Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

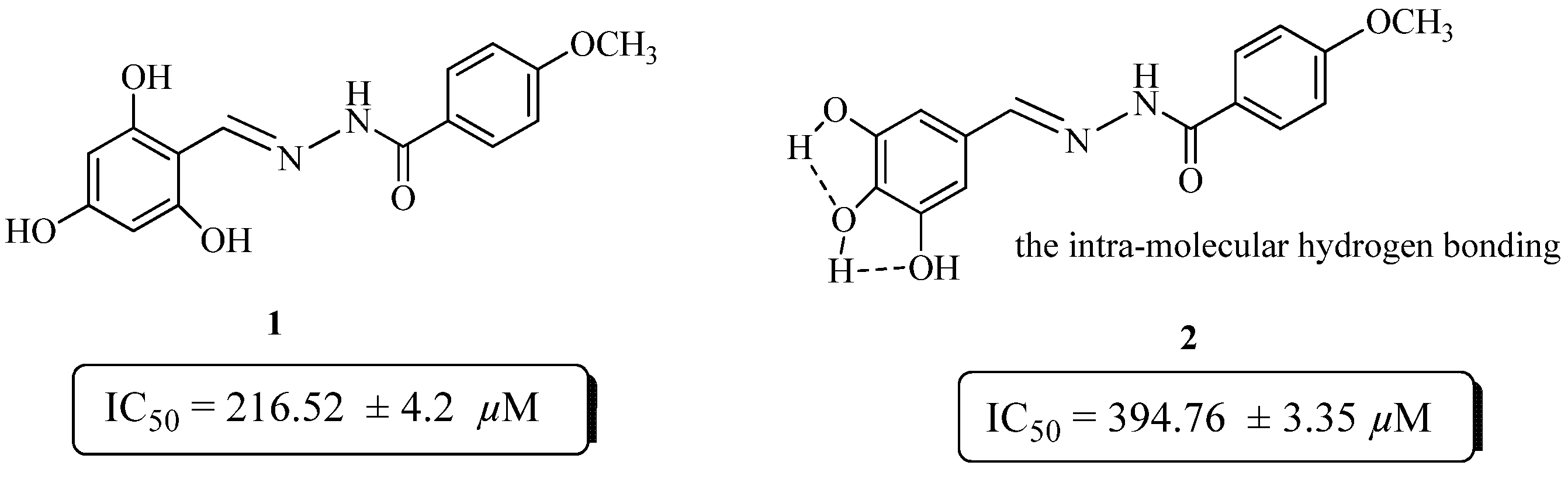
2.2. Antiglycation Activity
Structure Activity Relationship
| Compounds | IC50 (µM ± SEM a) | Compounds | IC50 (µM ± SEM a) |
|---|---|---|---|
| 1 | 216.52 ± 4.2 | 16 | NA b |
| 2 | 394.76 ± 3.35 | 17 | 474.97 ± 19.14 |
| 3 | 289.58 ± 2.64 | 18 | 718.96 ± 10.7 |
| 4 | 307.1 ± 6.08 | 19 | NA b |
| 5 | 420.40 ± 3.3 | 20 | NA b |
| 6 | 227.75 ± 0.53 | 21 | NA b |
| 7 | 242.53 ± 6.1 | 22 | NA b |
| 8 | 347.62 ± 5.8 | 23 | NA b |
| 9 | NA b | 24 | NA b |
| 10 | 657.75 ± 14.0 | 25 | NA b |
| 11 | 287.79 ± 1.59 | 26 | NA b |
| 12 | 399.90 ± 7.9 | 27 | NA b |
| 13 | NA b | 28 | NA b |
| 14 | 649.18 ± 18.5 | 29 | NA b |
| 15 | 748.71 ± 7.8 | 30 | NA b |
| Standard Rutin c | 294.5 ± 1.50 | ||
3. Experimental
3.1. General Information
3.2. Experimental Protocol
3.2.1. Synthesis of 4-Methoxybenzohydrazide
3.2.2. General Procedure for the Synthesis of 4-Methoxybenzohydrazone Derivatives
3.2.3. Protocol for Antiglycation Activity
3.3. Software/Statistical
4. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
References
- Cimerman, Z.; Miljanić, S.; Galic, N. Schiff Bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croat. Chem. Acta 2000, 73, 81–95. [Google Scholar]
- Musharraf, S.G.; Bibi, A.; Shahid, N.; Najam-ul-Haq, M.; Khan, M.; Taha, M.; Mughal, U.R.; Khan, K. Acylhydrazide and isatin Schiff bases as alternate UV-laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis. Am. J. Anal. Chem. 2012, 3, 779–789. [Google Scholar] [CrossRef]
- Tarafder, M.T.; Kasbollah, A.; Saravan, N.; Crouse, K.A.; Ali, A.M.; Tin, O.K. S-methyldithiocarbazate and its Schiff bases: Evaluation of bondings and biological properties. J. Biochem. Mol. Biol. Biophys. 2002, 6, 85–91. [Google Scholar] [CrossRef]
- Kabak, M.; Elmali, A.; Elerman, Y. Keto-enol tautomerism, conformations and structure of N-(2-hydroxy-5-methylphenyl), 2-hydroxybenzaldehyde-imine. J. Mol. Struct. 1999, 477, 151–158. [Google Scholar] [CrossRef]
- Küçükgüzel, I.; Küçükgüzel, Ş.G.; Rollas, S.; Otuk-Saniş, G.; Özdemir, O.; Bayrak, İ.; Altuğ, T.; Stables, J.P. Synthesis of some 3-(arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity. Il Farmaco 2004, 59, 839–891. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; de Clercq, E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm. Acta Helv. 1999, 74, 11–17. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Loncle, C.; Brunel, J.M.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Öztürk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef]
- Todeschini, A.R.; de Miranda, A.L.; Silva, C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and evaluation of analgesic, anti-inflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998, 33, 189–199. [Google Scholar] [CrossRef]
- Melnyk, P.; Leroux, V.; Sergheraert, C.; Grellier, P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg. Med. Chem. Lett. 2006, 16, 31–35. [Google Scholar] [CrossRef]
- Leite, L.F.C.C.; Ramos, M.N.; Da Silva, J.B.P.; Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic profile of novel N-containing heterocycle derivatives: Arylidene 3-phenyl-1,2,4-oxadiazole-5-carbohydrazide. Il Farmaco 1999, 54, 747–757. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; Da Silva, K.C.; Léda, P.H.; de Miranda, A.L.; Fraga, C.A.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Cunha, A.C.; Figueiredo, J.M.; Tributino, J.L.M.; Miranda, A.L.P.; Castro, H.C.; Zingali, R.B.; Fraga, C.A.M.; de Souza, M.C.B.V.; Ferreira, V.F.; Barreiro, E.J. Antiplatelet properties of novel N-substituted-phenyl-1,2,3-triazole-4-acylhydrazone derivatives. Bioorg. Med. Chem. 2003, 11, 2051–2059. [Google Scholar] [CrossRef]
- Kaymakçıoğlu, K.B.; Oruç, E.E.; Unsalan, S.; Kandemirli, F.; Shvets, N.; Rollas, S.; Anatholy, D. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef]
- Terzioglu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b]-[1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Sawada, Y.; Yanai, T.; Nakagawa, H.; Tsukamoto, Y.; Tamagawa, Y.; Yokoi, S.; Yanagi, M.; Toya, T.; Sugizaki, H.; Kato, Y.; et al. Synthesis and insecticidal activity of benzoheterocyclic analogues of N'-benzoyl-N-(tert-butyl)benzohydrazide: Part 1. Design of benzoheterocyclic analogues. Pest Manag. Sci. 2003, 59, 25–35. [Google Scholar] [CrossRef]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Khan, K.M.; Jaafar, F.M.; Samreen; Siddiqui, S.; Choudhary, M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013, 23, 3463–3466. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Rollas, S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Il Farmaco 2002, 57, 583–587. [Google Scholar] [CrossRef]
- Greenfield, R.S.; Kaneko, T.; Daues, A.; Edson, M.A.; Fitzgerald, K.A.; Olech, L.J.; Grattan, J.A.; Spitalny, G.L.; Braslawsky, G.R. Evaluation in vitro of adriamycin immunoconjugates synthesized using an acid-sensitive hydrazone linker. Cancer Res. 1990, 50, 6600–6007. [Google Scholar]
- Caffery, C.R.; Schanz, M.; Nkemgu-Njinkeng, J.; Brush, M.; Hansell, E.; Cohen, F.E.; Flaherty, T.M.; Mckerrow, J.H.; Steverding, D. Screening of acyl hydrazide proteinase inhibitors for antiparasitic activity against Trypanosoma brucei. Int. J. Anitimicrob. Agents 2002, 19, 227–231. [Google Scholar] [CrossRef]
- Khan, K.M.; Shujaat, S.; Rahat, S.; Hayat, S.; Atta-ur-Rahman; Choudhary, M.I. β-N-Cyanoethyl acyl hydrazide derivatives: A new class of β-glucuronidase inhibitors. Chem. Pharm. Bull. Jpn. 2002, 50, 1443–1446. [Google Scholar] [CrossRef]
- Mansour, A.K.; Eid, M.M.; Khalil, N.S.A.M. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- Patole, J.; Sandbhor, U.; Padhye, S.; Deobagkar, D.N.; Anson, C.E.; Powell, A. Structural chemistry and in vitro antitubercular activity of acetylpyridine benzoyl hydrazone and its copper complex against Mycobacterium smegmatis. Bioorg. Med. Chem. Lett. 2003, 13, 51–55. [Google Scholar] [CrossRef]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation end products-role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Brownlee, M. Glycation and complications Diabetes. Diabetes 1994, 43, 836–841. [Google Scholar] [CrossRef]
- Peppa, M.; Uribarri, J.; Vlassara, H. Glucose, advanced glycation end products, and diabetes complications: What is new and what works. Clin. Diabetes 2003, 21, 186–187. [Google Scholar] [CrossRef]
- Monnier, V.M. Intervention against the Maillard reaction in vivo. Arch. Biochem. Biophys. 2003, 419, 1–15. [Google Scholar] [CrossRef]
- Vasan, S.; Foiles, P.; Founds, H. Therapeutic potential of breakers of advanced glycation end product–protein crosslinks. Arch. Biochem. Biophys. 2003, 419, 89–96. [Google Scholar] [CrossRef]
- Hunt, J.V.; Bottoms, M.A.; Mitchinson, M.J. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem. J. 1993, 291, 529–535. [Google Scholar]
- Ahmed, M.S.; Ahmed, N. Antiglycation properties of aged garlic extract: Possible role in prevention of diabetic complications. Am. Soc. Nutr. 2006, 136, 796–799. [Google Scholar]
- Gugliucci, A. Glycation as the glucose link to diabetic complications. J. Am. Osteopath. Assoc. 2000, 100, 621–634. [Google Scholar]
- Gugliucci, A.; Menini, T. The polyamines spermine and spermidine protect proteins from structural and functional damage by AGE precursors: A new role for old molecules. Life Sci. 2003, 72, 2603–2616. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Adhikari, A.; Rasheed, S.; Marasini, B.P.; Hussain, N.; Kaleem, W.A.; Atta-ur-Rahman. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of α-glucosidase enzyme and protein glycation. Phytochem. Lett. 2011, 4, 404–406. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Ali, M.; Wahab, A.; Khan, A.; Rasheed, S.; Shyaula, S.L.; Rahman, A.U. New antiglycation and enzyme inhibitors from Parmotrema cooperi. Sci. China Chem. 2011, 54, 1926–1931. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Choudhary, M.I.; Basha, F.Z.; Abbas, G.; Khan, S.N.; Shah, S.A.A. Science at the interface of chemistry and biology: Discoveries of α-glucosidase inhibitors and antiglycation agents. Pure Appl. Chem. 2007, 79, 2263–2268. [Google Scholar] [CrossRef]
- Ayatollahi, S.A.M.; Kobarfard, F.; Asgarpanah, J.; Choudhary, M.I. Antiglycation activity of Otostegia persica (Burm) Boiss. Afr. J. Biotechnol. 2010, 9, 3645–3648. [Google Scholar]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide schiff bases: Synthesis and antiglycation activity. J. Pak. Chem. Soc. 2013, 35, 929–937. [Google Scholar]
- Khan, K.M.; Rahim, F.; Ambreen, N.; Taha, M.; Khan, M.; Jahan, H.; Najeebullah, U.; Shaikh, A.; Iqbal, S.; Perveen, S.; et al. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. [Google Scholar] [CrossRef]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Rahim, F.; Jahun, H.; Perveen, S.; Choudhary, M.I. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med. Chem. 2011, 7, 572–580. [Google Scholar] [CrossRef]
- Khan, K.M.; Khan, M.; Ambreen, N.; Taha, M.; Rahim, F.; Rasheed, S.; Saied, S.; Shafi, H.; Perveen, S.; Choudhary, M.I. Oxindole derivatives: Synthesis and antiglycation activity. Med. Chem. 2013, 9, 681–688. [Google Scholar] [CrossRef]
- Khan, K.M.; Khan, M.; Ali, M.; Taha, M.; Rasheed, S.; Perveen, S.; Choudhary, M.I. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg. Med. Chem. 2009, 17, 7795–7801. [Google Scholar] [CrossRef]
- Zeb, A.; Malik, I.; Rasheed, S.; Choudhary, M.I.; Basha, F.Z. Metronidazole esters: A new class of antiglycation agents. Med. Chem. 2012, 8, 846–852. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jaafar, F.M.; Aziz, A.N.; Yousuf, S. (E)-N'-(3,4Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide monohydrate. Acta Cryst. 2013, E69, o490. [Google Scholar]
- Baharudin, M.S.; Taha, M.; Ismail, N.H.; Shah, S.A.A. (E)-N'-(4-Chlorobenzylidene)-2-methoxybenzohydrazide. Acta Cryst. 2013, E69, o276. [Google Scholar]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. (E)-2-Methoxy-N'-(2,4,6-trihydroxybenzylidene)Benzohydrazide. Acta Cryst. 2013, E69, o277. [Google Scholar]
- Taha, M.; Ismail, N.H.; Jaafar, F.M.; Khan, K.M.; Yousuf, S. (E)-2,4-Dimethyl-N'-(2-methylbenzylidene) benzohydrazide. Acta Cryst. 2013, E69, o400. [Google Scholar]
- Baharudin, M.S.; Taha, M.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. N-[(E)-2-Hydroxy-5-methoxybenzylidene]-2-methoxybenzohydra-zide. Acta Cryst. 2012, E68, o3255. [Google Scholar]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. N'-[(E)-2,3-Dihydroxybenzylidene]-2-methoxybenzohydrazide. Acta Cryst. 2012, E68, o3256. [Google Scholar]
- Taha, M.; Naz, H.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. (E)-4-Methoxy-N'-[(pyridin-4-yl)methylidene] benzohydrazide monohydrate. Acta Cryst. 2012, E68, o2778. [Google Scholar]
- Taha, M.; Naz, H.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. (E)-N-(3,4-Dimethoxybenzylidene)-4-methoxybenzohydrazide. Acta Cryst. 2012, E68, o2780. [Google Scholar]
- Naz, H.; Taha, M.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. Methyl (E)-3,5-dimethoxy-2-{[2-(4-methoxybenzoyl)hydrazin-1-ylidene]- methyl}benzoate. Acta Cryst. 2012, E68, o2671. [Google Scholar]
- Taha, M.; Naz, H.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. (E)-4-Methoxy-N'-(3,4,5-trihydroxybenzylidene)benzohydrazide methanol monosolvate. Acta Cryst. 2012, E68, o2846. [Google Scholar]
- Cho, S.J.; Roman, G.; Yeboah, F.; Konishi, Y. The road to advanced glycation end products: A mechanistic perspective. Curr. Med. Chem. 2007, 14, 1653–1671. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Taha, M.; Naz, H.; Rasheed, S.; Ismail, N.H.; Rahman, A.A.; Yousuf, S.; Choudhary, M.I. Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity. Molecules 2014, 19, 1286-1301. https://doi.org/10.3390/molecules19011286
Taha M, Naz H, Rasheed S, Ismail NH, Rahman AA, Yousuf S, Choudhary MI. Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity. Molecules. 2014; 19(1):1286-1301. https://doi.org/10.3390/molecules19011286
Chicago/Turabian StyleTaha, Muhammad, Humera Naz, Saima Rasheed, Nor Hadiani Ismail, Aqilah Abd Rahman, Sammer Yousuf, and Muhammad Iqbal Choudhary. 2014. "Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity" Molecules 19, no. 1: 1286-1301. https://doi.org/10.3390/molecules19011286
APA StyleTaha, M., Naz, H., Rasheed, S., Ismail, N. H., Rahman, A. A., Yousuf, S., & Choudhary, M. I. (2014). Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity. Molecules, 19(1), 1286-1301. https://doi.org/10.3390/molecules19011286





