2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as Novel Antifungal Lead Compounds: Biological Evaluation and Structure-Activity Relationships
Abstract
:1. Introduction
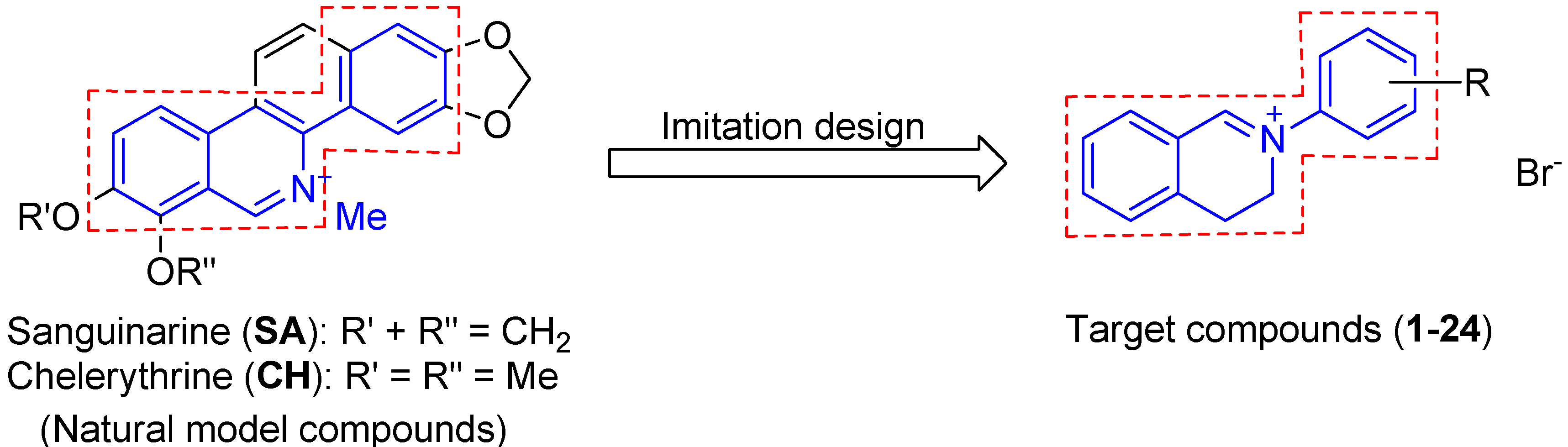
2. Results and Discussion
2.1. Screening of Antifungal Activity in Vitro
| Compound | Linear growth inhibitory rates (means, %) ** | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | R | A.A. | C.L. | P.O. | F.S. | V.M. | F.O.N. | F.O.V. |
| 1 | H | 56.8 h | 76.7 ef | 50.6 hi | 23.5 k | 32.4 l | 50.8 h | 25.6 m |
| 2 | o-F | 78.4 b | 95.1 a | 69.9 b | 85.4 b | 80.8 bc | 80.1 c | 71.3 c |
| 3 | m-F | 66.4 def | 86.9 bcd | 62.2 cdef | 48.5 ghij | 54.2 i | 44.1 i | 43.1 h |
| 4 | p-F | 78.4 b | 73.4 fg | 62.2 cdef | 59.7 def | 66.4 g | 71.1 de | 59.5 e |
| 5 | o-Cl | 84.1 a | 84.1 cd | 67.9 bc | 59.2 def | 72.9 ef | 74.0 f | 55.8 f |
| 6 | m-Cl | 68.3 d | 84.5 cd | 60.9 def | 62.1 de | 72.1 ef | 36.6 j | 34.9 ij |
| 7 | p-Cl | 78.4 b | 89.0 bc | 64.1 bcde | 55.8 defgh | 61.2 g | 74.0 f | 65.1 d |
| 8 | o-Br | 78.1 b | 92.0 ab | 82.3 a | 93.9 a | 71.7 ef | 91.7 b | 74.6 b |
| 9 | p-Br | 72.1 c | 69.4 g | 62.8 cde | 49.0 ghij | 55.5 i | 65.9 f | 61.5 e |
| 10 | o-I | 65.9 def | 81.6 de | 60.3 def | 55.3 defghi | 70.8 f | 67.3 ef | 52.8 f |
| 11 | m-I | 64.2 f | 84.9 cd | 59.0 defg | 54.3 efghi | 53.3 i | 28.5 k | 25.6 m |
| 12 | p-I | 60.6 g | 73.4 fg | 53.8 gh | 47.0 ij | 77.3 cd | 44.1 i | 43.6 h |
| 13 | o-CF3 | 64.0 f | 73.9 fg | 65.4 bcd | 53.8 efghi | 55.9 i | 48.4 hi | 48.2g |
| 14 | m-CF3 | 64.9 ef | 86.1 bcd | 63.5 cde | 47.5 hij | 47.2 j | 35.1 j | 31.3 kl |
| 15 | p-CF3 | 67.8 de | 87.7 bcd | 58.3 efg | 44.6 j | 38.5 k | 36.1 j | 33.8 jk |
| 16 | o-NO2 | 11.1 m | 4.0 l | 12.8 n | −12.2 n | 2.7 op | 5.3 m | 8.2 o |
| 17 | m-NO2 | 73.1 c | 73.9 fg | 70.4 b | 56.81 defg | 47.2 j | 56.9 g | 52.8 f |
| 18 | p-NO2 | 63.5 f | 60.8 h | 62.2 cdef | 58.7 def | 82.3 ab | 36.1 j | 37.9 i |
| 19 | o-Me | 51.0 i | 58.7 ghi | 32.7 l | −0.6 m | 2.3 p | 13.8 l | 10.8 o |
| 20 | m-Me | 11.6 m | 41.6 k | 19.2 m | 2.6 m | 11.6 n | 8.1 m | 11.3 o |
| 21 | p-Me | 60.1 g | 57.1 ghi | 35.3 kl | 13.5 l | 6.6 o | 33.2 j | 21.0 n |
| 22 | o-OH | 47.2 k | 53.8 ij | 44.9 ij | 53.4 fghi | 11.0 n | 25.2 k | 20.0 n |
| 23 | p-OH | 58.2 gh | 87.7b cd | 55.8 fgh | 73.3 c | 76.0 de | 35.1 j | 30.8 kl |
| 24 | p-OMe | 55.8 h | 57.5 hi | 46.8 i | 22.7 k | 25.0 m | 36.6 j | 29.7 l |
| SA | — | 47.7 jk | 58.7 hi | 40.7 jk | 56.4 defg | 69.1 fg | 50.5 h | 59.7 e |
| CH | — | 50.3 ij | 48.7 j | 50.1 hi | 63.5 d | 57.1 i | 63.2 f | 47.6 g |
| TBZ | — | 16.4 l | 9.3 l | 6.4 o | 81.5 b | 86.0 a | 100.0 a | 100.0 a |
2.2. Structure-Activity Relationship
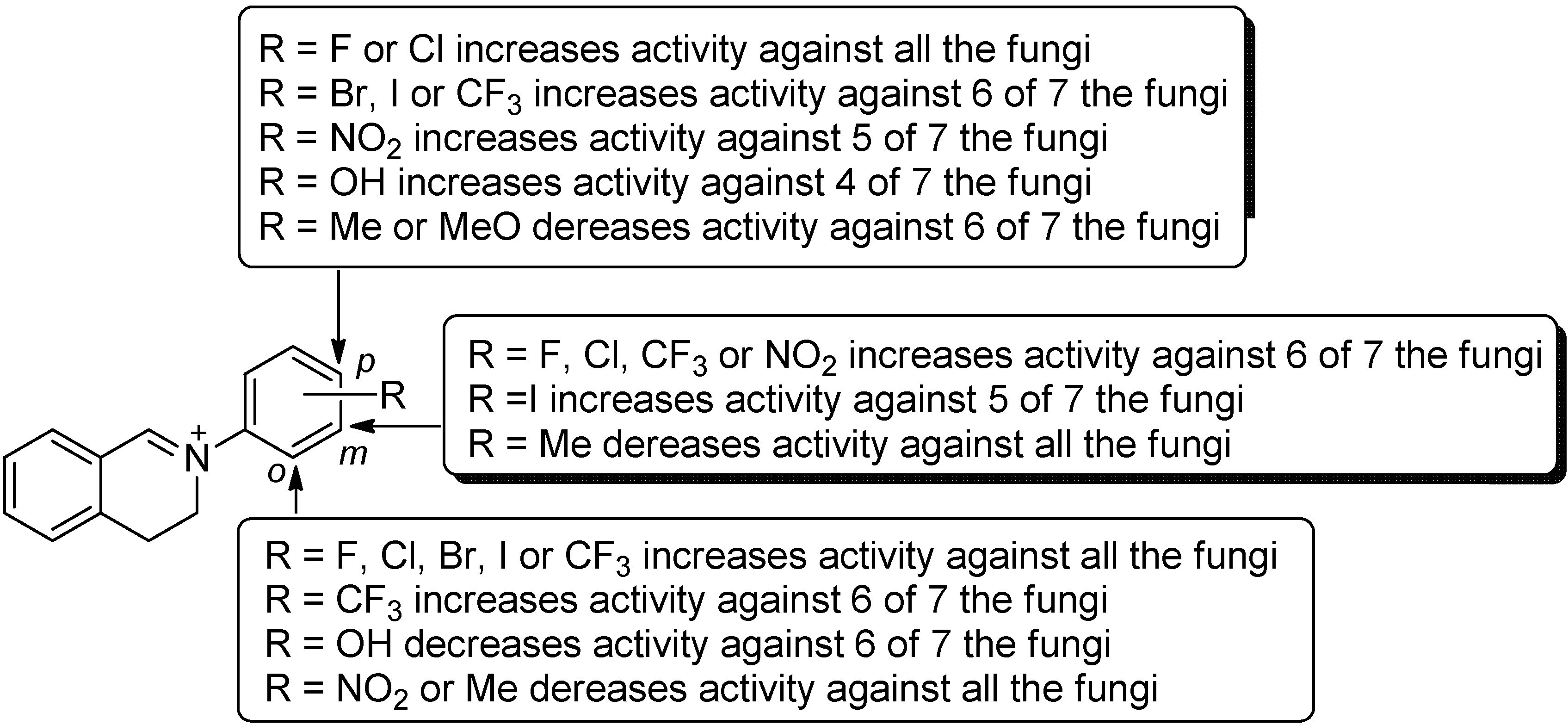
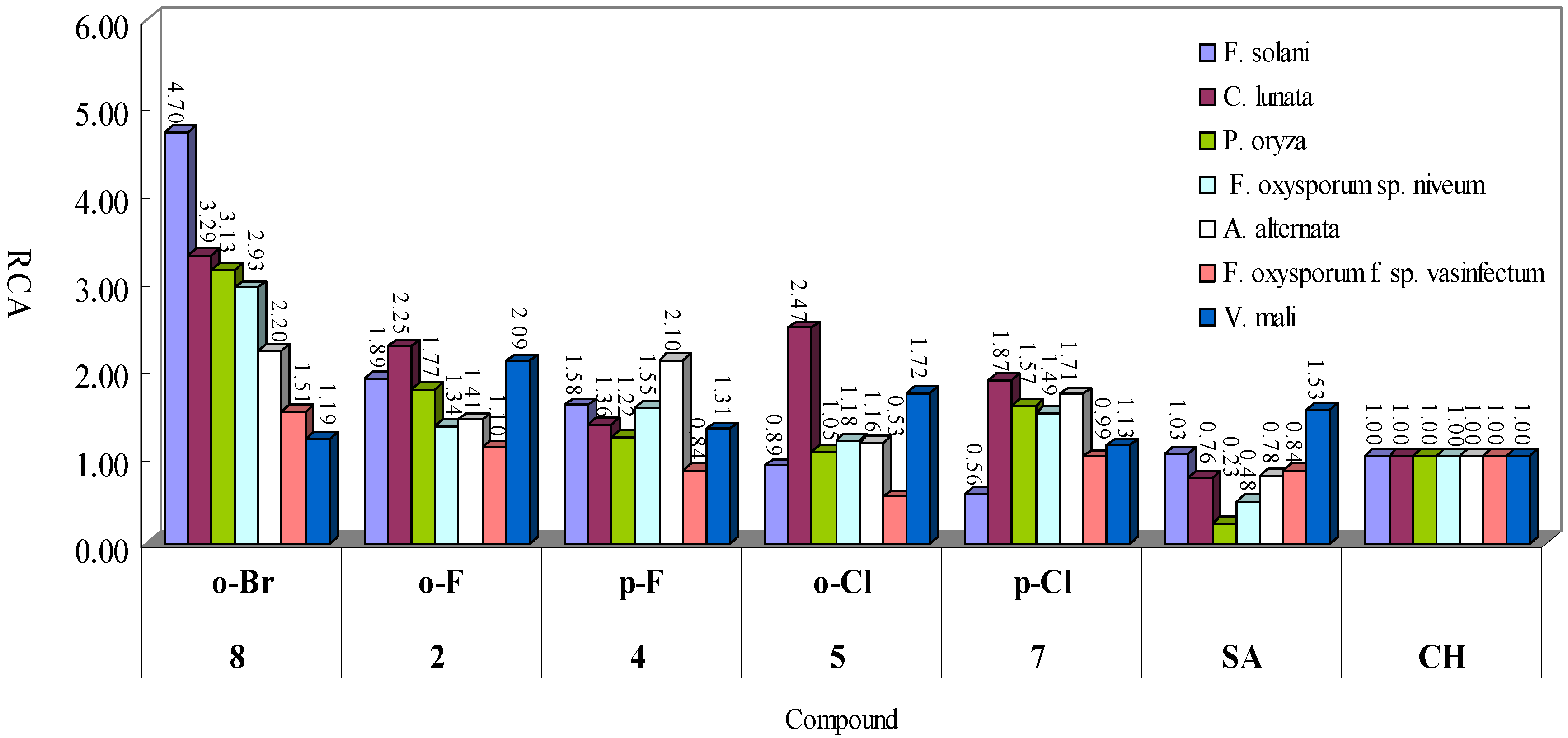
2.3. Antifungal Toxicity
| Fungus | Compd. | Toxicity regression equation * | R2 | EC50 | 95%CI ** of EC50 | |
|---|---|---|---|---|---|---|
| (μg/mL) | (μmol/L) | |||||
| F.O.V. | 2 (o-F) | y = 1.4300x + 3.0769 | 0.9780 | 22.12 | 72.2 | 20.74–23.60 |
| 4 (p-F) | y = 2.6168x + 0.4853 | 0.9381 | 53.12 | 173.5 | 42.10–67.02 | |
| 5 (o-Cl) | y = 1.2450x + 3.0099 | 0.9433 | 39.67 | 123.0 | 36.31–72.17 | |
| 7 (p-Cl) | y = 2.3044x + 1.3235 | 0.9971 | 39.39 | 122.1 | 39.29–40.05 | |
| 8 (o-Br) | y = 1.0996x + 3.7981 | 0.9958 | 12.39 | 33.8 | 12.09–12.70 | |
| SA | y = 1.6623x + 2.4605 | 0.9730 | 33.71 | 73.4 | 23.26–48.85 | |
| CH | y = 3.0902x − 0.3177 | 0.9737 | 52.58 | 110.6 | 29.43–93.95 | |
| F.O.N. | 2 (o-F) | y = 2.4275x + 1.3864 | 0.9769 | 30.80 | 100.6 | 29.98–31.64 |
| 4 (p-F) | y = 2.4139x + 1.5676 | 0.9669 | 26.42 | 86.3 | 25.36–27.52 | |
| 5 (o-Cl) | y = 1.3796x + 3.2094 | 0.9373 | 19.86 | 61.6 | 18.05–37.91 | |
| 7 (p-Cl) | y = 2.7227x + 0.9408 | 0.9988 | 30.97 | 96.0 | 30.88–31.06 | |
| 8 (o-Br) | y = 1.8107x + 3.1516 | 0.9414 | 10.49 | 28.6 | 7.48–14.71 | |
| SA | y = 1.4175x + 2.5893 | 0.9853 | 50.20 | 109.3 | 43.44–58.01 | |
| CH | y = 1.7052x + 2.5090 | 0.9864 | 28.89 | 60.8 | 23.77–35.12 | |
| V.M. | 2 (o-F) | y = 2.3031x + 1.8962 | 0.9836 | 22.27 | 72.7 | 21.71–26.42 |
| 4 (p-F) | y = 2.3521x + 1.3300 | 0.9162 | 36.33 | 118.7 | 33.79–59.37 | |
| 5 (o-Cl) | y = 2.0946x + 2.0847 | 0.9651 | 24.65 | 76.4 | 23.21–26.18 | |
| 7 (p-Cl) | y = 1.4730x + 2.9048 | 0.9749 | 26.45 | 82.0 | 25.87–30.72 | |
| 8 (o-Br) | y = 1.1731x + 3.4768 | 0.9575 | 19.88 | 54.2 | 16.03–24.66 | |
| SA | y = 1.9432x + 2.2639 | 0.9880 | 25.59 | 55.7 | 17.19–38.09 | |
| CH | y = 1.9658x + 1.8575 | 0.9541 | 39.68 | 83.5 | 16.17–97.36 | |
| F.S. | 2 (o-F) | y = 2.1583x + 2.0918 | 0.9938 | 22.25 | 72.7 | 22.05–23.64 |
| 4 (p-F) | y = 1.2769x + 3.4716 | 0.9846 | 15.74 | 51.4 | 15.52–17.30 | |
| 5 (o-Cl) | y = 1.0950x + 3.4884 | 0.9583 | 24.02 | 74.5 | 22.95–32.70 | |
| 7 (p-Cl) | y = 0.9805x + 3.5004 | 0.9534 | 33.84 | 104.9 | 29.63–38.65 | |
| 8 (o-Br) | y = 2.1420x + 2.9683 | 0.9833 | 8.88 | 24.2 | 8.04–9.81 | |
| SA | y = 2.1928x + 1.4479 | 0.9893 | 41.68 | 90.8 | 31.65–54.89 | |
| CH | y = 1.3614x + 3.0620 | 0.9466 | 26.52 | 55.8 | 15.92–44.19 | |
| A.A. | 2 (o-F) | y = 1.3559x + 3.2997 | 0.9835 | 17.95 | 58.6 | 17.50–21.32 |
| 4 (p-F) | y = 2.6327x + 1.3957 | 0.9876 | 23.39 | 76.4 | 22.96–26.56 | |
| 5 (o-Cl) | y = 1.1348x + 3.5676 | 0.9738 | 18.29 | 56.7 | 17.57–24.00 | |
| 7 (p-Cl) | y = 1.3719x + 3.3852 | 0.9813 | 15.03 | 46.6 | 14.60–18.29 | |
| 8 (o-Br) | y = 1.1204x + 3.9040 | 0.9953 | 9.51 | 25.9 | 9.25–9.78 | |
| SA | y = 2.2140x + 1.1814 | 0.9550 | 53.03 | 115.5 | 28.22–99.67 | |
| CH | y = 2.6508x + 0.5078 | 0.9586 | 49.51 | 104.2 | 21.52–113.92 | |
| C.L. | 2 (o-F) | y = 1.3293x + 3.6629 | 0.9725 | 10.14 | 33.1 | 7.28–14.11 |
| 4 (p-F) | y = 1.0827x + 3.7694 | 0.9198 | 13.70 | 44.7 | 12.10–31.73 | |
| 5 (o-Cl) | y = 1.3088x + 3.7443 | 0.9169 | 9.11 | 28.2 | 7.10–11.69 | |
| 7 (p-Cl) | y = 1.4152x + 3.4244 | 0.9675 | 12.98 | 40.2 | 12.33–18.34 | |
| 8 (o-Br) | y = 1.7125x + 3.3707 | 0.9373 | 8.94 | 24.4 | 6.38–12.53 | |
| SA | y = 1.5699x + 2.5652 | 0.9621 | 35.55 | 77.4 | 23.37–54.07 | |
| CH | y = 0.9154x + 3.9048 | 0.9621 | 15.72 | 33.1 | 6.08–40.66 | |
| P.O. | 2 (o-F) | y = 1.0351x + 3.7446 | 0.9531 | 16.33 | 53.3 | 15.63–21.97 |
| 4 (p-F) | y = 1.4434x + 2.8066 | 0.9892 | 33.08 | 108.0 | 32.78–35.15 | |
| 5 (o-Cl) | y = 0.8119x + 3.9161 | 0.9818 | 21.63 | 67.0 | 20.66–22.82 | |
| 7 (p-Cl) | y = 1.6338x + 2.6110 | 0.9893 | 28.99 | 89.9 | 28.73–30.81 | |
| 8 (o-Br) | y = 1.4194x + 3.4358 | 0.9806 | 12.65 | 34.46 | 11.15–14.35 | |
| SA | y = 0.8326x + 3.3309 | 0.9754 | 101.09 | 220.1 | 72.61–140.73 | |
| CH | y = 1.6792x + 2.1968 | 0.9629 | 46.71 | 98.3 | 39.01–55.93 | |
3. Experimental
3.1. General
3.2. Assay of Antifungal Activity in vitro
3.3. Statistic Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Teng, P.S.; Willocquet, L.; Nutter, F.W., Jr. Quantification and modeling of crop losses: A review of purposes. Annu. Rev. Phytopathol. 2006, 44, 89–112. [Google Scholar]
- Bräse, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar]
- Bai, Y.B.; Zhang, A.L.; Tang, J.J.; Gao, J.J. Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 2013, 61, 2789–2795. [Google Scholar]
- Wedge, D.E.; Camper, N.D. Biologically active natural products. In Agrochemicals and Pharmaceuticals; Cutler, H.G., Cutler, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–15. [Google Scholar]
- Dostál, J; Slavík, J. Some aspects of the chemistry of quaternary benzo[c]phenanthridine alkaloids. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier Scicence B.V: Oxford, UK, 2002; Volume 27, Part H; pp. 155–184. [Google Scholar]
- Miao, F.; Yang, X.J.; Ma, Y.N.; Zheng, F.; Song, X.P.; Zhou, L. Structural modification of sanguinarine and chelerythrine and their in vitro acaricidal activity against Psoroptes cuniculi. Chem. Pharm. Bull. 2012, 60, 1508–1513. [Google Scholar]
- Lenfeld, J.; Kroutil, M.; Maršálek, E.; Slavík, J.; Preininger, V.; Šimánek, V. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 1981, 43, 161–165. [Google Scholar] [CrossRef]
- Psotova, J.; Vecera, R.; Zdarilova, A.; Anzenbacherova, E.; Kosina, P.; Svobodova, A.; Hrbac, J.; Jirovsky, D.; Stiborova, M.; Lichnovsky, V.; et al. Safety assessment of sanguiritrin, Alkaloid fraction of Macleaya cordata, in rats. Vet. Med. 2006, 51, 145–155. [Google Scholar]
- Kosina, P.; Walterova, D.; Ulrichova, J.; Lichnovsky, V.; Stiborova, M.; Rydlova, H.; Vicar, J.; Krecman, V.; Brabec, M.J.; Simanek, V. Sanguinarine and chelerythrine: Assessment of safety on pigs in ninety days feeding experiment. Food Chem. Toxicol. 2004, 42, 85–91. [Google Scholar] [CrossRef]
- Ahsan, H.; Reagan-Shaw, S.; Breur, J.; Ahmad, N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007, 249, 198–208. [Google Scholar] [CrossRef]
- Meng, F.Y.; Zuo, G.Y.; Hao, X.Y.; Wang, G.C.; Xiao, H.T.; Zhang, J.Q.; Xu, G.L. Antifungal activity of the benzo[c]phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009, 125, 494–496. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, D.M.; Zhou, C.D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011, 25, 863–875. [Google Scholar] [CrossRef]
- Kerry, M.A.; Duval, O.; Waigh, R.D.; Mackay, S.P. The role of the iminium bond in the inhibition of reverse transcriptase by quaternary benzophenanthridines. J. Pharm. Pharmacol. 1998, 50, 1307–1315. [Google Scholar] [CrossRef]
- Eun, J.P.; Koh, G.Y. Suppression of angiogenesis by the plant alkaloid, Sanguinarine. Biochem. Biophys. Res. Commun. 2004, 317, 618–624. [Google Scholar] [CrossRef]
- Cho, K.M.; Yoo, I.D.; Kim, W.G. 8-Hydroxydihydrochelerythrine and 8-hydroxydihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. 2006, 29, 2317–2320. [Google Scholar] [CrossRef]
- Yao, J.Y.; Li, X.L.; Shen, J.-Y.; Pan, X.Y.; Hao, G.J.; Xu, Y.; Ying, W.L.; Ru, H.S.; Liu, X.L. Isolation of bioactive components from Chelidonium majus L. with activity against Trichodina sp. Aquaculture 2011, 318, 235–238. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhou, Z.; Jiang, D.X.; Han, J.; Wang, J.F.; Zhao, L.W.; Li, J. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2010, 171, 305–313. [Google Scholar] [CrossRef]
- Nyangulu, J.M.; Hargreaves, S.L.; Sharples, S.L.; Mackay, S.P.; Waigh, R.D.; Duval, O.; Mberu, E.K.; Watkins, W.M. Antimalarial benzo[c]phenanthridines. Bioorg. Med. Chem. Lett. 2005, 15, 2007–2010. [Google Scholar] [CrossRef]
- Yang, X.J.; Miao, M.; Yao, Y.; Cao, F.J.; Yang, R.; Ma, Y.N.; Qin, B.F.; Zhou, L. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules 2012, 17, 13026–13035. [Google Scholar]
- Nakanishi, T.; Suzuki, M.; Saimoto, A.; Kabasawa, T.J. Structural considerations of NK109, An antitumor benzo[c]phenanthridine alkaloid. J. Nat. Prod. 1999, 62, 864–867. [Google Scholar] [CrossRef]
- Ma, Y.N.; Yang, X.J.; Pan, L.; Hou, Z.; Geng, H.L.; Song, X.P.; Zhou, L.; Miao, F. Synthesis of 2-aryl-3,4-dihydroisoquinolin-2-ium bromides and their in vitro acaricidal activity against Psoroptes cuniculi. Chem. Pharm. Bull. 2013, 61, 204–211. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–24 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hou, Z.; Yang, R.; Zhang, C.; Zhu, L.-F.; Miao, F.; Yang, X.-J.; Zhou, L. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as Novel Antifungal Lead Compounds: Biological Evaluation and Structure-Activity Relationships. Molecules 2013, 18, 10413-10424. https://doi.org/10.3390/molecules180910413
Hou Z, Yang R, Zhang C, Zhu L-F, Miao F, Yang X-J, Zhou L. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as Novel Antifungal Lead Compounds: Biological Evaluation and Structure-Activity Relationships. Molecules. 2013; 18(9):10413-10424. https://doi.org/10.3390/molecules180910413
Chicago/Turabian StyleHou, Zhe, Rui Yang, Cen Zhang, Li-Fei Zhu, Fang Miao, Xin-Juan Yang, and Le Zhou. 2013. "2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as Novel Antifungal Lead Compounds: Biological Evaluation and Structure-Activity Relationships" Molecules 18, no. 9: 10413-10424. https://doi.org/10.3390/molecules180910413




