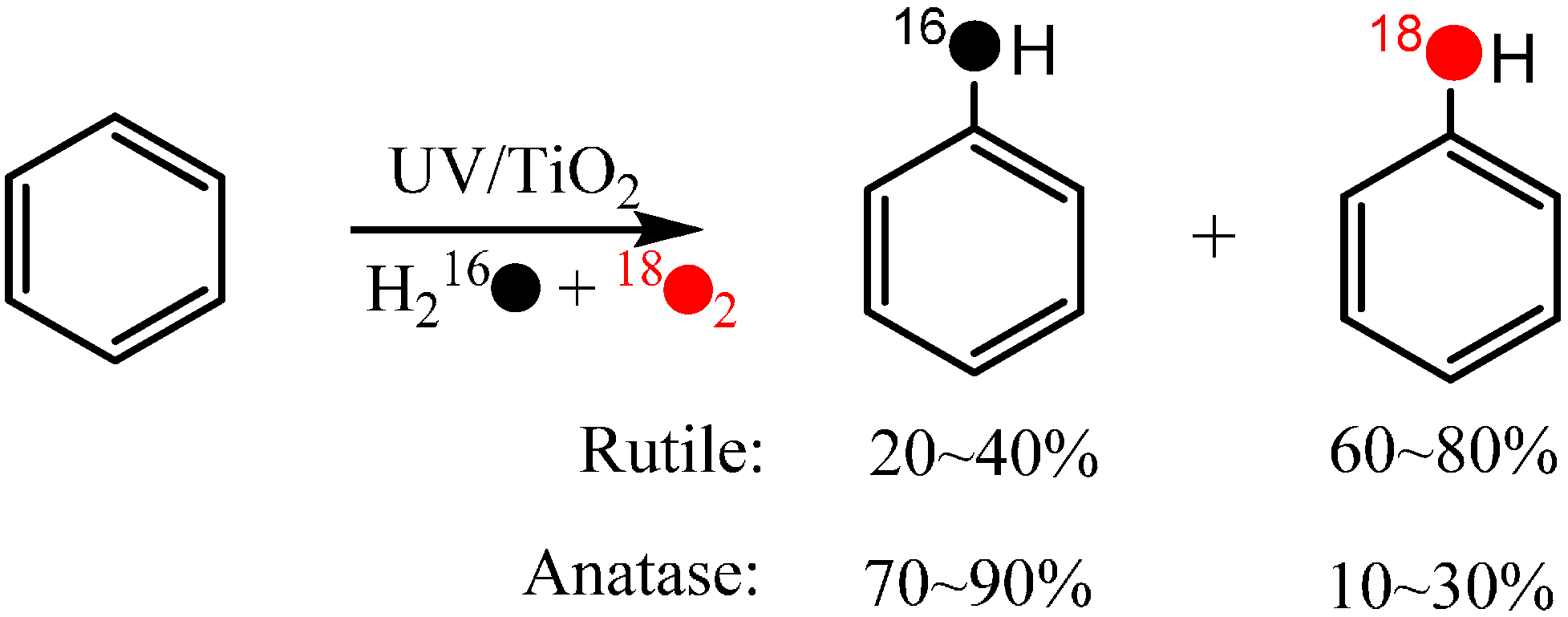
During the TiO
2 photocatalytic degradation of aromatic compounds, hydroxylation products are always the main detected intermediates. It is also accepted that the hydroxylation process is the primary one, and sometimes even the rate-determining step of the whole photocatalytic degradation reaction of aromatic compounds. Usually, the hydroxylation of aromatics is believed to be initiated by direct oxidation by h
vb+ followed by hydrolysis or the attack of ·OH (formed from the oxidation of water by h
vb+) in the photocatalytic systems. According to both pathways, the O-atom of the hydroxyl group in the hydroxylated product should come from H
2O. However, while using the
18O-isotope labeling method (H
218O and
18O
2) to investigate the process of photocatalytic oxidation of benzene to CO
2 in aqueous solution, Matsumura and coworkers [
51] found that the oxygen atoms of molecular oxygen were introduced into the phenol hydroxylation products (
Scheme 2).
Scheme 2.
TiO
2 photocatalytic oxidation of benzene to phenolin aerated aqueous solution [
51].
Scheme 2.
TiO
2 photocatalytic oxidation of benzene to phenolin aerated aqueous solution [
51].
Moreover, the incorporation of O atoms from O2 exhibited great differences between the anatase and rutile systems, i.e., 10%–30% and 60%–80% of the O atoms in the phenol are from O2 in the anatase and rutile systems, respectively. This observation has at least two implications: (1) dioxygen can participate directly in the oxidation process and incorporate it’s O-atom into the hydroxylation products; (2) the extent of the O2-incorporation is dependent on the photocatalytic conditions, such the crystal phase of the photocatalyst.
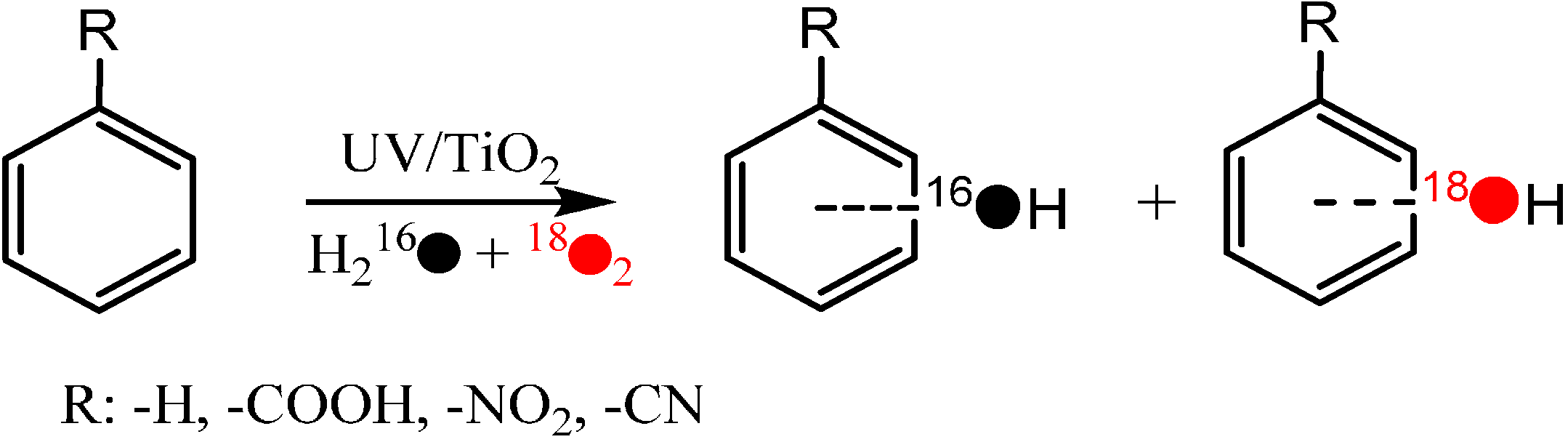
In order to unravel the detailed pathway of oxygen incorporation from O
2 in photocatalytic hydroxylation of aromatic compounds, the photocatalytic hydroxylation of several model substrates, such as benzoic acid, benzene, nitrobenzene, and benzonitrile were investigated by the isotope labeling method to trace the origin of the O atoms (from oxidant O
2 or solvent H
2O) in the hydroxyl groups of their corresponding hydroxylation products (
Scheme 3) [
41]. The results showed that, as reported by Matsumura
et al., the O atoms in the hydroxyl groups of the hydroxylation products can originate from both H
2O and O
2, and their contributions are comparable to each other in the hydroxylation process. More importantly, the percentage of hydroxylation products from O
2 was found to depend markedly on the reaction conditions [
41], such as the irradiation time and substrate concentration. The percentage of O
2-incorporation in hydroxybenzoic acid (BA-OH), for example, increased from 33.0% to 40.1% as the irradiation time increased from 1 h to 2 h at an initial benzoic acid concentration of 3 mM. In addition, when the initial concentration of benzoic acid increased from 3 mM to 25 mM, the percentage of O
2-incorporation in BA-OH decreased from 33.0% to 13.4% for the same irradiation time. Such a dependence of the percentage of O
2-incorporation in hydroxylation products on the irradiation time and initial substrate concentration was also observed in the photocatalytic hydroxylation of nitrobenzene and benzonitrile.
Scheme 3.
The photocatalytic hydroxylation of benzene derivatives on TiO
2 (P25) in aerated aqueous solution [
41].
Scheme 3.
The photocatalytic hydroxylation of benzene derivatives on TiO
2 (P25) in aerated aqueous solution [
41].
It was also observed that when benzoic acid and benzene, which have different adsorption abilities on TiO
2, coexisted in the same reaction system, the percentage of O
2-incorporation in their hydroxylation products was different [
41]. The addition of benzene to benzoic acid lowered slightly the percentage of O
2-incorporation in BA-OH, while the addition of benzoic acid in benzene notably increased the percentage of O
2-incorporation into its phenol hydroxylation product. The condition-dependence of the percentage of O
2-incorporation provides an excellent opportunity for us to probe further into the role and the mechanism of O
2 in the photocatalytic reaction.
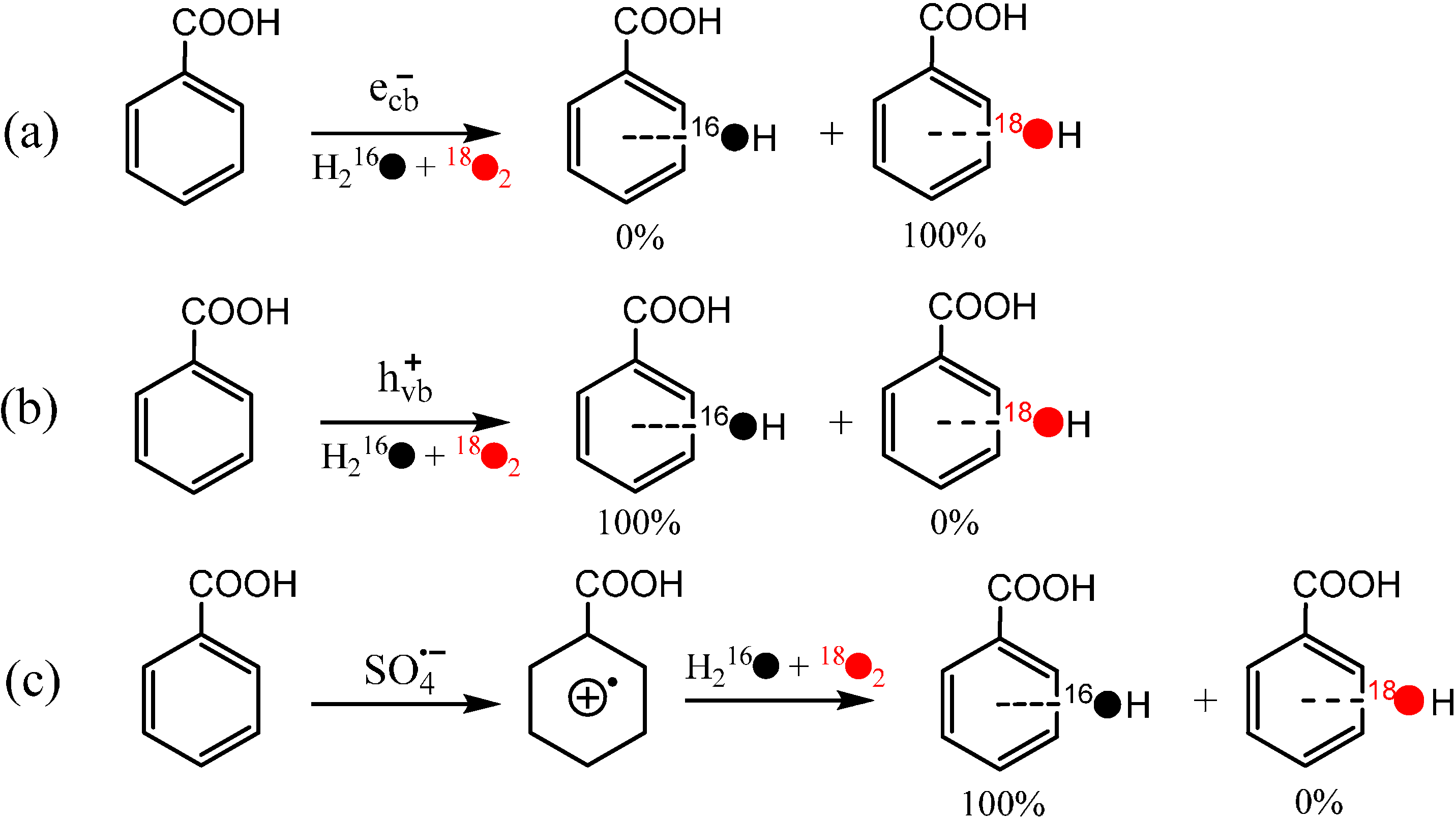
Further, by selective removal of the reactive species generated from the water oxidation by h
+vb or from O
2 reduction by e
−cb, the effect of every reactive species on the isotope distribution of the hydroxylated product was investigated systematically. When formic acid was used to selectively remove the h
+vb, the hydroxylation reaction still occurred. Moreover, nearly all O atoms in the hydroxyl groups of the hydroxylated products of benzoic acid came from O
2 in this situation (
Scheme 4a). On the other hand, by employing the benzoquinone to scavenge e
−cb or oxidize O
2− back to O
2, the hydroxyl O atoms almost all originated from the H
2O solvent (
Scheme 4b). Such a sharp contrast in the isotope distribution indicates that h
+vb is indispensable to H
2O incorporation and H
2O cannot participate in the hydroxylation of aromatic compounds if the oxidation of H
2O by h
+vb is blocked, while e
−cb is indispensable to O
2 incorporation. These observations do not support the earlier proposed mechanism that the direct reaction between O
2 and the substrate radical species formed by hole oxidation or HO
∙-adduct is the main O
2-incorporation pathway in the hydroxylation process, but imply that the O
2 has to be activated by e
−cb before its incorporation. This argument is further confirmed by the isotope experiment of directly oxidation benzoic acid to its cation radical by the one-electron oxidant SO
4∙−, in which the hydroxyl O atoms were observed to nearly all from H
2O (
Scheme 4c).
Scheme 4.
(
a) The hydroxlyation of benzoic acid initiated by e
−cb; (
b) the hydroxlylation of benzoic acid initiated by h
+vb; (
c) the hydroxlylation of benzoic acid via the path of one-electron oxidation by SO
4∙− [
41].
Scheme 4.
(
a) The hydroxlyation of benzoic acid initiated by e
−cb; (
b) the hydroxlylation of benzoic acid initiated by h
+vb; (
c) the hydroxlylation of benzoic acid via the path of one-electron oxidation by SO
4∙− [
41].
In addition, the accumulation of H2O2 was observed to be dependent on the concentration of substrates. The higher concentration of benzoic acid led to the slower consumption of H2O2, which is attributed to the competitive adsorption on TiO2 between H2O2 and benzoic acid. The different percentages of O2-incorporation in their hydroxylation products between benzoic acid and benzene, which have different adsorption abilities on TiO2, also demonstrate that the adsorption is a key factor that determines the O2-incorporation. All the experimental results indicate that the reaction on the surface tends to incorporate the more O atoms from H2O into the product, while the hydroxylation of the unabsorbed substrate leads to the formation of the product containing the more O2-derived O atoms. Therefore, both the pathways of the direct oxidation by h+vb with further hydrolysis (only from H2O) on the surface and the addition of ∙OH (from both H2O and O2) in bulk solution are important in the TiO2 photocatalytic hydroxylation of aromatics.
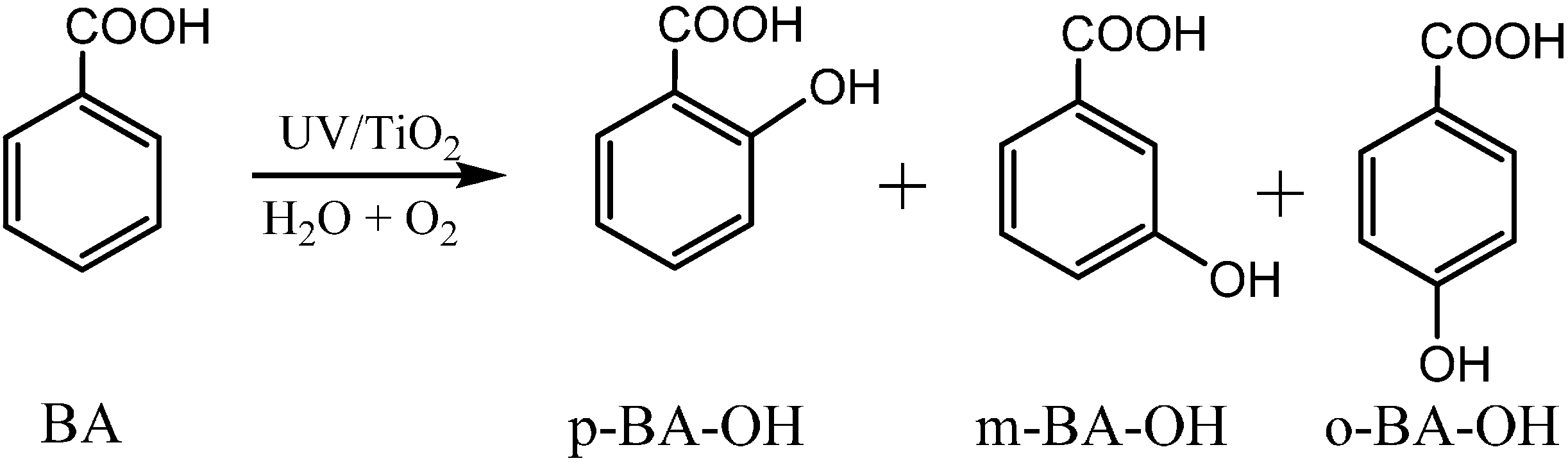
For substituted aromatic rings, different regioisomeric hydroxylated products, which usually exhibit different biological toxicity and secondary reactivity, can be formed during the photocatalytic hydroxylation. By detailed analysis of the monohydroxylated products of benzoic acid, it was found that three isomers of BA-OH,
i.e.,
meta-(
m-),
para- (
p-), and
ortho- (
o-) BA-OH, were all formed during the phtotocatalytic hydroxylation reaction (
Scheme 5) [
52]. The isotopic labeling experiments to trace the change of the oxygen source of the formed isomeric hydroxylated intermediates showed that the proportions of oxygen atom of the three hydroxylated isomers were remarkably different. The proportion of O atom of
m-BA-OH from H
2O was higher than those of
p- and
o-BA-OHs. These observations are somewhat unexpected, because the hydroxylation reaction should have the same oxygen source if the same active species and hydroxylated mechanism are responsible for the hydroxylation in the same photocatalytic system. In addition, the difference in the isotopic abundance of product isomers increased stably with the decrease of partial pressure of O
2 (P
O2) and with the increase of substrate concentrations. The analysis of the monohydroxylated products of benzoic acid indicated that three isomers of BA-OH have different yields, and that the yield distributions of these three monohydroxylated products changed with P
O2 and substrate concentration. The formation of
m-BA-OH was depressed relative to the
p- and
o-BA-OH with the increase of P
O2, while the high substrate concentration favored the formation of
m-BA-OH and disfavored the formation of
p- and
o-BA-OH, which is consistent with the changes in the isotope abundance.
Scheme 5.
The formation of the three regioisomeric monohydroxylated products of benzoic acid:
meta-hydroxyl benzoic acid (
m-BA-OH),
para-hydroxyl benzoic acid (
p-BA-OH), and
ortho- hydroxyl benzoic acid (
o-BA-OH) [
52].
Scheme 5.
The formation of the three regioisomeric monohydroxylated products of benzoic acid:
meta-hydroxyl benzoic acid (
m-BA-OH),
para-hydroxyl benzoic acid (
p-BA-OH), and
ortho- hydroxyl benzoic acid (
o-BA-OH) [
52].
The theoretical calculation indicated that the standard reduction potentials (E°
vs. NHE) of the HO-adduct benzoic acid radicals at
p- and
o-positions are −0.22 and −0.27 V, respectively, while
m-HO-BA radical has an E° of −0.66 V. The redox potentials of formed
p- and
o-BA-OH adduct radicals are below the bottom of conduction band of TiO
2 (−0.29 V), and these adduct radicals can be easily reduced by e
−cb. In contrast, the reduction of
m-BA-OH radical by e
−cb is impossible because of the more negative reduction potential of
m-BA-OH adduct radical. Confirming this observation, the redistribution of electron density in the presence of extra e
−cb indicates that TiO
2 with adsorbed HO-adduct radical in the the
p- and
o- positions of benzoic acid, the added electron distributes mainly on the HO-BA radical, whereas this electron spreads predominantly over the d-orbits of Ti-atoms, which make up the conduction band of TiO
2, when
m-HO-BA radical is adsorbed on the TiO
2 cluster [
52]. Evidently, the reduction of
m-BA-OH radical by e
−cb occurs on the surface of TiO
2, where the O atoms of hydroxylated products predominantly come from H
2O. However, in the bulk solution where no reductive e
−cb is available, all three HO-adduct radicals would transform into the corresponding hydroxylated products. Accordingly, any factor that can influence the adsorption of substrates and the accumulation of e
−cb would change the yield distribution and O-origin of three isomeric hydroxylated products. For example, the lower P
O2 and higher substrate concentration all would exaggerate the accumulation of e
−cb, which favors the reduction of
p- and
o-BA-OH radicals. As a result, the relative ratio of
m-BA-OH will increase. The concept that the e
−cb can selectively recombine the formed surface HO-adduct radicals back to the original substrate (
Scheme 6) implicates that we can modulate the hydroxylated intermediates distribution in the photocatalytic degradation of aromatic pollutants by tuning the Fermi level of the photocatalyst or controlling the accumulation amount of e
−cb or surface modification.
Scheme 6.
The selective reduction of HO-adduct radicals by conduction band electrons (e
−cb) back to the original substrate [
52].
Scheme 6.
The selective reduction of HO-adduct radicals by conduction band electrons (e
−cb) back to the original substrate [
52].
Another example for the isotope-labeling study on photocatalytic mechanism comes from the modulation of O
2 reduction pathway by pendant proton relay [
42]. In the photocatalytic oxidation of benzoic acid and benzene, it was found that the O
2-incorporation in the hydroxylation products was markedly depressed by the addition of phosphate, which can adsorb strongly on the surface of TiO
2. Further, the isotope-labeling analysis (H
216O and
18O
2) indicated that nearly 50% of the O-atoms of hydroxyl groups in BA-OH were derived from O
2 for pristine TiO
2, while almost no O-atoms from O
2 were detected for the phosphate system with 2 mM phosphates. Similarly, for the photocatalytic oxidation of the weak-adsorbed benzene the significantly decreased O-atom incorporation from O
2 into phenol was also observed upon addition of phosphate (from about 50% to 10%) [
42]. Since the O
2-incorporation is attributed to the sequential reduction of O
2 by e
−cb, as mentioned above, the depression in O
2-incorporation is an indication of new reduction pathway of O
2 by e
−cb in the presence of phosphates. Indeed, a detailed examination on the formation and decomposition of H
2O
2 during photoctalytic oxidation showed that the little H
2O
2 was generated in the presence of phosphates, while plenty of H
2O
2 was accumulated in the pristine TiO
2 system. The following electrochemical experiments confirmed that O
2 in the phosphate systems is reduced to H
2O via direct four electrons reduction (Equation (1)), while the pathway of the sequential single electron reduction to H
2O
2 (Equations (2–5)) is bypassed. Such a change in the O
2 reduction pathway is proposed to result from the management of surface proton by formation of “pendant proton relay structure” in which the surface-adsorbed phosphate has a pendant acid/base site [
42]:
3. Photocatalytic Cleavage of Aryl-Ring on TiO2 Surface
Another essential step on the way to the complete mineralization of aromatic pollutants into CO
2 is ring-opening, which involves the C-C bond cleavage of aryl rings and is more complex than the hydroxylation process. Accordingly, our understanding on the mechanism of cleavage of aryl ring is even poorer. Many researchers still believe that the ∙OH radicals are the main active oxygen species in the ring-opening step due to their high oxidative ability [
6]. However, recent electrochemical studies suggested that the aromatic rings cannot be efficiently cleaved by the ∙OH radicals in the absence of O
2 [
7], indicating the ∙OH radicals are not a good active species for the cleavage of aryl rings. Other reports [
54,
55,
56,
57,
58,
59] also argued that the reaction of the O
2-derived species, such as superoxide or singlet oxygen, with aromatics is the main pathway in photocatalytic ring-opening step. It is also considered that both the ∙OH radicals and O
2 are both important in the aromatic ring cleavage,
i.e., ∙OH radicals attack the aromatic ring, and the cleavage results from the reaction of the radicals formed by OH-attack and O
2 [
55,
60]. As a matter of fact, whether ∙OH radical or O
2 ultimately breaks the C-C bond of aromatic ring is still experimentally under debate and the mechanism of the ring-opening reaction in most reports remains somewhat speculative. Another popular mechanism in the ring-opening step for TiO
2 photocatalysis is that the superoxide radical anion, generated from the reduction of O
2 by e
cb−, reacts with substrate radical cation which is formed from oxidation of the substrate by h
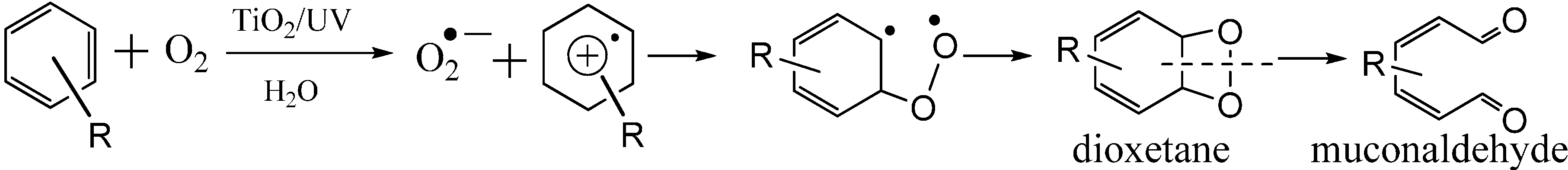
vb+, to form a dioxetane intermediate, then homolytic cleavage of the C-C bond leads to the formation the muconaldehyde (
Scheme 7) [
33,
55,
58,
61,
62,
63,
64].
Scheme 7.
The previously proposed mechanism for TiO2 photocatalytic cleavage of aryl-ring via a dioxetane intermediate.
Scheme 7.
The previously proposed mechanism for TiO2 photocatalytic cleavage of aryl-ring via a dioxetane intermediate.
However, no direct evidence was provided so far to support this proposal. In aqueous solution, the detected initial ring-opening products are always carboxylic acids or carboxylic acid derivatives, instead of the expected dialdehyde, probably because the aldehyde is an unstable intermediate and it can be rapidly converted into a stable carboxylic acid under photocatalytic conditions. Another possibility for the low yield of the aldehyde was attributed to the hydroxylation of the aromatic ring before the cleavage, while the ring cleavage still occurs via a dioxetane intermediate process. Recently, Matsumura
et al. [
65] indeed detected the muconaldehyde intermediate during the cleavage of benzene rings by TiO
2 photocatalyst although its yield is also very low in aqueous solution. Unfortunately, they could not track the origin of O atoms introduced into the muconaldehyde due to the fast oxygen exchange between carbonyl groups of the muconaldehyde and water. In order to track the ring-open pathway by avoiding the oxygen exchange between the products and the solvent, the
ortho-dihydroxybenzenes were used as model substrates, because the oxidative cleavage of
ortho-dihydroxy-benzenes would form directly the muconic acid which cannot exchange its oxygen atom with H
2O. Therefore, the behaviours of oxygen in the ring-opening products can be definitely tracked by the
18O-labeling methods in the aqueous TiO
2 photocatalytic system. Further, among the numerous
ortho-dihydroxyl substituted benzenes, 3,5-di-
tert-butylcatechol (DTBC) is an ideal molecular probe for the ring-cleavage, because its substituents can distinguish the ring-opening position and the steric effect of bulky
t-butyl groups also can induce the primary ring-opening reaction with unexpectedly high yields [
53]. Moreover, the pathway for oxidative cleavage of aromatic ring of DTBC has been extensively studied in the systems of catechol oxygenases and its artificially synthesised iron-containing analogues.
As illustrated in
Scheme 8, the main oxidation products are generally divided into three groups: (1) products
2 and
3 are the primary ring-opening products without the loss of a carbon atom, and they are generally intradiol products, which means that the O-atom was inserted into the C-C bond between the two
ortho hydroxyls; (2) products
4 and
5 have a loss of one carbon relative to their matrix and are generally extradiol products because the O-atom is inserted into the C-C bond out of the two hydroxyls; and (3) products
6–
8 are the products by simple oxidation of the DTBC in which no aromatic C-C bond is completely broken. The yields of these products are known to change over a wide range, dependent on the reaction conditions, oxidation systems and reaction mechanisms.
Scheme 8.
The structures of the main intermediate products of photocatalytic cleavage of 3,5-di-tert-butylcatechol (DTBC) by TiO
2 (P25) in aerated, water/acetonitrile mixed solution. The main products are divided into three groups and the values in brackets indicate the highest yields of the corresponding products [
53].
Scheme 8.
The structures of the main intermediate products of photocatalytic cleavage of 3,5-di-tert-butylcatechol (DTBC) by TiO
2 (P25) in aerated, water/acetonitrile mixed solution. The main products are divided into three groups and the values in brackets indicate the highest yields of the corresponding products [
53].
Under the optimized reaction conditions [
53], the total yield of the identified products during photocatalytic oxidation of DTBC could account for nearly 75% of the initial DTBC, and the initial ring-opening products (
2+
3+
4+
5) had a yield of 30% (
Scheme 8), which means that most of the products were recovered after the photocatalytic reaction. In addition, the isotope exchange experiment of the initial ring-opening products
2–
5 with H
218O or
18O
2 showed that the oxygen isotopic exchange or the photoinduced oxygen isotopic exchange process was slow and can be ignored under the present experimental conditions. All these characteristics of the oxidation products of DTBC make it possible to conveniently determine the oxygen sources and accurately quantify the ring-opening products by oxygen-18 isotope labeling experiments. Among these products, products
2 and
3 are the intermediates that most directly reflect the aromatic ring-opening mechanism. The product
2 had the highest yield among all of the cleavage products (~25%). In addition, it has been widely accepted in the literature and confirmed by our experiments that product
2 is the precursor of product
3, through the hydrolysis and subsequently addition reactions. Therefore, product
2 bears the direct information of the primary ring-opening process and is the most desirable intermediates to trace the oxygen source by isotope method.
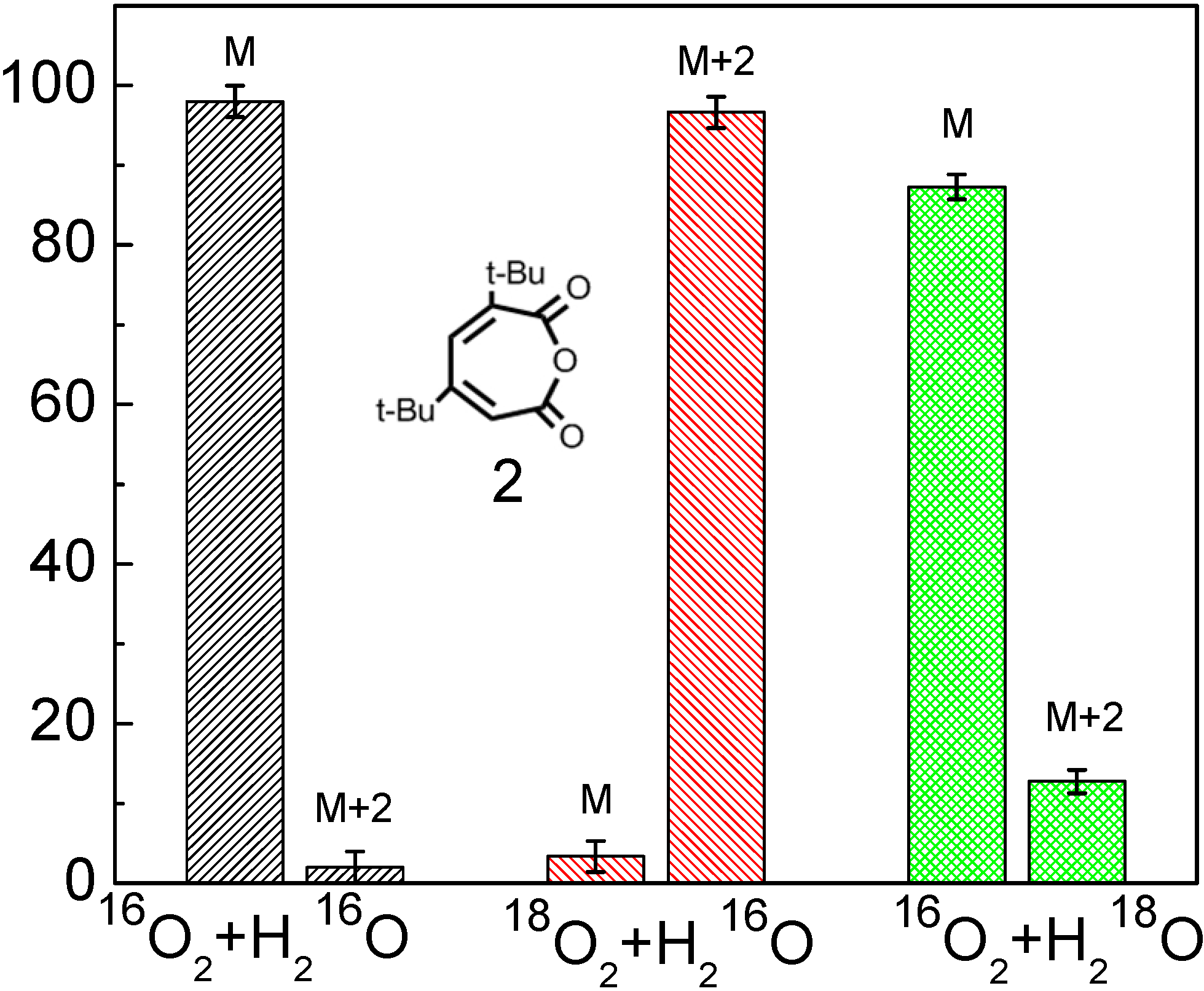
The isotope-labeling result showed that the inserted O atom (the bridging oxygen in anhydride functional group) in the ring of product
2 was from the O
2 in both
18O
2/H
216O and
16O
2/H
218O systems (
Figure 1), which provides the most direct information on how the O
2 cleaves the aromatic ring in the photocatalytic reaction [
53].
Figure 1.
The oxygen-isotope distribution of product
2 under the various isotope conditions. In each panel, the horizontal axis represents the three isotope conditions: (1) Natural
16O
2 and H
216O; (2)
18O
2 and H
216O; (3)
16O
2 and H
218O; the vertical axis represents the oxygen-isotope distribution ratio (%); M, M+2, M+4 denote products including 0, 1, 2 atoms of
18O in place of
16O [
53].
Figure 1.
The oxygen-isotope distribution of product
2 under the various isotope conditions. In each panel, the horizontal axis represents the three isotope conditions: (1) Natural
16O
2 and H
216O; (2)
18O
2 and H
216O; (3)
16O
2 and H
218O; the vertical axis represents the oxygen-isotope distribution ratio (%); M, M+2, M+4 denote products including 0, 1, 2 atoms of
18O in place of
16O [
53].
This observation seems not to support the earlier proposal of the usual ring opening pathway via a dioxetane intermediate (
Scheme 7). If the TiO
2 photocleavage of aromatics is through the hemolysis of dioxetane intermediate, the major ring-opening products should be a diacid (muconic acid) or acid-aldehydes. However, in the primary ring-opening products, only product
2 was detected in the photocatalytic reaction. No evidence for muconic acid formation could be obtained, neither by HPLC-ESI nor GC-MS detection of the compound itself or of its silylated derivatives. Two possibilities may explain this observation: muconic acid is formed but rapidly converts to product
2, or muconic acid is not formed at all and product
2 is directly derived from the insertion of an O atom in the C-C bond of DTBC. The former can be excluded because only one O atom was labeled in product
2 (
Scheme 9a). If the product
2 were derived from lactonization of muconic acid, it is impossible for product
2 to be completely preserved with one labeled O atom, because the two oxygen atoms in the carboxylate anions are equal (
Scheme 9b). Therefore, the isotopic labeling experiments indicate that the inserting O-atom of O
2 into the C-C bond can lead to the cleavage of the aromatic rings in photocatalytic systems.
This single oxygen insertion from O
2 was also found to be dominant in the formation of products
4 and
5 by using
16O
2/H
218O or
18O
2/H
216O. These results imply that the O
2-incorporation into the aryl ring is through a single-oxygen insertion rather than the insertion of both oxygen atoms of the O
2 as proposed earlier [
33,
55,
58,
61,
62,
63,
64]. This incorporation of a single oxygen atom from O
2 is analogous to the reaction of oxygenases in biological systems (
Scheme 9c), which leads to distinct intradiol or extradiol ring-opening products, respectively, depending on the initial sites of O
2 coordination [
66,
67,
68,
69]. It is expected that the activation and insertion of O
2 into aromatic ring in the present case is dependent on its coordination to the Ti–sites on the TiO
2 surface, just as in the active centre of oxygenases.
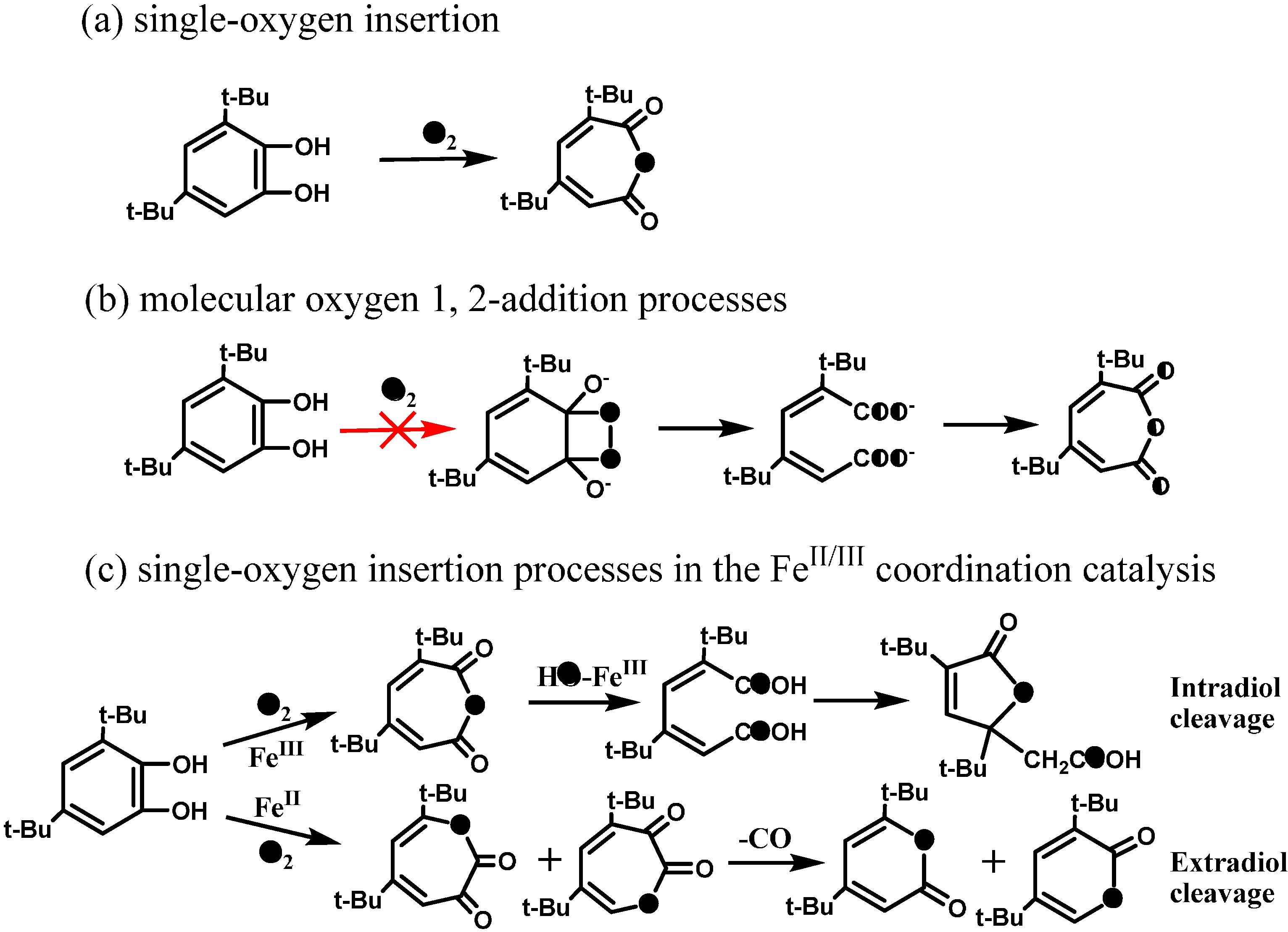
Scheme 9.
The oxygen-isotope distribution of product
2 via the (
a) single oxygen insertion process or (
b) molecular oxygen 1,2-addition process. (
c) the single oxygen incorporationprocesses in biological systems [
53].
Scheme 9.
The oxygen-isotope distribution of product
2 via the (
a) single oxygen insertion process or (
b) molecular oxygen 1,2-addition process. (
c) the single oxygen incorporationprocesses in biological systems [
53].
Also interestingly, it was found that the different TiO
2 particles with identical crystal structures and similar defect concentrations in bulk, but different exposed Ti-site coordination on the surface exhibited different the distribution in the intradiol products (
2 and
3) and extradiol products (
4 and
5) [
53]. The ratio of intradiol to extradiol products had a good correlation with the ratio of Ti-
4c to Ti-
3c sites. Since the relative amounts of the exposed Ti-
4c to Ti-
3c sites would be largely dependent on the TiO
2 particle size, the ratio can be determined by the inherent geometric size of the particles. The most noteworthy fact was that even for the smallest size of TiO
2 particles (~9.7 nm), the formation of intradiol products still exceeds that of extradiol products. This was consistent with the geometrical proportions of Ti-
4c and Ti-
3c sites on the surface of any size of TiO
2 nanocrystals. Thus, it was proposed that particle size determines the surface Ti-site coordination state, and then determines the chemoselectivity via the different activation pathways of O
2 [
53]. The high proportion of the intradiol products must be yielded on the steps (Ti-
4c) or kinks, where the O
2 should be activated and incorporated by the anchored DTBC radicals since there is no available Ti-site left for O
2 coordination and reduction (
Scheme 10a). Similarly, a small proportion of the extradiol products should be delivered from the corners (Ti-
3c) or partial oxygen vacancies (also Ti-
3c) with the smallest distribution proportion on the surface, and the activation of O
2 was performed by Ti
3+ (or e
cb−) because there is an available Ti-site left for O
2 coordination (
Scheme 10b). Finally, the Ti center channels the decomposition of these proxy adducts and leads to the ring-opening products by inducing the cleavage of O-O bond (
Scheme 10). The mechanism suggests that it is the molecular oxygen that breaks the C-C bond of the aromatics. The surface-mediated aromatic ring cleavage mechanism appears in TiO
2 photocatalytic system shows the site coordination, steric hindrance and stereoelectronic effects play more important roles than the single oxidation potential. It also provides further understanding of the essence of heterogeneous photocatalytic oxidation, namely the final conduction band electron or Ti-sites activating dioxygen.
Scheme 10.
Proposed mechanism for singly O-atom incorporation in the photocatalytic cleavage of catechol by TiO
2. (
a) intradiol cleavage via the anchored DTBC radicals active dioxygen at the step or edge, (
b) extradiol cleavage via Ti-sites active dioxygen on the corner [
53].
Scheme 10.
Proposed mechanism for singly O-atom incorporation in the photocatalytic cleavage of catechol by TiO
2. (
a) intradiol cleavage via the anchored DTBC radicals active dioxygen at the step or edge, (
b) extradiol cleavage via Ti-sites active dioxygen on the corner [
53].
4. TiO2 Photocatalytic Decarboxylation of Carboxylic Acids
As discussed above, the observed initial ring-opening products of aromatic compounds in aerated aqueous solutions are always carboxylic acids or carboxylic acid derivatives. Thus decarboxylation of these carboxylic acids is one of the most important steps for complete mineralization of organic pollutants. The most accepted mechanism for decarboxylation is through the Photo-Koble reactions [
70], which is initiated by the hole oxidation of the carboxylic acids to release the CO
2 and alkyl radicals. In the presence of O
2, the alkyl radicals react with O
2 to form the peroxyl radicals, and then decompose to hydroxylated and carbonylated intermediates via quaternion peroxide intermediates (Russell mechanism) [
71]. However, the experimental results for the degradation of saturated monocarboxylic acids (from acetic acid (C
2) to valeric (C
5)) in aerated aqueous solution by TiO
2 photocatalysis indicated that the photocatalytic oxidation of the acids led to the release of CO
2 and the formation of carboxylate acid with one less carbon, that is, a C
5 acid sequentially formed C
4 products, then C
3 and so forth (
Scheme 11) [
44,
72], but little hydroxylated and carbonylated intermediates were detected. This means that there are other possible mechanisms for the TiO
2 photocatalytic decarboxylation of carboxylic acids other than the Photo-Koble process and Russell mechanism.
Scheme 11.
Stepwise cleavages of C1-C2 bonds in carboxylic acids.
Scheme 11.
Stepwise cleavages of C1-C2 bonds in carboxylic acids.
Since this decarboxylation breaks the C
1-C
2 bond of the original acid and establishes a new carboxyl group in the immediately smaller acid, it is feasible to directly trace these processes by using the 18-oxygen isotopic labeling method. The photocatalytic decarboxylation of propionic acid was carried out in H
218O solution to observe the
18O profile of the produced acetic acid [
44]. The isotope-labeling results showed that both the oxygen atoms of O
2 and H
2O can incorporate into the acetic acid. The percentage of the O
2-incorporation can reach as much as 42% at a conversion of 25.1%. To obtain information about the intermediates generated during decarboxylation, diffuse reflectance FTIR measurements (DRIFTS) were employed to monitor
in-situ the oxidative decarboxylation process of propionic acid. During irradiation, an absorption peak of C=O stretch of α-keto group of pyruvic acid was observed. This peak shifted from 1772 cm
−1 to 1726 cm
−1 when the 18-oxygen isotope labeling of H
218O was introduced, indicating that pyruvic acid is the intermediate of decarboxylation of propionic acid, and the oxygen atom of α-keto group comes from H
2O (Equation (6)) [
44]:
The isotope labeling results on the decarboxylation of propionic acid showed the O atom in O
2 was incorporated into the product acetic acid, while O
2 did not participate into the formation of the intermediate pyruvic acid (Equation (7)) [
44]. Thus O
2 should be introduced during the oxidation of pyruvic acid to acetic acid. By using pyruvic acid as the model substrate, the 18-oxygen labeling experiment in
18O
2/ H
216O was carried out to examine the transformation of pyruvic acid to acetic acid by TiO
2 photocatalysis. The oxygen atom of O
2 was largely incorporated into the acetic acid, and at least one oxygen atom of the substrate pyruvic acid was preserved in the carboxyl group of the formed acetic acid. The proportion of O
2-incorporation greatly depended on the reaction conditions, because the O
2-involved decarboxylation competes with the hole/OH radical-promoted decarboxylation process. The electrochemical experiment further confirmed that, in the absence of holes/OH radicals, O
2 could independently cleave the C
1-C
2 bond of pyruvic acid to generate acetic acid with 100% selectivity at a negative bias, while no reaction was observed in the case of propionic acid [
44]. A reasonable explanation for such a difference is the different modes of coordination of propionic acid and pyruvic acid on the TiO
2 surface. An α-keto acid adsorbs on the TiO
2 surface via bidentate coordination with the Ti sites, which is favorable for the incorporation of O
2 into the C
1-C
2 bond, likely through a Crigee rearrangement (
Scheme 12). However, for propionic acids, it can only chemisorb via monodentate coordination which is not favorable for O
2-incorporation.
Scheme 12.
A possible pre-coordination mechanism for dioxygen incorporation into the product during the decarboxylation of α-keto acids [
44].
Scheme 12.
A possible pre-coordination mechanism for dioxygen incorporation into the product during the decarboxylation of α-keto acids [
44].
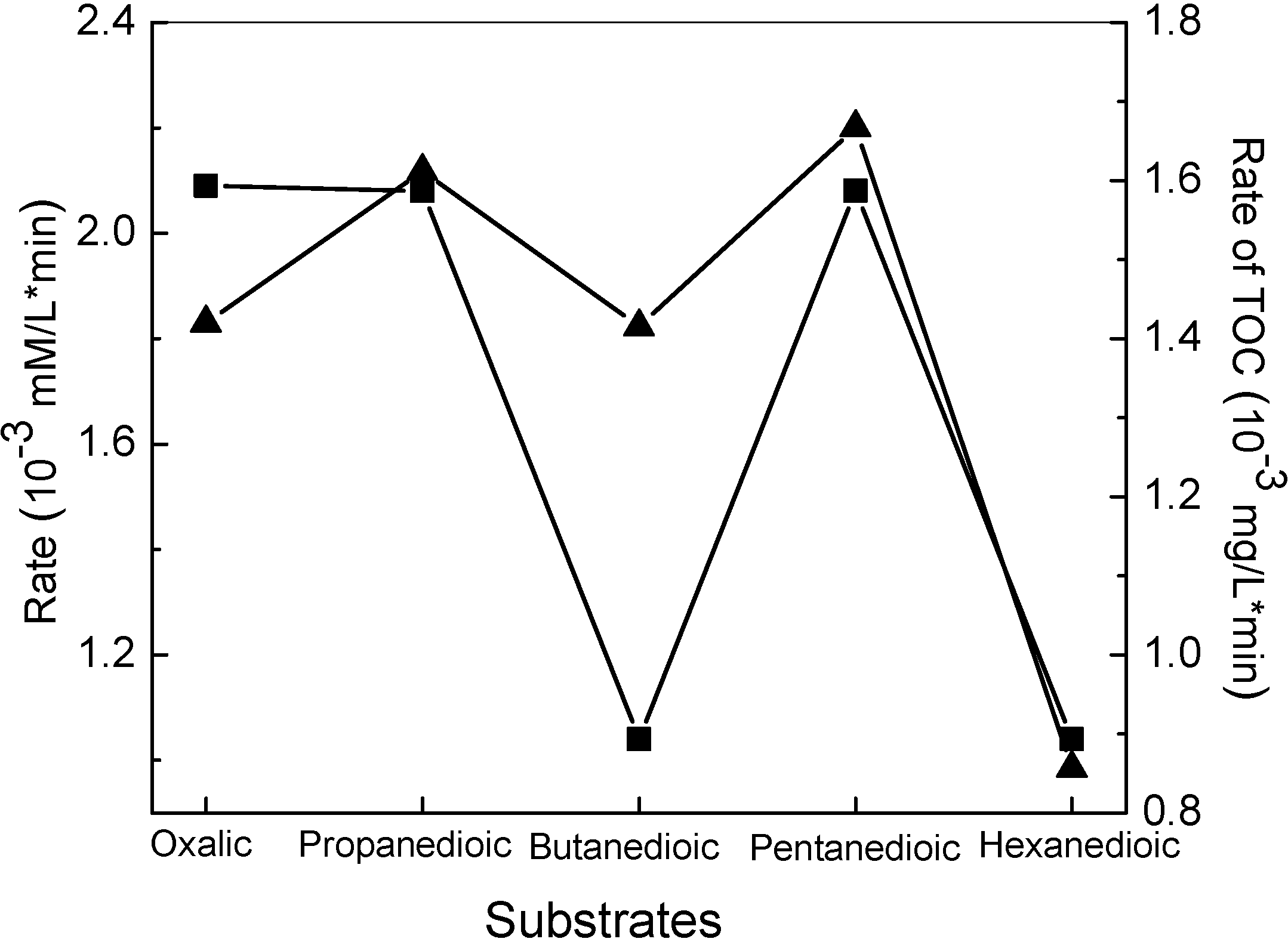
For the decarboxylation of dicarboxylic acids, the process was also found to proceed through stepwise loss fo carbon atoms [
43]. More interestingly, the dicarboxylic acids with an even number of carbon atoms (e-DAs) always degraded more slowly than those acids with an odd number of carbon atoms (o-DAs) (
Figure 2).
Figure 2.
Average rates for both full conversion (■) and TOC removal (▲) by TiO2-based photocatalysis for the five dicarboxylic acids as a function of their carbon number [
43].
Figure 2.
Average rates for both full conversion (■) and TOC removal (▲) by TiO2-based photocatalysis for the five dicarboxylic acids as a function of their carbon number [
43].
The attenuated total reflection FTIR (ATR-FTIR) combined with the
13C labeling method showed that both carboxyl groups of the e-DAs acids coordinate to TiO
2 through bidentate chelating forms. However, for the o-DAs acids, only one carboxyl group coordinates to TiO
2 in a bidentate chelating manner and the other carboxyl group interacts with the surface in a monodentate mode. Further 18-oxygen labeling experiments showed that the photocatalytic oxidation of o-DAs and e-DAs had different oxygen sources in the carboxyl group of the formed decarboxylated products of dicarboxylic acid [
43]. For o-DAs, which have two adsorption different modes: the asymmetrical bidentate and monodentate chelating mode, only the oxygen from H
2O was incorporated into the initial decarboxylated products (
Scheme 13a). In contrast, for the e-DAs, which exhibit symmetrical bidentate chelating mode on TiO
2 surface both O
2 and H
2O contributed to their decarboxylated products (
Scheme 13b). All these results indicated that the coordination patterns of the substrates on the TiO
2 surface are very important in the TiO
2 photocatalytic decarboxylation of saturated moncarboxylic or dicarboxylic acids and are sometimes the main factor that determines the active species, such as h
+vb/∙OH and e
−cb/O
2, to cleave the C-C or C-H bond of the substrates.
Scheme 13.
Schematic diagrams of photoctalytic decarboxylation of dicarboxylic acids. (
a) Pentanedioic acid represents dicarboxylic acids with an odd number of carbon atoms (o-DAs); (
b) butanedioic acid represents dicarboxylic acids with an even number of carbon atoms (e-DAs) [
43].
Scheme 13.
Schematic diagrams of photoctalytic decarboxylation of dicarboxylic acids. (
a) Pentanedioic acid represents dicarboxylic acids with an odd number of carbon atoms (o-DAs); (
b) butanedioic acid represents dicarboxylic acids with an even number of carbon atoms (e-DAs) [
43].