Dillapiole, Isolated from Peperomia pellucida, Shows Gastroprotector Activity against Ethanol-Induced Gastric Lesions in Wistar Rats
Abstract
:1. Introduction
2. Results and Discussion
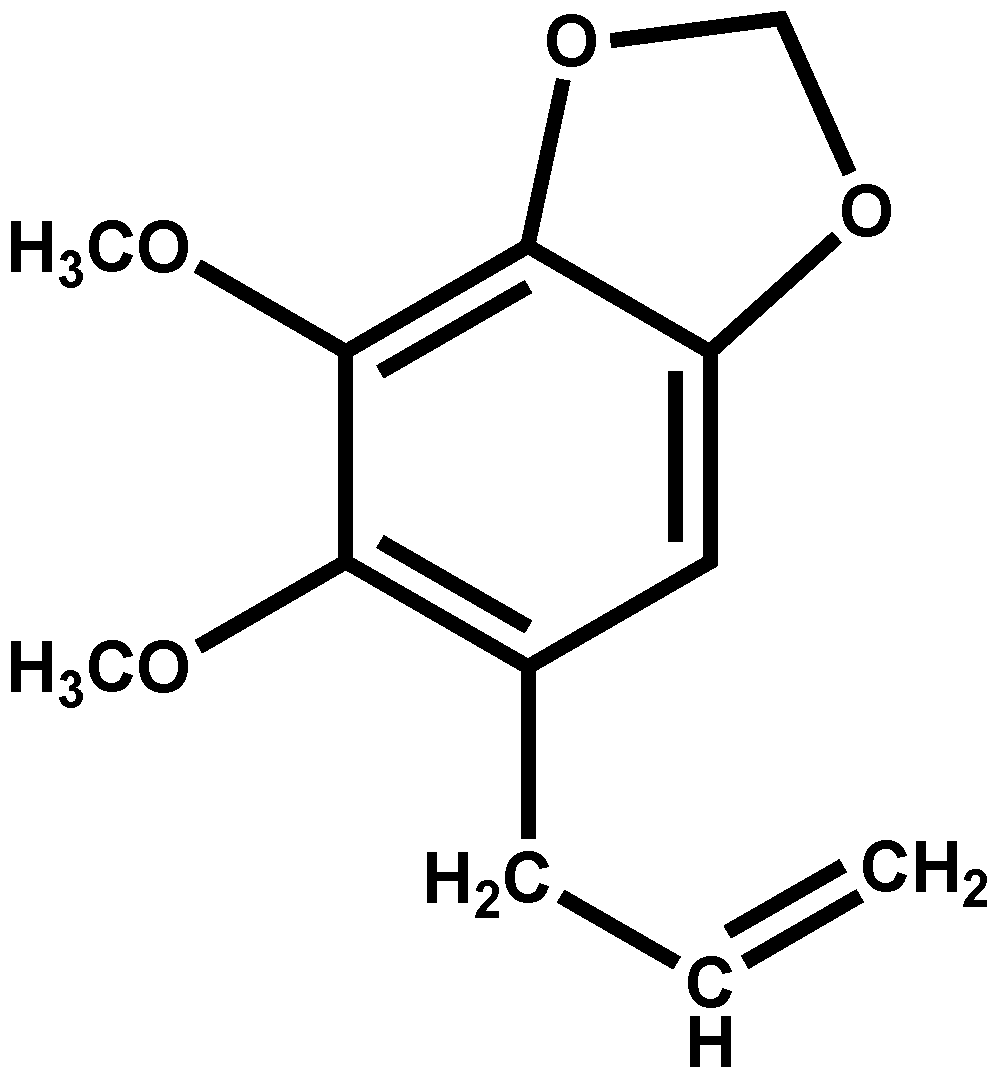
2.1. Bioassay-Guided Fractionation and Isolation of Dillapiole
| Treatment | Dose (mg/kg) | n | UI (mm2) | Gastroprotection (%) |
|---|---|---|---|---|
| Control | --- | 8 | 85.4 ± 9.4 | --- |
| Hexane extract | 30 | 8 | 39.1 ± 5.1 * | 54.1 ± 6.1 |
| 100 | 8 | 36.7 ± 3.2 * | 56.9 ± 3.7 | |
| Dichloromethane extract | 30 | 8 | 34.7 ± 5.2 * | 59.2 ± 6.1 |
| 100 | 8 | 15.0 ± 4.8 * | 82.3 ± 5.6 | |
| Methanol extract | 30 | 8 | 69.9 ± 7.9 | 18.0 ± 7.5 |
| 100 | 8 | 81.0 ± 6.2 | 5.1 ± 5.1 | |
| Carbenoxolone | 100 | 8 | 21.8 ± 3.9 * | 72.0 ± 5.0 |
| Treatment | Dose (mg/kg) | n | UI (mm2) | Gastroprotection (%) |
|---|---|---|---|---|
| Control | --- | 8 | 72.0 ± 12.4 | --- |
| F1 | 100 | 8 | 9.7 ± 4.3 * | 86.4 ± 6.0 |
| F2 | 100 | 8 | 36.5 ± 6.7 * | 49.3 ± 9.3 |
| F3 | 100 | 8 | 47.4 ± 7.5 * | 48.0 ± 11.2 |
| F4 | 100 | 8 | 27.6 ± 7.2* | 61.6 ± 9.6 |
| F5 | 100 | 8 | 42.5 ± 7.2 | 40.0 ± 10.0 |
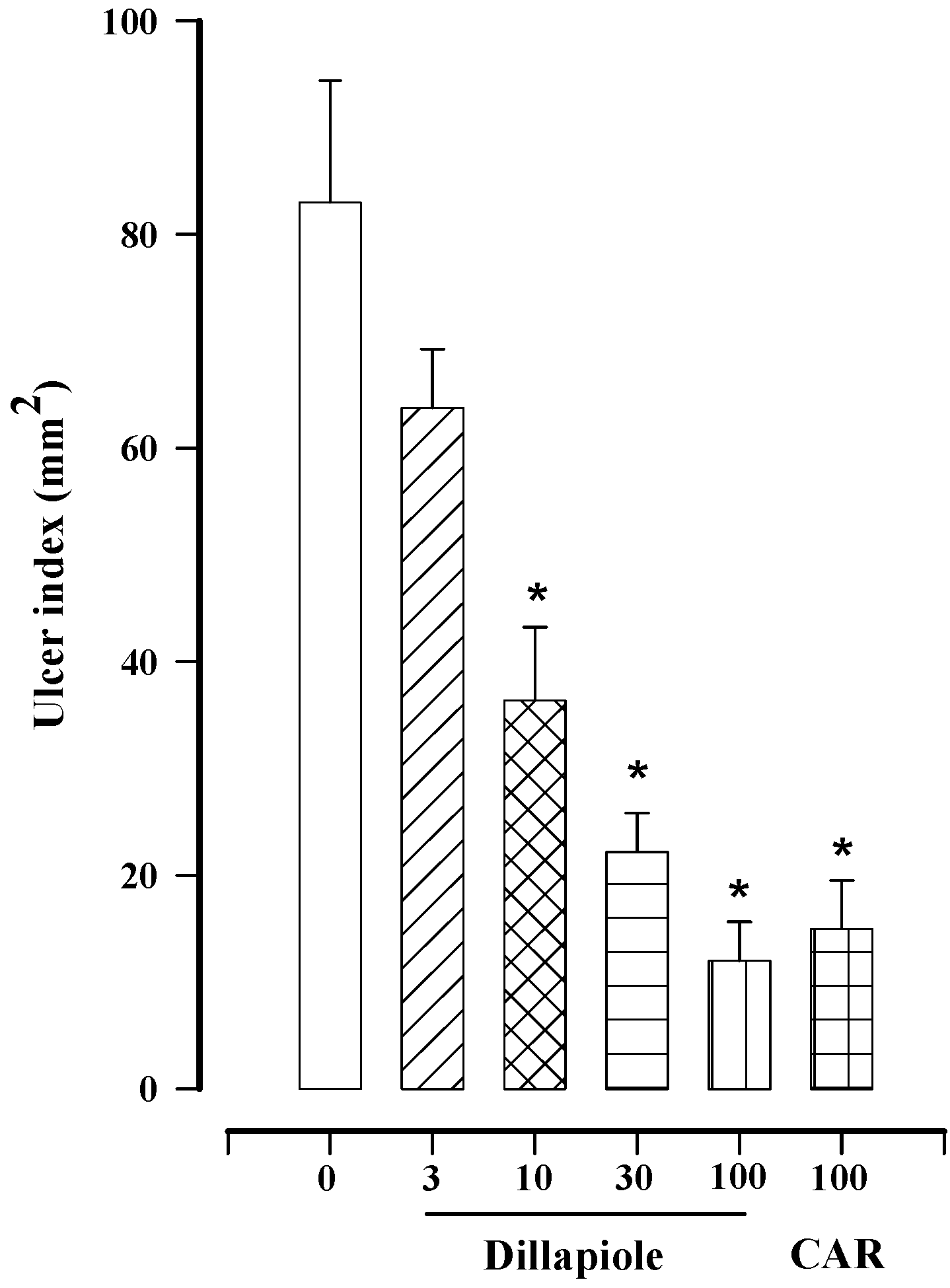
2.2. Effect of Indomethacin, L-NAME, and NEM on the Gastroprotection of Dillapiole
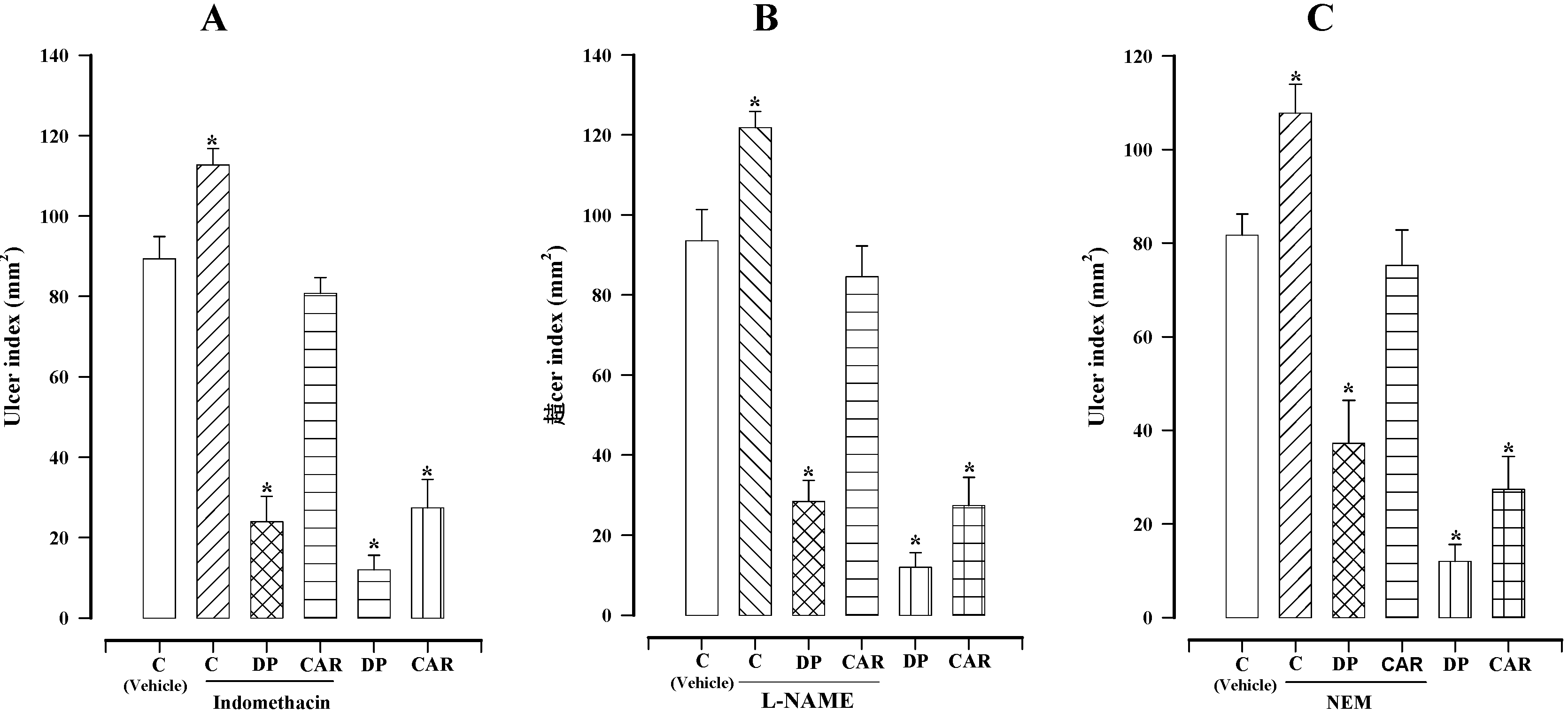
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Preliminary Fraction
3.4. Animals
3.5. Drugs and Dosage
3.6. Acute Gastric Ulcer Induced by Absolute Ethanol
3.7. Ethanol-Induced Gastric Mucosal Lesions in Indomethacin Pretreated Rats
3.8.Ethanol-Induced Gastric Mucosal Lesions in L-NAME Pretreated Rats
3.9. Ethanol-Induced Gastric Mucosal Lesions in NEM Pretreated Rats
3.10. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Klopell, F.C.; Lemos, M.; Sousa, J.P.B.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; Andrade, S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z. Naturforsch. C 2007, 62, 537–542. [Google Scholar]
- Barros, M.P.; Lemos, M.; Maistro, E.L.; Leite, M.F.; Sousa, J.P.; Bastos, J.K.; Andrade, S.F. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J. Ethnopharmacol. 2008, 120, 372–377. [Google Scholar] [CrossRef]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; Portella, R.de L.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.; da Luz, S.C.; Boligon, A.A.; et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- Ineu, R.P.; Pereira, M.E.; Aschner, M.; Nogueira, C.W.; Zeni, G.; Rocha, J.B. Diphenyl diselenide reverses gastric lesions in rats: Involvement of oxidative stress. Food Chem.Toxicol. 2008, 46, 3023–3029. [Google Scholar]
- Sánchez-Mendoza, M.E.; Reyes-Ramírez, A.; Cruz Antonio, L.; Martínez-Jiménez, L.; Rodríguez-Silverio, J.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer compound, tagitinin C, from Tithonia diversifolia: Role of nitric oxide, prostaglandins and sulfhydryls. Molecules 2011, 16, 665–674. [Google Scholar] [CrossRef]
- Loc, N.H.; Bach, N.H.; Kim, T.G.; Yang, M.S. Tissue culture and expression of Escherichia coli heat-labile enterotoxin B subunit in transgenic Peperomia pellucida. Protein Expr.Purif. 2010, 72, 82–86. [Google Scholar] [CrossRef]
- de Fátima Arrigoni-Blank, M.; Dmitrieva, E.G.; Franzotti, E.M.; Antoniolli, A.R.; Andrade, M.R.; Marchioro, M. Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). J. Ethnopharmacol. 2004, 91, 215–218. [Google Scholar] [CrossRef]
- Mutee, A.F.; Salhimi, S.M.; Yam, M.F.; Lim, C.P.; Abdullah, G.Z.; Ameer, O.Z.; Abdulkarim, M.F.; Asmawi, M.Z. In vivo anti-inflammatory and in vitro antioxidant activities of Peperomia pellucida. Int. J. Pharmacol. 2010, 6, 686–690. [Google Scholar] [CrossRef]
- Abdul Hamid, R.; Zakaria, N.; Zuraini, A. Anti-ulcer activity of aqueous etanol extract of Peperomia pellucida in Spregue dawley rats. Planta Med. 2007, 73, 455. [Google Scholar]
- Cicció-Alberti, J.F.; Ballestero, C.M. Constituyentes volátiles de las hojas y espigas de Piper aduncum (Piperaceae) de Costa Rica. Rev. Biol. Trop. 1997, 45, 783–790. [Google Scholar]
- Bayma, J.C.; Arruda, M.S.; Müller, A.H.; Arruda, A.C.; Canto, W.C. A dimeric ArC2 compound from Peperomia pellucida. Phytochemistry 2000, 55, 779–782. [Google Scholar] [CrossRef]
- Belzile, A.S.; Majerus, S.L.; Podeszfinski, C.; Guillet, G.; Durst, T.; Arnason, J.T. Dillapiol derivatives as synergists: Structure-activity relationship analysis. Pestic. Biochem. Physiol. 2000, 66, 33–36. [Google Scholar] [CrossRef]
- Maxia, A.; Falconieri, D.; Piras, A.; Porcedda, S.; Marongiu, B.; Frau, M.A.; Goncalves, M.J.; Cabral, C.; Cavaleiro, C.; Salgueiro, L. Chemical composition and antifungal activity of essential oils and supercritical CO2 extracts of Apium nodiflorum (L.) Lag. Mycopathologia 2012, 174, 61–67. [Google Scholar] [CrossRef]
- Parise-Filho, R.; Pastrello, M.; Pereira Camerlingo, C.E.; Silva, G.J.; Agostinho, L.; de Souza, T.; Motter Magri, F.M.; Ribeiro, R.R.; Brandt, C.A.; Polli, M.C. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm. Biol. 2011, 49, 1173–1179. [Google Scholar] [CrossRef]
- Arai, I.; Hamasaka, Y.; Futaki, N.; Takahashi, S.; Yoshikawa, K.; Higuchi, S.; Otomo, S. Effect of NS-398, a new nonsteroidal anti-inflammatory agent, on gastric ulceration and acid secretion in rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 8, 259–270. [Google Scholar]
- Kargman, S.; Charleson, S.; Cartwright, M. Characterization of prostaglandin GrH synthase 1 and 2 in rat, dog, monkey and human gastrointestinal tracts. Gastroenterology 1996, 3, 445–454. [Google Scholar]
- Schmassmann, A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 43–51. [Google Scholar] [CrossRef]
- Cryer, B.; Feldman, M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 413–421. [Google Scholar] [CrossRef]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Wallace, J.L. Mechanisms of protection and healing: current knowledge and future research. Am. J. Med. 2001, 110, S19–S23. [Google Scholar] [CrossRef]
- Beserra, A.M.; Calegari, P.I.; Souza Mdo, C.; Dos Santos, R.A.; Lima, J.C.; Silva, R.M.; Balogun, SO.; Martins, D.T. Gastroprotective and ulcer-healing mechanisms of ellagic acid in experimental rats. J. Agric. Food Chem. 2011, 59, 6957–6965. [Google Scholar] [CrossRef]
- Vera-Arzave, C.; Cruz-Antonio, L.; Arrieta, J.; Cruz-Hernández, G.; Velázquez-Méndez, A.M.; Reyes-Ramírez, A.; Sánchez-Mendoza, M.E. Gastroprotection of suaveolol, isolated from Hyptis suaveolens, against ethanol-induced gastric lesions in Wistar rats: role of prostaglandins, nitric oxide and sulfhydryls. Molecules 2012, 17, 8917–8927. [Google Scholar] [CrossRef]
- Ancha, H.; Ojeas, H.; Tedesco, D.; Ward, A.; Harty, R.F. Somatostatin-induced gastric protection against ethanol: Involvement of nitric oxide and effects on gastric mucosal blood flow. Regul. Pept. 2003, 110, 107–113. [Google Scholar] [CrossRef]
- Chandranath, S.I.; Bastaki, S.M.; Singh, J. A comparative study on the activity of lansoprazole, omeprazole and PD- 136450 on acidified ethanol- and indomethacin-induced gastric lesions in the rat. Clin. Exp. Pharmacol. Physiol. 2002, 29, 173–180. [Google Scholar] [CrossRef]
- Avila, J.R.; de la Lastra, C.A.; Martin, M.J.; Motilva, V.; Luque, I.; Delgado, D.; Esteban, J.; Herrerias, J. Role of endogenous sulphydryls and neutrophil infiltration in the pathogenesis of gastric mucosal injury induced by piroxicam in rats. Inflamm. Res. 1996, 45, 83–88. [Google Scholar] [CrossRef]
- Sample Availability: A sample of dillapiole is available upon request.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rojas-Martínez, R.; Arrieta, J.; Cruz-Antonio, L.; Arrieta-Baez, D.; Velázquez-Méndez, A.M.; Sánchez-Mendoza, M.E. Dillapiole, Isolated from Peperomia pellucida, Shows Gastroprotector Activity against Ethanol-Induced Gastric Lesions in Wistar Rats. Molecules 2013, 18, 11327-11337. https://doi.org/10.3390/molecules180911327
Rojas-Martínez R, Arrieta J, Cruz-Antonio L, Arrieta-Baez D, Velázquez-Méndez AM, Sánchez-Mendoza ME. Dillapiole, Isolated from Peperomia pellucida, Shows Gastroprotector Activity against Ethanol-Induced Gastric Lesions in Wistar Rats. Molecules. 2013; 18(9):11327-11337. https://doi.org/10.3390/molecules180911327
Chicago/Turabian StyleRojas-Martínez, Raúl, Jesús Arrieta, Leticia Cruz-Antonio, Daniel Arrieta-Baez, Antonio Magdiel Velázquez-Méndez, and María Elena Sánchez-Mendoza. 2013. "Dillapiole, Isolated from Peperomia pellucida, Shows Gastroprotector Activity against Ethanol-Induced Gastric Lesions in Wistar Rats" Molecules 18, no. 9: 11327-11337. https://doi.org/10.3390/molecules180911327





