In Vivo and in Vitro Effects of Secondary Metabolites against Xanthomonas campestris pv. campestris
Abstract
:1. Introduction
2. Results
2.1. In Vitro Effect of Gluconapin and Its Isothiocyanate
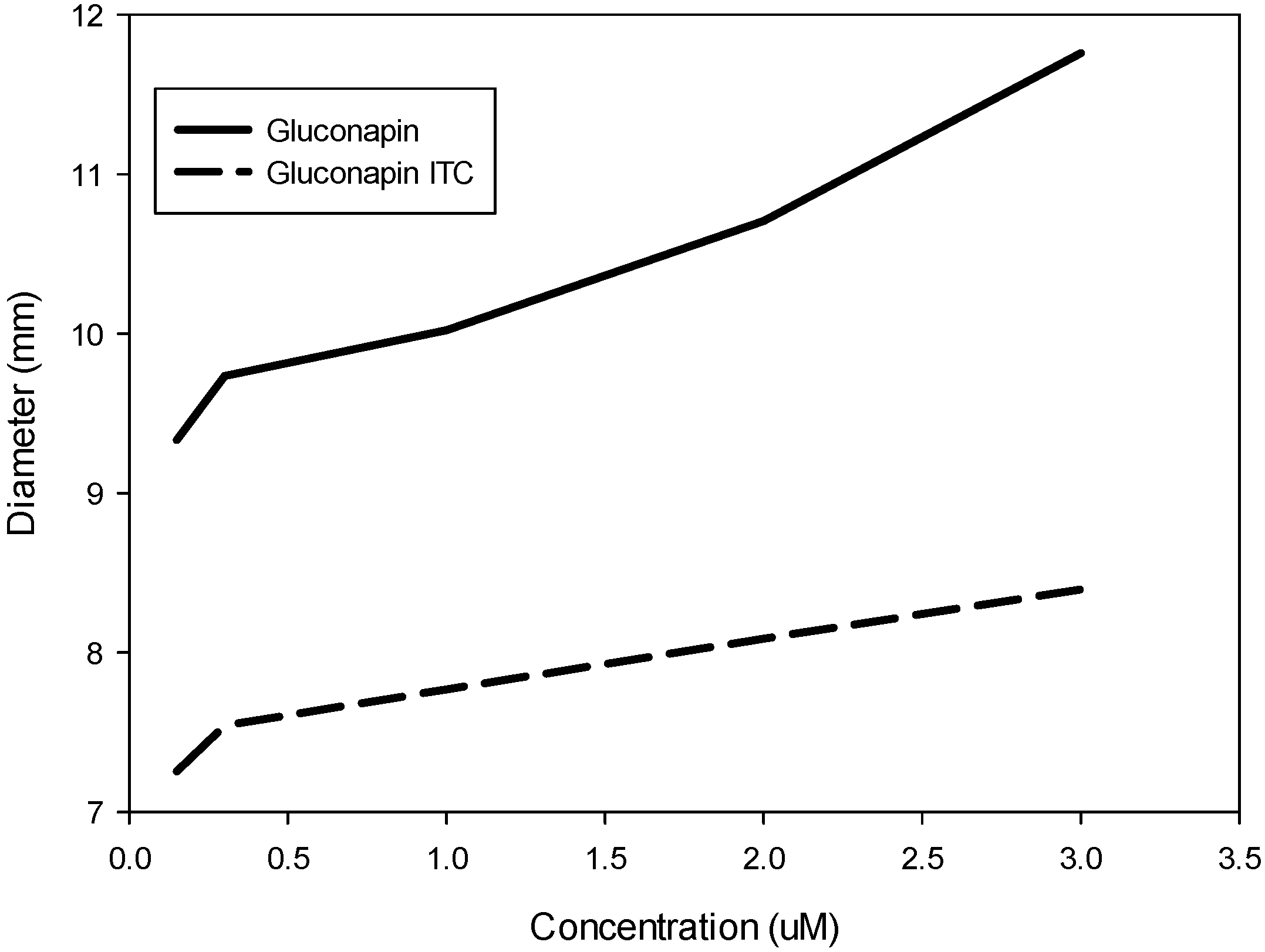
2.2. In Vitro Effect of Glucosinolates and Phenolic Compounds in Methanolic Extracts from Leaves
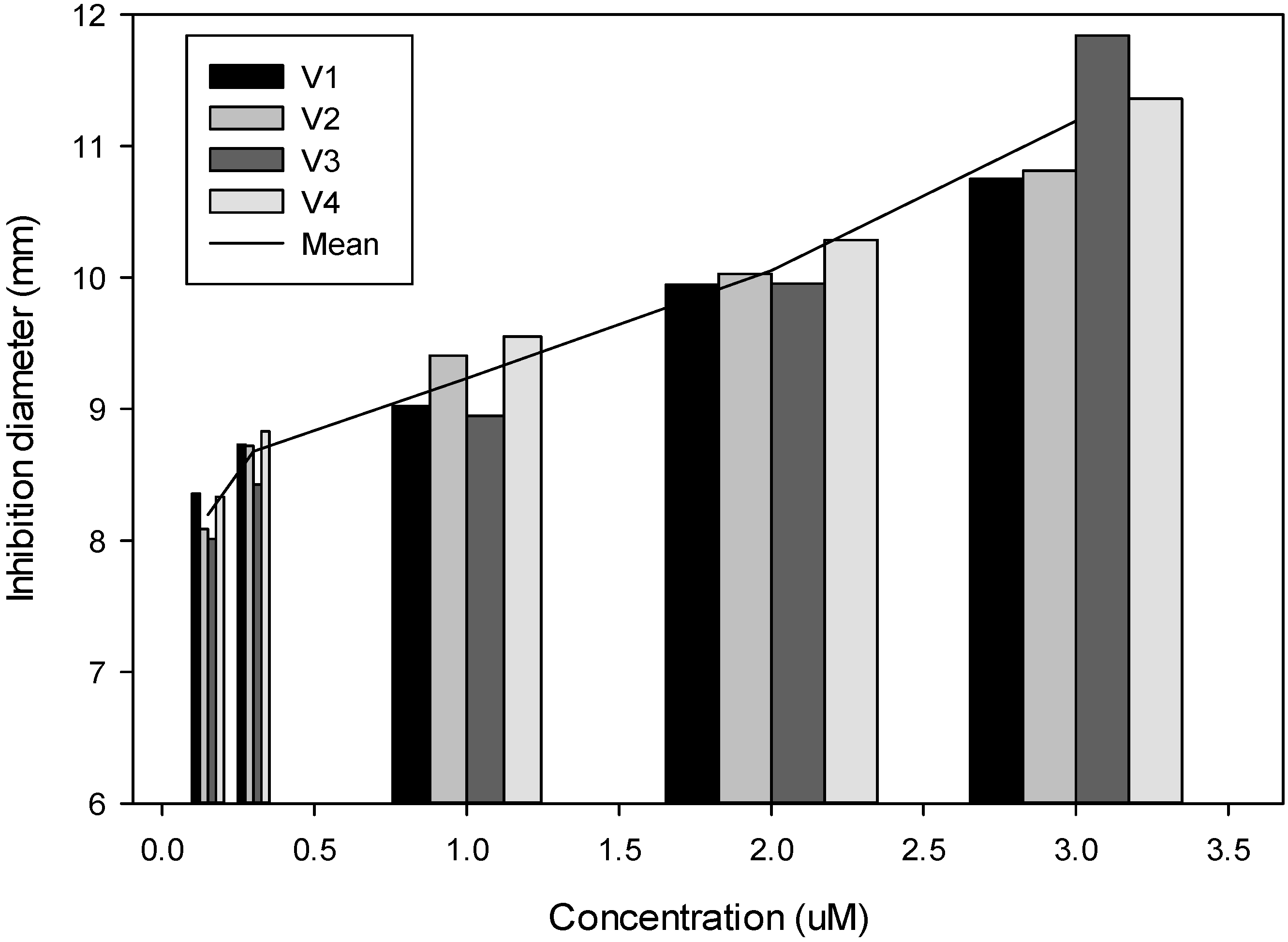
2.3. Effect of Plant Glucosinolates and Phenolic Compounds
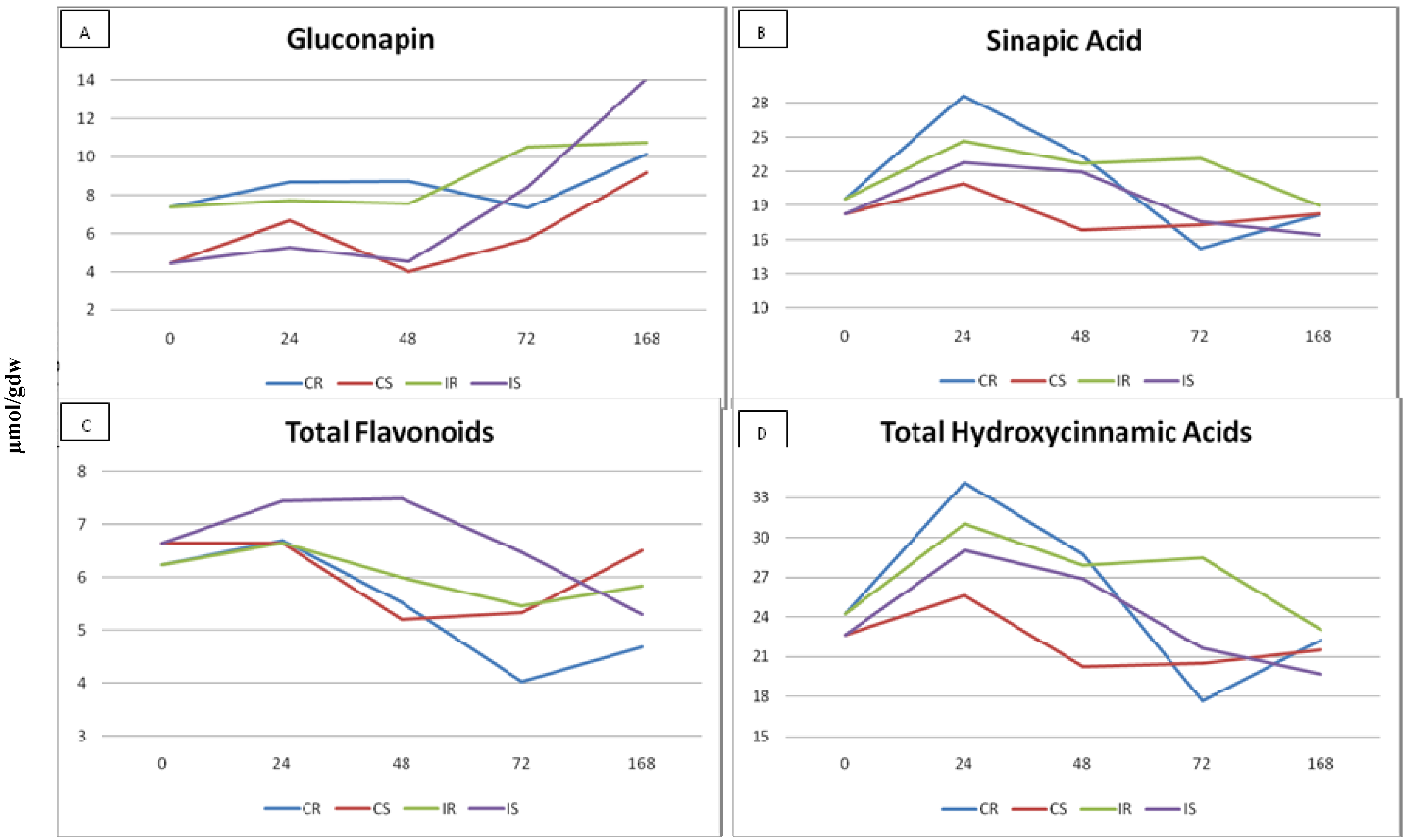
| Compound | Time 0 (0 h) | Time 1 (24 h) | Time 2 (48 h) | Time 3 (72 h) | Time 4 (168 h) |
|---|---|---|---|---|---|
| GIB | 0.060 ** | 0.040 | 0.435 | 0.005 | 0.334 |
| PRO | 0.004 | 1.884 * | 1.168 ** | 0.145 | 0.168 |
| GNA | 12.877 ** | 6.329 * | 15.742 * | 5.737 | 8.894 |
| OHGBS | 0.045 | 0.034 | 0.021 | 0.012 | 0.011 |
| GBN | 0.004 | 0.001 | 0.011 | 0.010 | 0.030 |
| GBS | 0.000 | 0.036 | 0.029 | 0.003 | 0.011 * |
| GNT | 0.001 | 0.005 | 0.007 | 0.001 | 0.011 * |
| NEOGBS | 0.000 | 0.000 | 0.000 * | 0.001 | 0.000 |
| TGLS | 17.579 ** | 13.987 ** | 20.074 * | 5.912 | 6.037 |
| CQAC | 0.000 | 0.001 | 0.008 | 0.003 | 0.005 |
| PCOQAC | 0.000 | 0.000 | 0.028 ** | 0.004 | 0.021 ** |
| F1 | 0.062 | 0.066 * | 0.246 ** | 0.132 | 0.042 |
| F2 | 0.104 | 0.183 | 0.640 ** | 0.252 | 0.135 * |
| F3 | 0.001 | 0.006 | 0.013 | 0.039 | 0.050 ** |
| F4 | 0.020 | 0.015 | 0.026 | 0.058 | 0.057 * |
| F5 | 0.001 | 0.003 | 0.020 * | 0.009 ** | 0.008 |
| F6 | 0.000 | 0.003 | 0.008 * | 0.003 | 0.002 |
| AC1 | 0.000 | 0.528 ** | 0.405 ** | 0.063 | 0.033 |
| AC2 | 0.135 | 0.646 | 1.229 | 0.793 | 0.304 |
| SA | 2.509 | 33.127 | 25.903 * | 13.874 | 1.955 |
| S1 | 0.000 | 0.007 | 0.104 | 0.038 | 0.021 |
| S2 | 0.000 | 0.009 | 0.174 ** | 0.029 | 0.021 |
| TFLAV | 0.248 | 0.459 | 3.053 ** | 1.981 | 1.178 |
| THYDROX | 3.745 | 38.488 | 44.937 * | 26.346 | 3.362 |
| TPHEN | 2.089 | 37.815 | 55.227 * | 35.745 | 4.537 |
| Compound | Resistant Inoculated | Resistant Control | Susceptible Inoculated | Susceptible Control | LSD |
|---|---|---|---|---|---|
| PRO | 0.000b | 0.793a | 0.000b | 1.263a | 0.650 |
| GNA | 7.567ab | 8.730a | 4.563bc | 4.010c | 3.389 |
| NEOGBS | 1.077a | 1.060c | 1.070ab | 1.053c | 0.012 |
| TGLS | 15.170ab | 17.203a | 11.480b | 12.540b | 4.086 |
| PCOQAC | 0.067b | 0.203a | 0.000b | 0.000b | 0.111 |
| F1 | 1.113b | 0.890b | 1.493a | 0.883b | 0.234 |
| F2 | 1.743b | 1.430b | 2.477a | 1.430b | 0.393 |
| F5 | 0.537b | 0.463b | 0.650a | 0.493b | 0.108 |
| F6 | 0.713a | 0.677a | 0.703a | 0.597b | 0.074 |
| AC1 | 1.533a | 1.097b | 1.780a | 1.000b | 0.378 |
| SA | 22.673a | 23.330a | 21.930a | 16.880b | 4.531 |
| S2 | 0.150bc | 0.553a | 0.000c | 0.350ab | 0.280 |
| TFLAV | 5.990b | 5.528b | 7.487a | 5.207b | 0.948 |
| THYDROX | 27.890a | 28.717a | 26.907a | 20.240b | 5.706 |
| TPHEN | 33.877a | 34.247a | 34.390a | 25.447b | 6.236 |
3. Discussion
4. Experimental
4.1. In Vitro Effect of GNA and Its Isothiocyanate
4.2. In Vitro Effect of Glucosinolates and Phenolic Compounds in Methanolic Extract from Leaves
4.3. Effect of Plant Glucosinolates and Phenolic Compounds
4.4. Extraction and Determination of Glucosinolates and Phenolic Compounds
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor (accessed on 6 September 2013).
- Kim, J.; Jung, Y.; Song, B.; Bong, Y.-S.; Ryu, D.H.; Lee, K.-S.; Hwang, G.-S. Discrimination of cabbage (Brassica rapa ssp. pekinensis) cultivars grown in different geographical areas using 1H-NMR-based metabolomics. Food Chem. 2013, 137, 68–75. [Google Scholar]
- Bong, Y.-S.; Shin, W.-J.; Gautam, M.K.; Jeong, Y.-J.; Lee, A.-R.; Jang, C.-S.; Lim, Y.-P.; Chung, G.-S.; Lee, K.-S. Determining the geographical origin of Chinese cabbages using multielement composition and strontium isotope ratio analyses. Food Chem. 2012, 135, 2666–2674. [Google Scholar]
- Dias, J.S.; Nogueira, P.; Corvo, L. Evaluation of a core collection of Brassica rapa vegetables for resistance to Xanthomonas campestris pv. campestris. Afr. J. Agric. Res. 2010, 5, 2972–2980. [Google Scholar]
- Vicente, J.G.; Conway, J.; Roberts, S.J.; Taylor, J.D. Identification and origin of Xanthomonas campestris pv. campestris races and related pathovars. Phytopathology 2001, 91, 492–499. [Google Scholar]
- Fargier, E.; Manceau, C. Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol. 2007, 56, 805–818. [Google Scholar]
- Lema, M.; Elena Cartea, M.; Sotelo, T.; Velasco, P.; Soengas, P. Discrimination of Xanthomonas campestris pv. campestris races among strains from northwestern Spain by Brassica spp. genotypes and rep-PCR. Eur. J. Plant Pathol. 2012, 133, 159–169. [Google Scholar]
- Lema, M.; Cartea, M.E.; Francisco, M.; Velasco, P.; Soengas, P. Screening for resistance to black rot in a Spanish collection of Brassica rapa. Plant Breed. 2013. submitted. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar]
- Agrawal, A.A.; Kurashige, N.S. A role for isothyociantes in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 2003, 29, 1403–1415. [Google Scholar]
- Rahmanpour, S.; Backhouse, D.; Nonhebel, H.M. Induced tolerance of Sclerotinia sclerotiorum to isothiocyanates and toxic volatiles from Brassica species. Plant Pathol. 2009, 58, 479–486. [Google Scholar]
- Fan, Z.X.; Lei, W.X.; Sun, X.L.; Yu, B.; Wang, Y.Z.; Yang, G.S. The association of Sclerotinia sclerotiorum resistance with glucosinolates in Brassica napus double-low DH population. J. Plant Pathol. 2008, 90, 43–48. [Google Scholar]
- Bending, G.D.; Lincoln, S.D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biol. Biochem. 2000, 32, 1261–1269. [Google Scholar]
- Giamoustaris, A.; Mithen, R. Glucosinolates and disease resistance in oilseed rape (Brassica napus ssp. oleifera). Plant Pathol. 1997, 46, 271–275. [Google Scholar]
- Lazzeri, L.; Manici, L.M. Allelopathic effect of glucosinolate containing plant green manure on Pythium sp. and total fungal population in soil. HortScience 2001, 36, 1283–1289. [Google Scholar]
- Motisi, N.; Montfort, F.; Dore, T.; Romillac, N.; Lucas, P. Duration of control of two soilborne pathogens following incorporation of above- and below-ground residues of Brassica juncea into soil. Plant Pathol. 2009, 58, 470–478. [Google Scholar]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Palva, E.T. Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 2006, 46, 758–767. [Google Scholar]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Monteiro, A.A.; Simoes, M.; Rosa, E.A.S.; Bennett, R.N. Initial in vitro evaluations of the antibacterial activities of glucosinolate enzymatic hydrolysis products against plant pathogenic bacteria. J. Appl. Microbiol. 2009, 106, 2096–2015. [Google Scholar]
- Mathur, V.; Ganta, S.; Raaijmakers, C.E.; Reddy, A.S.; Vet, L.E.M.; van Dam, N.M. Temporal dynamics of herbivore-induced responses in Brassica juncea and their effect on generalist and specialist herbivores. Entomol. Exp. Appl. 2011, 139, 215–225. [Google Scholar]
- Broekgaarden, C.; Voorrips, R.E.; Dicke, M.; Vosman, B. Transcriptional responses of Brassica nigra to feeding by specialist insects of different feeding guilds. Insect Sci. 2011, 18, 259–272. [Google Scholar]
- Hafidh, R.R.; Abdulamir, A.S.; Vern, L.S.; Bakar, F.A.; Abas, F.; Jahanshiri, F.; Sekawi, Z. Inhibition of growth of highly resistant bacterial and fungal pathogens by a natural product. Open Microbiol. J. 2011, 5, 96–106. [Google Scholar]
- Aires, A.; Dias, C.S.P.; Carvalho, R.; Oliveira, M.H.; Monteiro, A.A.; Simoes, M.V.; Rosa, E.A.S.; Bennett, R.N.; Saavedra, M.J. Correlations between disease severity, glucosinolate profiles and total phenolics and Xanthomonas campestris pv. campestris inoculation of different Brassicaceae. Sci. Hortic. 2011, 129, 503–510. [Google Scholar]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; Garcia-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar]
- O’Callaghan, K.J.; Stone, P.J.; Hu, X.J.; Griffiths, D.W.; Davey, M.R.; Cocking, E.C. Effects of glucosinolates and flavonoids on colonization of the roots of Brassica napus by Azorhizobium caulinodans ORS571. Appl. Environ. Microbiol. 2000, 66, 2185–2191. [Google Scholar]
- Buskov, S.; Serra, B.; Rosa, E.; Sorensen, H.; Sorensen, J.C. Effects of intact glucosinolates and products produced from glucosinolates in myrosinase-catalyzed hydrolysis on the potato cyst nematode (Globodera rostochiensis cv. Woll). J. Agric. Food Chem. 2002, 50, 690–695. [Google Scholar]
- Menard, R.; Larue, J.P.; Silue, D.; Thouvenot, D. Glucosinolates in cauliflower as biochemical markers for resistance against downy mildew. Phytochemistry 1999, 52, 29–35. [Google Scholar]
- Siemens, J.; Glawischnig, E.; Ludwig-Mueller, J. Indole glucosinolates and camalexin do not influence the development of the clubroot disease in Arabidopsis thaliana. J. Phytopathol. 2008, 156, 332–337. [Google Scholar]
- Siemens, J.; Nagel, M.; Ludwig-Muller, J.; Sacristan, M.D. The interaction of Plasmodiophora brassicae and Arabidopsis thaliana: Parameters for disease quantification and screening of mutant lines. J. Phytopathol. 2002, 150, 592–605. [Google Scholar]
- Kirkegaard, J.A.; Sarwar, M.; Wong, P.T.W.; Mead, A.; Howe, G.; Newell, M. Field studies on the biofumigation of take-all by Brassica break crops. Aust. J. Agric. Res. 2000, 51, 445–456. [Google Scholar]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation potential of brassicas - III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant Soil 1998, 201, 103–112. [Google Scholar]
- Tierens, K.; Thomma, B.P.H.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Cammue, B.P.A.; Broekaert, W.F. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of arabidopsis to microbial pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar]
- Jiang, H.; Song, W.; Li, A.; Yang, X.; Sun, D. Identification of genes differentially expressed in cauliflower associated with resistance to Xanthomonas campestris pv. campestris. Mol. Biol. Reports 2011, 38, 621–629. [Google Scholar] [CrossRef]
- Njoroge, S.M.C.; Vallad, G.E.; Park, S.-Y.; Kang, S.; Koike, S.T.; Bolda, M.; Burman, P.; Polonik, W.; Subbarao, K.V. Phenological and Phytochemical Changes Correlate with Differential Interactions of Verticillium dahliae with Broccoli and Cauliflower. Phytopathology 2011, 101, 523–534. [Google Scholar]
- Francisco, M.; Velasco, P.; Lema, M.; Cartea, M.E. Genotypic and environmental effects on agronomic and nutritional value of Brassica rapa. Agron. J. 2011, 103, 735–742. [Google Scholar] [CrossRef]
- Lema Marquez, M.; Teran, H.; Singh, S.P. Selecting common bean with genes of different evolutionary origins for resistance to Xanthomonas camplestris pv. phaseoli. Crop Sci. 2007, 47, 1367–1374. [Google Scholar]
- Velasco, P.; Francisco, M.; Moreno, D.A.; Ferreres, F.; Garcia-Viguera, C.; Cartea, M.E. Phytochemical Fingerprinting of Vegetable Brassica oleracea and Brassica napus by Simultaneous Identification of Glucosinolates and Phenolics. Phytochem. Anal. 2011, 22, 144–152. [Google Scholar] [CrossRef] [Green Version]
- SAS, SAS Online Doc, Version 9.2, SAS Institute Inc: Cary, NC, USA, 2008.
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Velasco, P.; Lema, M.; Francisco, M.; Soengas, P.; Cartea, M.E. In Vivo and in Vitro Effects of Secondary Metabolites against Xanthomonas campestris pv. campestris. Molecules 2013, 18, 11131-11143. https://doi.org/10.3390/molecules180911131
Velasco P, Lema M, Francisco M, Soengas P, Cartea ME. In Vivo and in Vitro Effects of Secondary Metabolites against Xanthomonas campestris pv. campestris. Molecules. 2013; 18(9):11131-11143. https://doi.org/10.3390/molecules180911131
Chicago/Turabian StyleVelasco, Pablo, Margarita Lema, Marta Francisco, Pilar Soengas, and María Elena Cartea. 2013. "In Vivo and in Vitro Effects of Secondary Metabolites against Xanthomonas campestris pv. campestris" Molecules 18, no. 9: 11131-11143. https://doi.org/10.3390/molecules180911131
APA StyleVelasco, P., Lema, M., Francisco, M., Soengas, P., & Cartea, M. E. (2013). In Vivo and in Vitro Effects of Secondary Metabolites against Xanthomonas campestris pv. campestris. Molecules, 18(9), 11131-11143. https://doi.org/10.3390/molecules180911131






