Synthesis, Half-Wave Potentials and Antiproliferative Activity of 1-Aryl-substituted Aminoisoquinolinequinones
Abstract
:1. Introduction
2. Results and Discussion
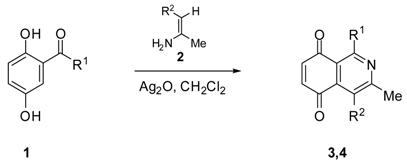
2.1. Chemistry
| Precursors | Product | R1 | R2 | Yield (%) a | |
|---|---|---|---|---|---|
| 1a | 2a | 3a | H | CO2Me | 86 b |
| 1b | 2a | 3b | phenyl | CO2Me | 60 |
| 1c | 2a | 3c | 2-thienyl | CO2Me | 67 |
| 1d | 2a | 3d | 2-furyl | CO2Me | 56 |
| 1a | 2b | 4a | H | COMe | 74 |
| 1b | 2b | 4b | phenyl | COMe | 53 |
| 1c | 2b | 4c | 2-thienyl | COMe | 72 |
| 1d | 2b | 4d | 2-furyl | COMe | 56 |
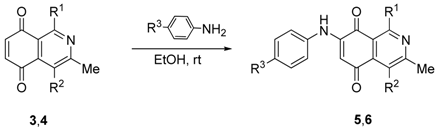
| Compound N° | R1 | R2 | R3 | Yield (%) a |
|---|---|---|---|---|
| 5a | H | CO2Me | H | 47 b |
| 5b | H | CO2Me | OMe | 36 b |
| 5c | phenyl | CO2Me | H | 57 |
| 5d | phenyl | CO2Me | OMe | 53 |
| 5e | thien-2-yl | CO2Me | H | 71 |
| 5f | thien-2-yl | CO2Me | OMe | 93 |
| 5g | fur-2-yl | CO2Me | H | 65 |
| 5h | fur-2-yl | CO2Me | OMe | 76 |
| 6a | H | COMe | H | 98 |
| 6b | phenyl | COMe | H | 70 |
| 6c | thien-2-yl | COMe | H | 77 |
| 6d | fur-2-yl | COMe | H | 91 |
| 6e | phenyl | COMe | OMe | 97 |
| 6f | thien-2-yl | COMe | OMe | 57 |
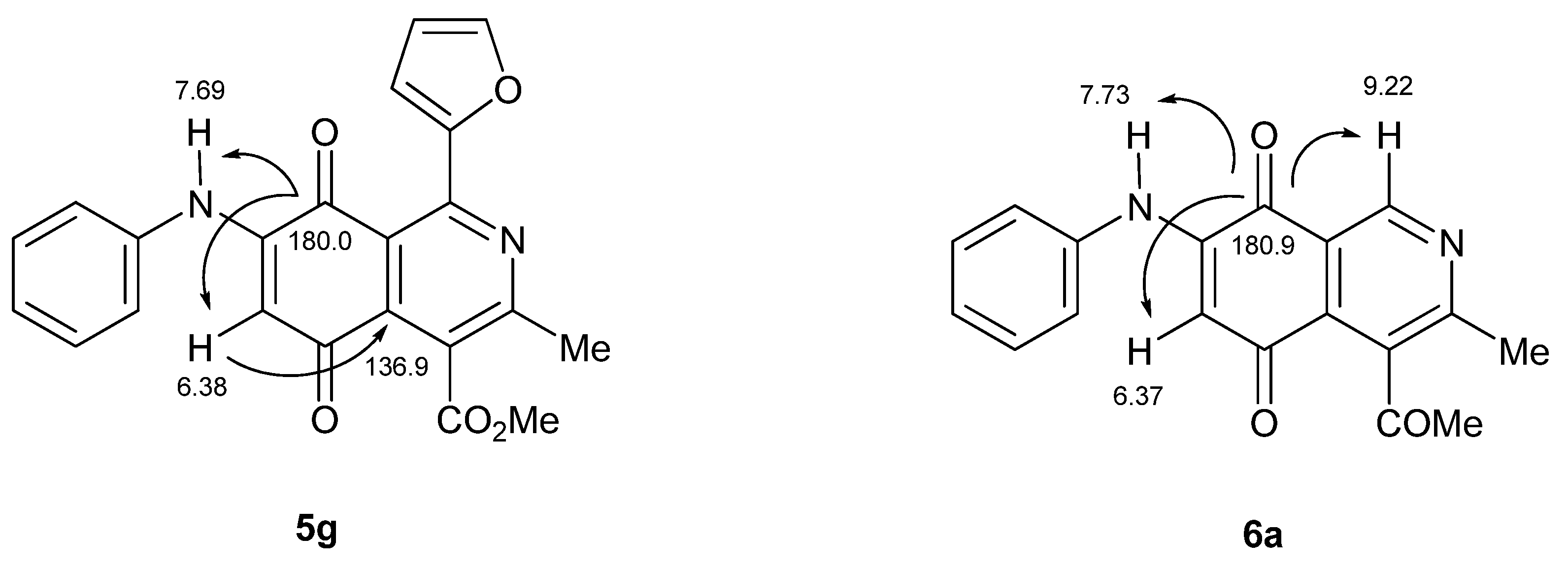
2.2. Electrochemical Results
| N° | R1 | R2 | −EI1/2 (mV) | 6- and/or-7H a |
|---|---|---|---|---|
| 3a | H | H | 352 | 7.04 |
| 3b | phenyl | H | 399 | 6.94 b |
| 3c | 2-thienyl | H | 392 | 6.99 |
| 3d | 2-furyl | H | 415 | 6.92 b |
| 4a | H | H | 344 | 7.05 b |
| 4b | phenyl | H | 416 | 6.96 b |
| 4c | 2-thienyl | H | 430 | 6.99 b |
| 4d | 2-furyl | H | 384 | 7.00 b |
| 5a | H | anilino | 563 | 6.39 |
| 5b | H | p-anisidino | 551 | 6.20 |
| 5c | phenyl | anilino | 560 | 6.39 |
| 5d | phenyl | p-anisidino | 583 | 6.23 |
| 5e | 2-thienyl | anilino | 565 | 6.39 |
| 5f | 2-thienyl | p-anisidino | 583 | 6.18 |
| 5g | 2-furyl | anilino | 554 | 6.38 |
| 5h | 2-furyl | p-anisidino | 577 | 6.21 |
| 6a | H | anilino | 464 | 6.37 |
| 6b | phenyl | anilino | 588 | 6.39 |
| 6c | 2-thienyl | anilino | 576 | 6.40 |
| 6d | 2-furyl | anilino | 533 | 6.36 |
| 6e | phenyl | p-anisidino | 570 | 6.21 |
| 6f | 2-thienyl | p-anisidino | 576 | 6.17 |
2.3. In Vitro Antiproliferative Activity of Phenylaminoisoquinolinequinones against Cancer Cell Lines
| N° | IC50 ± SEM a (μM) a | clogP e | ||
|---|---|---|---|---|
| AGS c | HL-60 d | |||
| 5a | 2.70 ± 0.60 | 1.10 ± 0.03 | 14.81 ± 0.74 | 0.74 |
| 5b | 2.80 ± 0.80 | 1.10 ± 0.10 | 3.80 ± 0.07 | 0.61 |
| 5c | 5.91 ± 0.36 | 2.52 ± 0.17 | 4.39 ± 0.26 | 2.84 |
| 5d | >100 | >100 | >100 | 2.71 |
| 5e | 9.89 ± 0.51 | 4.24 ± 0.21 | 5.19 ± 0.31 | 2.82 |
| 5f | 9.19 ± 0.53 | 3.28 ± 0.13 | 10.26 ± 0.09 | 2.69 |
| 5g | 4.72 ± 0.29 | 1.79 ± 0.11 | 5.0 ± 0.35 | 1.45 |
| 5h | 4.58 ± 0.35 | 1.83 ± 0.11 | 8.04 ± 0.49 | 1.33 |
| 6a | 3.67 ± 0.22 | 1.19 ± 0.07 | 1.24 ± 0.06 | 0.23 |
| 6b | 5.51 ± 0.22 | 2.21 ± 0.09 | 4.74 ± 0.37 | 2.33 |
| 6c | >100 | >100 | >100 | 2.31 |
| 6d | 5.72 ± 0.24 | 1.79 ± 0.11 | 8.19 ± 0.57 | 0.95 |
| 6e | 16.10 ± 1.11 | 4.66 ± 0.28 | 9.36 ± 0.72 | 2.20 |
| 6f | >100 | 4.28 ± 0.21 | >100 | 2.19 |
| Etoposide | 0.33 ± 0.02 | 0.58 ± 0.02 | 2.23 ± 0.09 | |
3. Experimental
3.1. General
3.2. Chemistry
3.3. General Procedure for the Synthesis of 7-Amino-1-arylisoquinolinquinone Derivatives
3.4. Cell Growth Inhibition Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Powis, G. Metabolism and reactions of quinoid anticancer agents. Pharmacol. Ther. 1987, 35, 57–162. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. J. Chem. Biol. Interact. 1991, 80, 1–14. [Google Scholar] [CrossRef]
- Paz, M.M.; Das, A.; Palom, Y.; He, Q.-Y.; Tomasz, M. Selective activation of mitomycin a by thiols to form DNA Cross-links and monoadducts: Biochemical basis for the modulation of mitomycin cytotoxicity by the quinone redox potential. J. Med. Chem. 2001, 44, 2834–2842. [Google Scholar] [CrossRef]
- Tudor, G.; Gutierrez, P.; Aguilera-Gutierrez, A.; Sausville, E.A. Cytotoxicity and apoptosis of benzoquinones: Redox cycling, cytochrome c release, and BAD protein expression. Biochem. Pharmacol. 2003, 65, 1061–1075. [Google Scholar] [CrossRef]
- Rao, K.V.; Biemann, K.; Woodward, R.B. The structure of streptonigrin. J. Am. Chem. Soc. 1963, 85, 2532–2533. [Google Scholar] [CrossRef]
- Gould, S.J.; Weimeb, S.M. Streptonigrin. Fortschr. Chem. Org. Naturst. 1982, 41, 77–111. [Google Scholar]
- Balitz, D.M.; Bush, J.A.; Bradner, W.T.; Doyle, T.W.; O’Herron, F.A.; Nettleton, D.E. Isolation of lavendamycin, a new antibiotic from streptomyces lavendulae. J. Antibiot. 1982, 35, 259–265. [Google Scholar] [CrossRef]
- Doyle, T.W.; Balitz, D.M.; Grulich, R.E.; Nettleton, D.E.; Gould, S.J.; Tann, C.H.; Mews, A.E. structure determination of lavendamycin, a new antitumor antibiotic from streptomyces lavendulae. Tetrahedron Lett. 1981, 22, 4595–4598. [Google Scholar] [CrossRef]
- Pettit, G.R.; Knight, J.C.; Collins, J.C.; Herald, D.L.; Pettit, R.K.; Boyd, M.R.; Young, V.G. Antineoplastic agents 430. Isolation and structure of cribrostatins 3, 4, and 5 from the republic of Maldives Cribrochalina species. J. Nat. Prod. 2000, 63, 793–798. [Google Scholar] [CrossRef]
- Milanowski, D.J.; Gustafson, K.R.; Kelley, J.A.; McMahon, J.B. Caulibugulones A–F, Novel antiproliferative isoquinoline quinones and iminoquinones from the marine bryozoan caulibugula intermis. J. Nat. Prod. 2004, 67, 70–73. [Google Scholar]
- Hawas, U.W.; Shaaban, M.; Shaaban, K.A.; Speitling, M.; Maier, A.; Kelter, G.; Fiebig, H.H.; Meiners, M.; Helmke, E.; Laatsch, H. Mansouramycins A–D, cytotoxic isoquinolinequinones from a marine streptomycete. J. Nat. Prod. 2009, 72, 2120–2124. [Google Scholar] [CrossRef]
- Lazo, J.S.; Aslan, D.C.; Southwick, E.C.; Cooley, K.A.; Ducruet, A.P.; Joo, B.; Vogt, A.; Wipf, P. Discovery and biological evaluation of a new family of potent inhibitors of the dual specificity protein phosphatase Cdc25. J. Med. Chem. 2001, 44, 4042–4049. [Google Scholar] [CrossRef]
- Mulchin, B.J.; Newton, C.G.; Baty, J.W.; Grasso, C.H.; Martin, W.J.; Walton, M.C.; Dangerfield, E.M.; Plunkett, C.H.; Berridge, M.V.; Harper, J.L.; et al. The anti-cancer, anti-inflammatory and tuberculostatic activities of a series of 6,7-substituted-5,8-quinolinequinones. Bioorg. Med. Chem. 2010, 18, 3238–3251. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Ibacache, J.A.; Arancibia, V.; Rodriguez, J.; Theoduloz, C. Studies on Quinones. Part 45. Novel 7-Aminoisoquinoline-5,8-quinone derivatives with antitumor properties on cancer cell lines. Bioorg. Med. Chem. 2009, 17, 2894–2901. [Google Scholar] [CrossRef]
- Delgado, V.; Ibacache, A.; Theoduloz, C.; Valderrama, J.A. Synthesis and in vitro cytotoxic evaluation of aminoquinones structurally related to marine isoquinolinequinones. Molecules 2012, 17, 7042–7056. [Google Scholar] [CrossRef]
- Delgado, V.; Ibacache, A.; Arancibia, V.; Theoduloz, C.; Jaime, A.; Valderrama, J.A. Synthesis and in vitro antiproliferative Activity of new phenylaminoisoquinolinequinones against cancer cell lines. Molecules 2013, 18, 721–734. [Google Scholar] [CrossRef]
- Valderrama, J.A.; González, M.F.; Pessoa-Mahana, D.; Tapia, R.A.; Fillion, H.; Pautet, F.; Rodríguez, J.A.; Theoduloz, C.; Schmeda-Hirschmann, G. Studies on quinones. Part 41: Synthesis and cytotoxicity of isoquinoline-containing polycyclic quinones. Bioorg. Med. Chem. 2006, 14, 5003–5011. [Google Scholar] [CrossRef]
- Benites, J.; Rios, D.; Díaz, P.; Valderrama, J.A. The solar-chemical photo-Friedel-Crafts heteroacylation of 1,4-quinones. Tetrahedron Lett. 2011, 52, 609–611. [Google Scholar] [CrossRef]
- Arenas, P.; Peña, A.; Ríos, D.; Benites, J.; Muccioli, G.; Buc, P.; Valderrama, J.A. Eco-friendly synthesis and antiproliferative evaluation of some oxygen substituted diaryl ketones. Molecules 2013, 18, 9818–9832. [Google Scholar] [CrossRef]
- Litvié, M.; Filipan, M.; Pogorelié, I.; Cepanec, I. Ammonium carbamate; mild, selective and efficient ammonia source for preparation of β-amino-α,β-unsaturated esters at room temperature. Green Chem. 2007, 7, 771–774. [Google Scholar]
- Prieto, Y.; Muñoz, M.; Arancibia, V.; Valderrama, M.; Lahoz, F.J.; Martín, M.L. Synthesis, structure and properties of ruthenium(II) complexes with quinolinedione derivatives as chelate ligands. Crystal structure of [Ru(CO)2Cl2(6-methoxybenzo[g]-quinoline-5,10-dione)]. Polyhedron 2007, 26, 5527–5532. [Google Scholar] [CrossRef]
- De Abreu, F.C.; de Ferraz, P.A.; Goulart, M.O.F. Some applications of electrochemistry in biomedical chemistry. Emphasis on the correlation of electrochemical and bioactive properties. J. Braz. Chem. Soc. 2002, 13, 19–35. [Google Scholar] [CrossRef]
- Aguilar-Martinez, M.; Cuevas, G.; Jimenez-Estrada, M.; González, I.; Lotina-Hennsen, B.; Macias-Ruvalcaba, N. An experimental and theoretical study of the substituent effects on the redox properties of 2-[(R-phenyl)amine]-1,4-naphthalenediones in acetonitrile. J. Org. Chem. 1999, 64, 3684–3694. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Van de Loosdrecht, A.A.; Beelen, R.H.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ibacache, J.A.; Delgado, V.; Benites, J.; Theoduloz, C.; Arancibia, V.; Muccioli, G.G.; Valderrama, J.A. Synthesis, Half-Wave Potentials and Antiproliferative Activity of 1-Aryl-substituted Aminoisoquinolinequinones. Molecules 2014, 19, 726-739. https://doi.org/10.3390/molecules19010726
Ibacache JA, Delgado V, Benites J, Theoduloz C, Arancibia V, Muccioli GG, Valderrama JA. Synthesis, Half-Wave Potentials and Antiproliferative Activity of 1-Aryl-substituted Aminoisoquinolinequinones. Molecules. 2014; 19(1):726-739. https://doi.org/10.3390/molecules19010726
Chicago/Turabian StyleIbacache, Juana Andrea, Virginia Delgado, Julio Benites, Cristina Theoduloz, Verónica Arancibia, Giulio G. Muccioli, and Jaime A. Valderrama. 2014. "Synthesis, Half-Wave Potentials and Antiproliferative Activity of 1-Aryl-substituted Aminoisoquinolinequinones" Molecules 19, no. 1: 726-739. https://doi.org/10.3390/molecules19010726