General Information
The 1H-NMR spectra were recorded on a Varian Mercury plus-400 MHz Fourier transform (FT)-NMR spectrometer, and chemical shift values were reported as δ ppm relative to tetramethylsilane (TMS). The 13C-NMR spectra were recorded on a Varian Mercury plus-400 MHz Fourier transform (FT)–NMR spectrometer, and chemical shift values were reported on δ ppm relative to CDCl3 or DMSO-d6. The mass spectra were recorded on an Agilent API 2000LC-MS/MS mass spectrometer. The HPLC chromatograms were recorded on a Waters 1525 HPLC instrument.
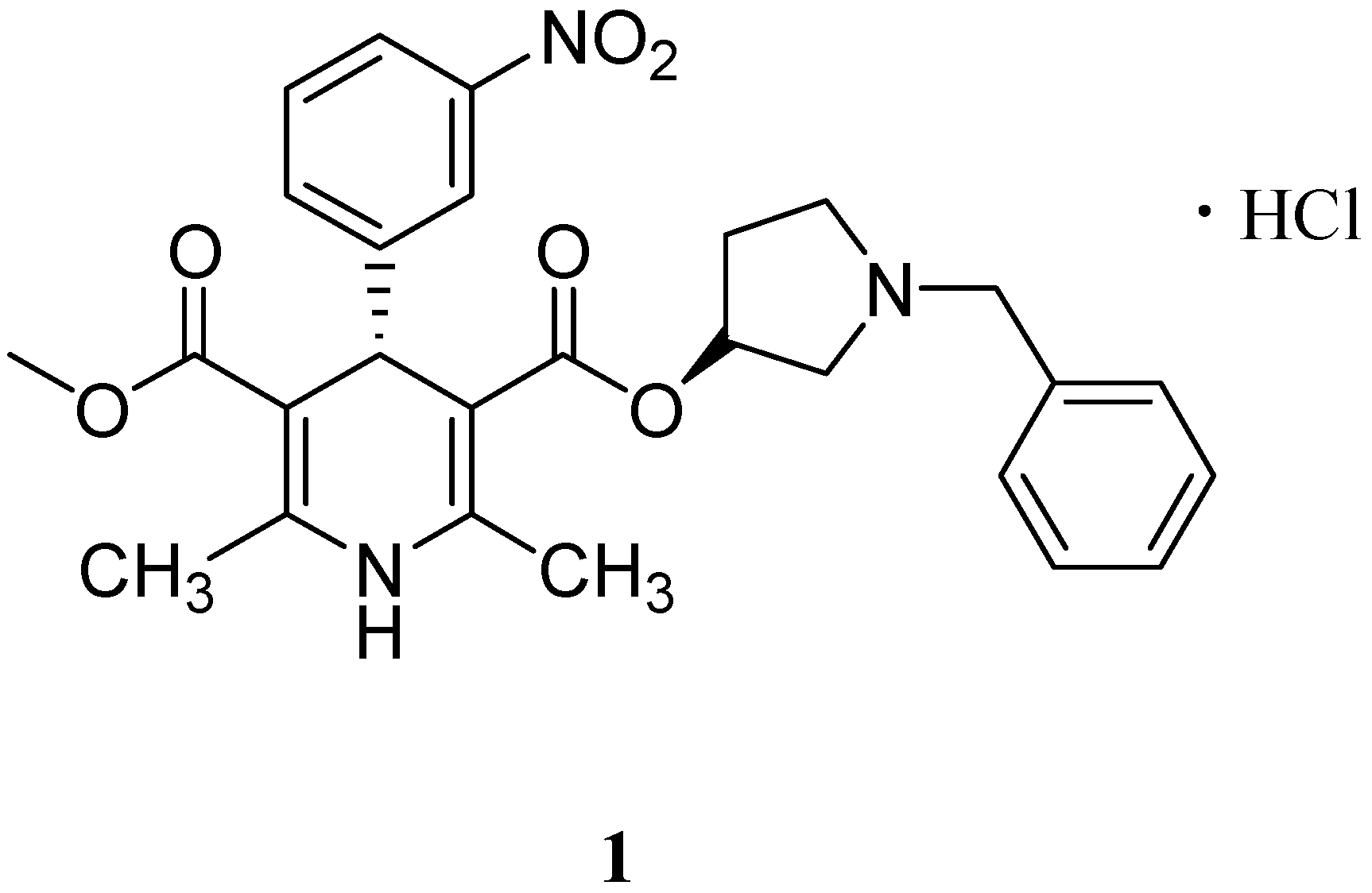
(3'S,4R)-1-Benzyl-3-pyrrolidinyl methyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridine-dicarboxylate (2) Phosphorus pentachloride (1.95 g, 0.02 mmol) was added slowly to a solution of (S)-5-(methoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid (13, 2.47 g, 0.0074 mol) in dichloromethane (DCM, 32.5 mL) at −20 °C, and stirred for 1 h below −15 °C. Then the mixture was cooled to −26 °C, and a solution of (S)-1-benzylpyrrolidin-3-ol (14, 1.35 g, 0.0076 mol) and dichloromethane (11.25 mL) were added to above mixture, and stirred for 2 h. The reaction mixture was washed with 5% sodium carbonate solution (150 mL) and water (150 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure at less than 35 °C. The residue was purified by flash column chromatography on silica gel, eluting with ethyl acetate/petroleum ether, 3:2, to yield 2 as a light yellow solid (1.5g, 41%). HPLC purity 92.9%. 1H-NMR (CDCl3) δ: 7.27–8.10 (m, 9H), 5.90 (s, 1H), 5.10–5.14 (m, 1H), 5.08 (s, 1H), 3.64 (s, 3H), 3.51–3.58 (m, 2H), 2.74–2.79 (m, 1H), 2.66–2.70 (m, 1H), 2.48–2.51 (m, 1H), 2.35 (s, 6H), 2.10–2.28 (m, 2H), 1.85-1.86 (m, 1H); 13C-NMR (CDCl3) δ: 167.5, 166.8, 149.8, 148.4, 144.9, 144.8, 138.8, 134.5, 128.7, 128.6, 128.2, 127.0, 122.9, 121.3, 103.3, 73.8, 60.1, 59.8, 52.6, 51.1, 39.9, 29.7, 19.6. MS (ESI−) m/z: 490.1 (M−H)−.
3-(R)-1-Benzylpyrrolidin-3-yl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl) pyridine-3,5-dicarboxylate (3) Manganese dioxide (3.5 g, 0.04 mol) was added to a solution of barnidipine (15, 2.0 g, 0.004 mol) in dichloromethane (150 mL), exposed to UV light, and stirred for 30 days at room temperature. The reaction mixture was filtered and concentrated. The residue was purified by flash column chromatography on silica gel, eluting with dichloromethane/ethanol, 40:1, followed by ethyl acetate/petroleum ether, 3:2, to afford 3 as a light yellow solid (0.6 g, 31%). HPLC purity 94.6%. 1H-NMR (CDCl3) δ: 7.13–8.10 (m, 9H), 4.96–4.99 (m, 1H), 3.34–3.47 (m, 5H), 2.46–2.51 (m, 8H), 2.21–2.27 (m, 1H), 2.11–2.14 (m, 1H), 1.92–2.03 (m, 1H), 1.37–1.43 (m, 1H). 13C-NMR (CDCl3) δ: 167.4, 166.7, 156.1, 156.0, 147.6, 143.4, 138.1, 137.8, 134.1, 128.1, 128.5, 128.0, 126.1, 126.1, 123.2, 123.0, 75.4, 59.6, 58.9, 52.2, 52.1, 31.1, 22.9. MS (ESI+) m/z: 490.0 (M+H)+.
(S)-3-Ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (4). Phosphorus pentachloride (1.66 g, 0.017 mol) was added to the solution of compound 12 (2.1 g, 0.006 mol) in dichloromethane (28 mL) below −20 °C, and stirred for 1 h. The reaction mixture was cooled to −30 °C, and was added ethanol (10 mL, 0.17 mol), and stirred for 4 h. The reaction mixture was poured into saturated sodium carbonate solution (200 mL), and extracted with dichloromethane (200 mL). The organic phase was washed with water (300 mL), dried over anhydrous magnesium sulfate and concentrated. The residue was purified by flash column chromatography on silica gel, eluting with ethyl acetate/petroleum ether, 3:2, to yield 4 as a light yellow solid (1.2 g, 55%). HPLC purity 99.7%. 1H-NMR (DMSO-d6) δ: 9.04 (s, 1H), 7.52-8.01 (m, 4H), 4.99 (s, 1H), 3.98–4.03 (m, 2H), 3.55 (s, 3H), 2.30 (s, 6H), 1.12–1.16 (m, 3H). 13C-NMR (DMSO-d6) δ: 167.7, 167.1, 150.7, 148.2, 147.2, 147.0, 134.7, 130.3, 122.3, 121.8, 101.7, 101.4, 59.9, 51.4, 41.0, 18.9 (2C), 14.7. MS (ESI−) m/z: 359.1 (M−H)−.
3-(2-Cyanoethyl) 5-ethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-pyridine-3,5-dicarboxylate (17) Ethyl 3-aminobut-2-enoate (16, 37.3 g, 0.29 mol) was added to the solution of compound 8 (85 g, 0.295 mol) in ethanol (216 mL), heated to reflux for 2 h. The reaction mixture was concentrated at 60 °C to about 100 mL, and cooled to 0–5 °C. The precipitate was collected by vacuum filtration and dried at 60 °C for 10 h to provide 17 (45.4 g, 39%). 1H-NMR (DMSO-d6) δ: 9.09 (s, 1H), 7.48–7.99 (m, 4H), 4.97(s, 1H), 4.09–4.13 (m, 2H), 3.93–4.0 (m, 2H), 2.77–2.83 (m, 2H), 2.27–2.30 (d, 6H), 1.10–1.15 (m, 3H). 13C-NMR (DMSO-d6) δ: 167.1, 166.6, 150.7(2C), 148.3, 146.8, 134.9, 130.3, 122.6, 121.8, 119.2, 102.2, 100.9, 59.9, 59.2, 41.0, 19.1, 18.8, 18.0, 14.7. MS (ESI−) m/z: 398.2 (M−H)−.
5-(Ethoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid (18) Compound 17 (45 g, 0.11 mol) was added to the mixture of 3.8% sodium hydroxide solution (350 g) and 1,2-dimethoxyethane (170 mL) at 30 °C, stirred for 2 h. The reaction mixture was diluted with water (115 mL), and extracted with dichloromethane (230 mL). The aqueous phase was acidized to pH 3.0 by 10% hydrochloric acid. The precipitate was filtered and washed with water, and dried at 90 °C for 2 h to afford 18 (31.8 g, 83.5%). 1H-NMR (DMSO-d6) δ:11.82 (s, 1H), 8.91 (s, 1H), 7.50–7.98 (m, 4H), 4.98 (s, 1H), 3.97–4.01 (m, 2H), 2.28 (s, 6H), 1.11–1.15 (m, 3H). 13C-NMR (DMSO-d6) δ: 169.1, 167.2, 150.9, 148.1, 147.2, 146.4, 134.8, 130.2, 122.4, 121.7, 102.3, 101.3, 59.8, 41.0, 18.9(2C), 14.7. MS (ESI−) m/z: 345.1 (M−H)−.
(R)-5-(Ethoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid (19) Cinchonine (29.4 g, 0.10 mol) was added to the solution of compound 18 (31.0 g, 0.09 mol) in N,N-dimethylformamide (5.7 mL), and heated 80 °C to get a clear solution. Water (38.4 mL) was added, and heated to 120 °C. The solution was cooled slowly to 20 °C and stirred for 12 h. The precipitate was filtered and washed with N,N-dimethylformamide/water (3:2) solution (12 mL). The filter mass was dissolved in 35% sodium hydroxide solution (92 mL), extracted with dichloromethane (80 mL). The aqueous phase was acidized to pH 2.0 with concentrated hydrochloric acid. The precipitate was collected and washed with water, and dried at 100 °C for 10 h to provide 19 (10.4 g, 33%). 1H-NMR (DMSO-d6) δ: 11.82 (s, 1H), 8.91 (s, 1H), 7.50–7.99 (m, 4H), 4.97 (s, 1H), 3.96–4.00 (m, 2H), 2.27 (s, 6H), 1.10–1.15 (m, 3H); 13C-NMR (DMSO-d6) δ: 169.1, 167.2, 150.9, 148.1, 147.2, 146.4, 134.8, 130.2, 122.4, 121.7, 102.3, 101.3, 59.8, 41.0, 18.9 (2C), 14.8. MS (ESI−) m/z: 345.1 (M−H)−.
(3'S,4S)-1-Benzyl-3-pyrrolidinyl ethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridine-dicarboxylate (5). Phosphorus pentachloride (3.5 g, 0.036 mol) was added to the solution of compound 19 (4.8 g, 0.014 mol) in dichloromethane (70 mL) below 0 °C, and stirred for 1 h. The reaction mixture was cooled to −20 °C, and was added the solution of (S)-1-benzylpyrrolidin-3-ol (14, 2.5 g, 0.014 mol) in dichloromethane (70 mL), and stirred for 2 h. The reaction mixture was poured into saturated sodium carbonate solution (275 mL), and extracted with dichloromethane (275 mL). The organic phase was washed with water (550 mL), dried over anhydrous magnesium sulfate and concentrated. The residue was purified by flash column chromatography on silica gel, eluting with ethyl acetate/petroleum ether, 3:2, to yield 5 as a light yellow solid (2.7 g, 38%). HPLC purity 98.2%. 1H-NMR (CDCl3) δ: 7.23–8.13 (m, 9H), 6.58 (br, 1H), 5.07–5.11 (m, 2H), 4.05–4.10 (m, 2H), 3.60–3.66 (m, 2H), 2.80–2.83 (m, 1H), 2.61–2.63 (m, 2H), 2.42–2.44 (m, 1H), 2.30 (s, 6H), 2.06–2.17 (m, 1H), 1.52–1.71 (m, 1H), 1.19–1.25 (m, 3H). 13C-NMR (CDCl3) δ: 167.1, 166.8, 150.0, 148.0, 145.2, 144.9, 138.7, 134.5, 128.5, 128.4, 128.1, 126.8, 122.9, 121.1, 103.0, 102.8, 77.0, 73.6, 60.0, 59.8, 52.4, 39.9, 31.7, 19.1, 14.1. MS (ESI−) m/z: 504.2 (M−H)−.
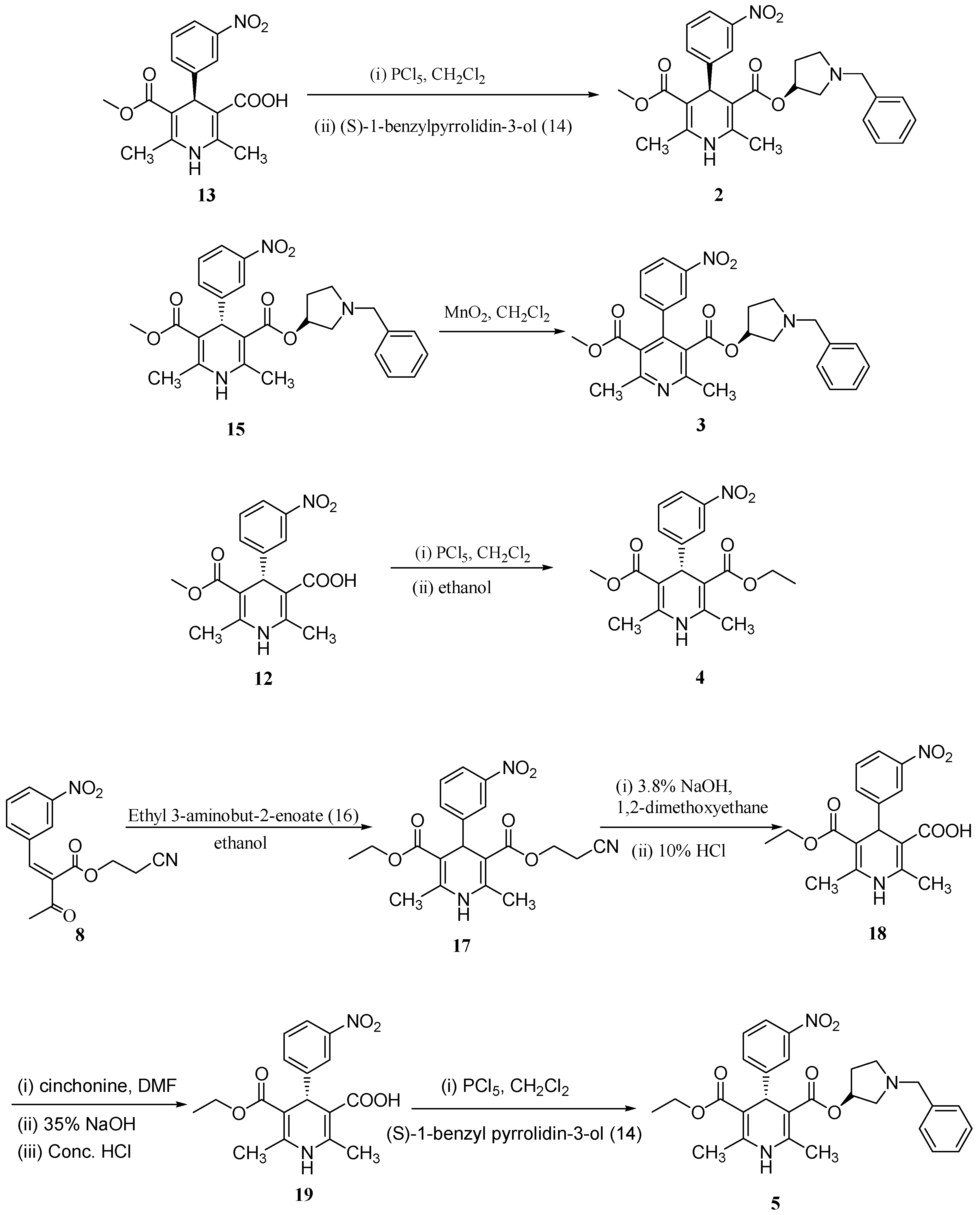
 −25.9 (c 0.005 g/mL, acetone)) was obtained in 75.3% yield by resolution of compound 11 using cinchonine as the resolving agent. Condensation of compound 12 with (S)-1-benzylpyrrolidin-3-ol (14), and subsequent salt formation of the resulting barnidipine (15), provided the desired barnidipine hydrochloride (1, optical purity 99.8%) in 65.5% yield (
−25.9 (c 0.005 g/mL, acetone)) was obtained in 75.3% yield by resolution of compound 11 using cinchonine as the resolving agent. Condensation of compound 12 with (S)-1-benzylpyrrolidin-3-ol (14), and subsequent salt formation of the resulting barnidipine (15), provided the desired barnidipine hydrochloride (1, optical purity 99.8%) in 65.5% yield (  +116.5 (c 0.01 g/mL, MeOH)).
+116.5 (c 0.01 g/mL, MeOH)).