Antidiabetic and Antioxidant Properties of Alkaloids from Catharanthus roseus (L.) G. Don
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
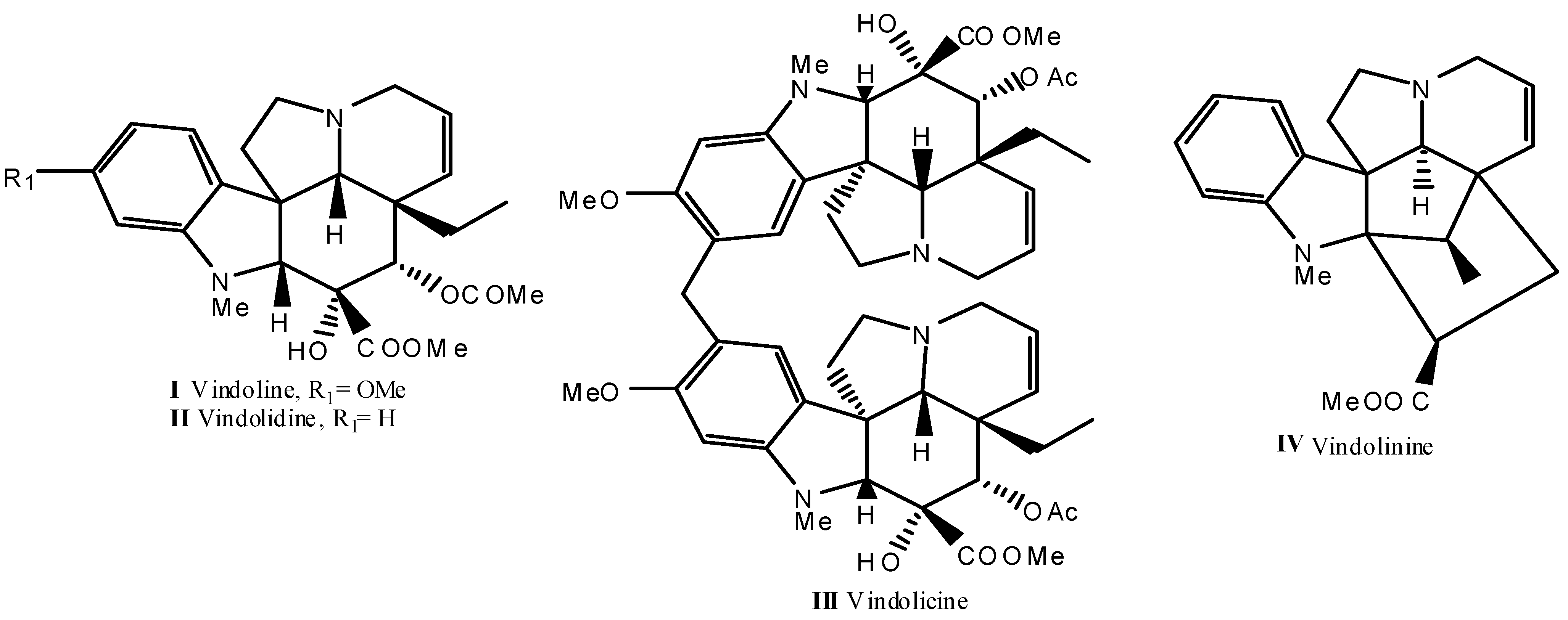
2.1.1. Identification of the Alkaloid Constituents
2.1.2. Effect of Alkaloids on β-TC6 Cell Viability
| Cytotoxicity (IC50) | DE | Vindoline | Vindolidine | Vindolicine | Vindolinine |
|---|---|---|---|---|---|
| µg/mL | 78.4 ± 12.2 | 82.1 ± 9.8 | 76.7 ± 8.1 | 68.0 ± 10.4 | 20.5 ± 3.6 |
| µM | - | 180.1 ± 21.5 | 180.1 ± 19.0 | 73.5 ± 11.3 | 57.6 ± 10.7 |
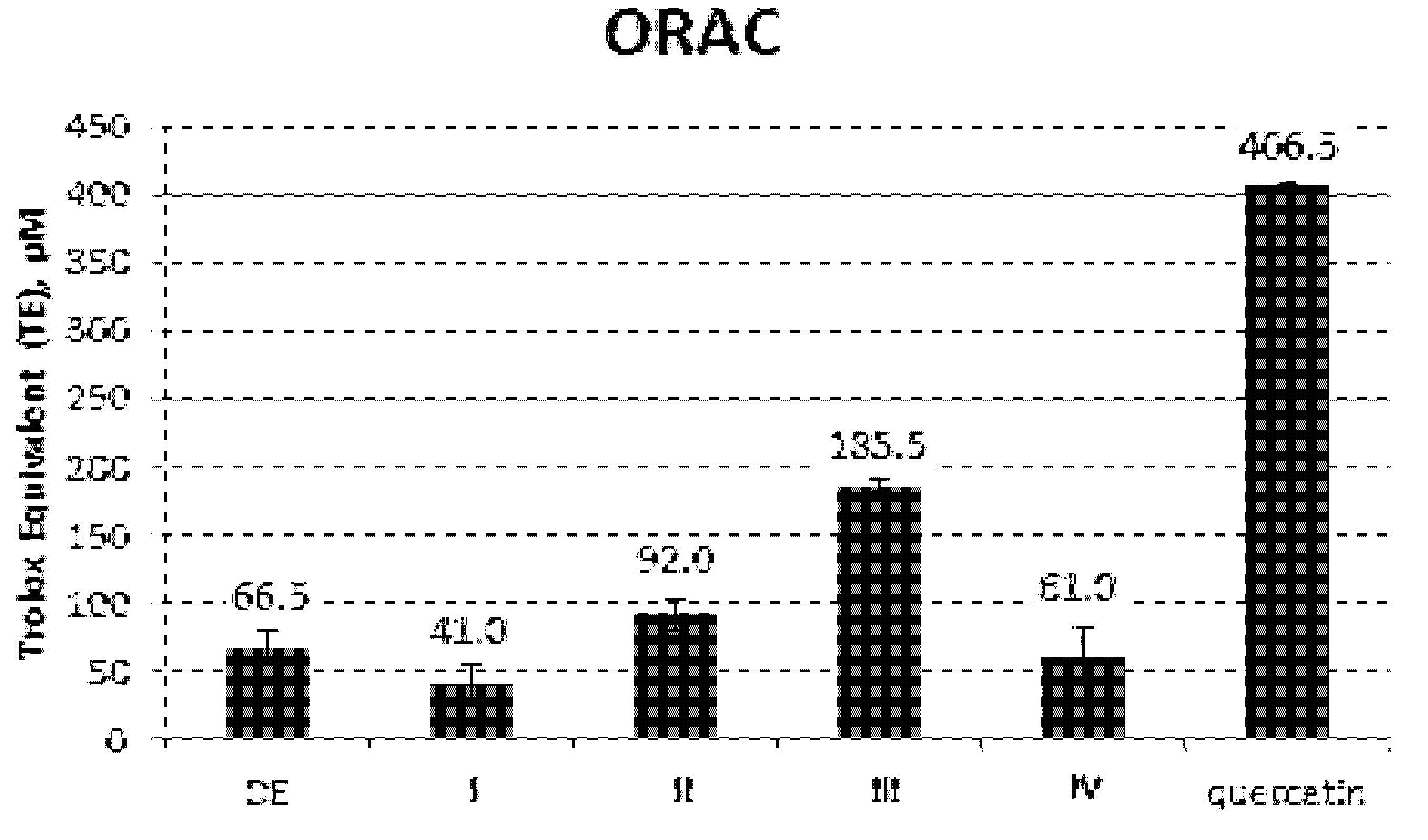
2.1.3. ORAC Evaluation
2.1.4. DPPH Assay

2.1.5. Effect of Alkaloids on H2O2-induced ROS Production in β-TC6 Cells

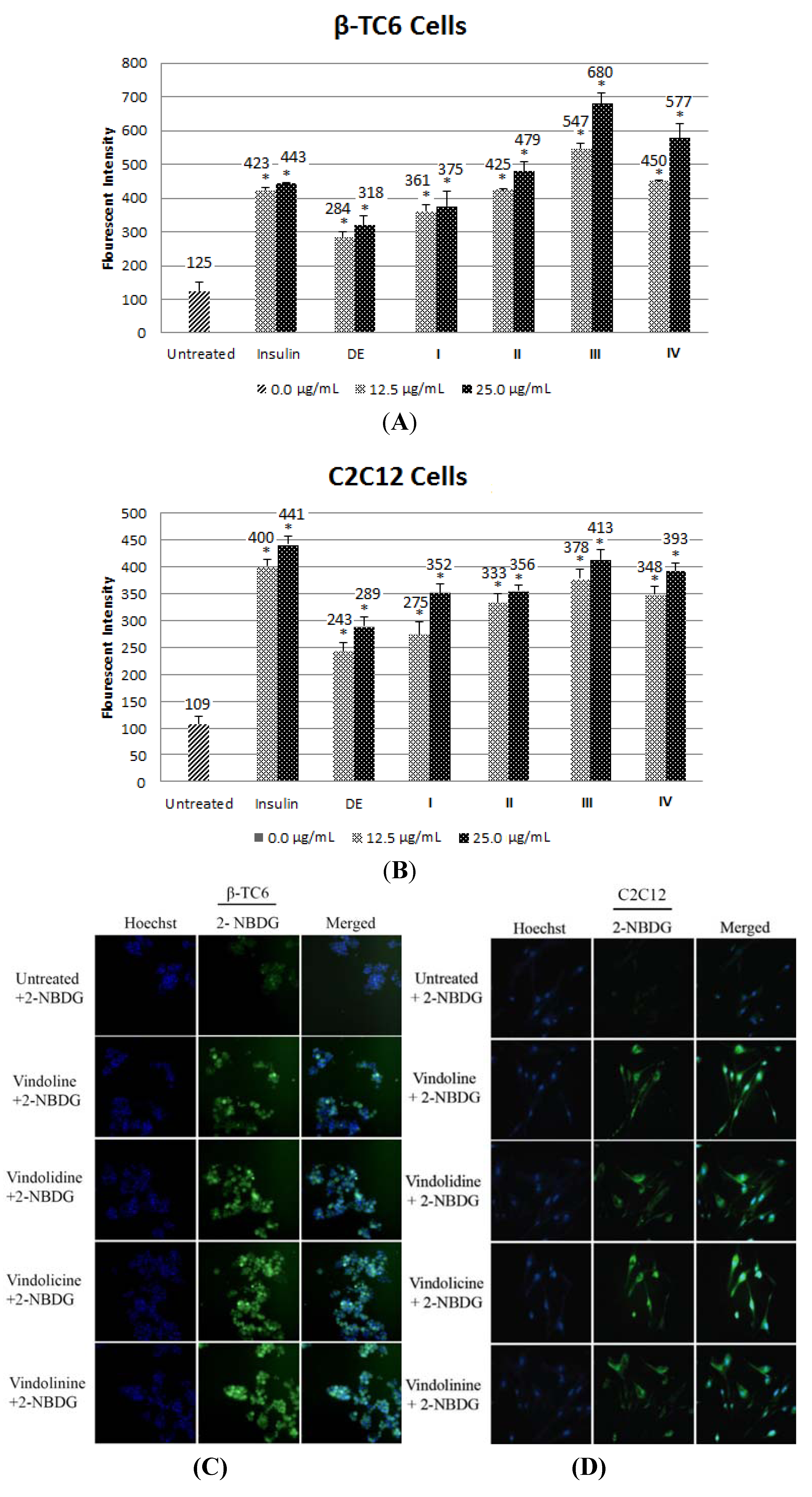
2.1.6. Effect of Alkaloid on Glucose Uptake in β-TC6 and C2C12 Cells
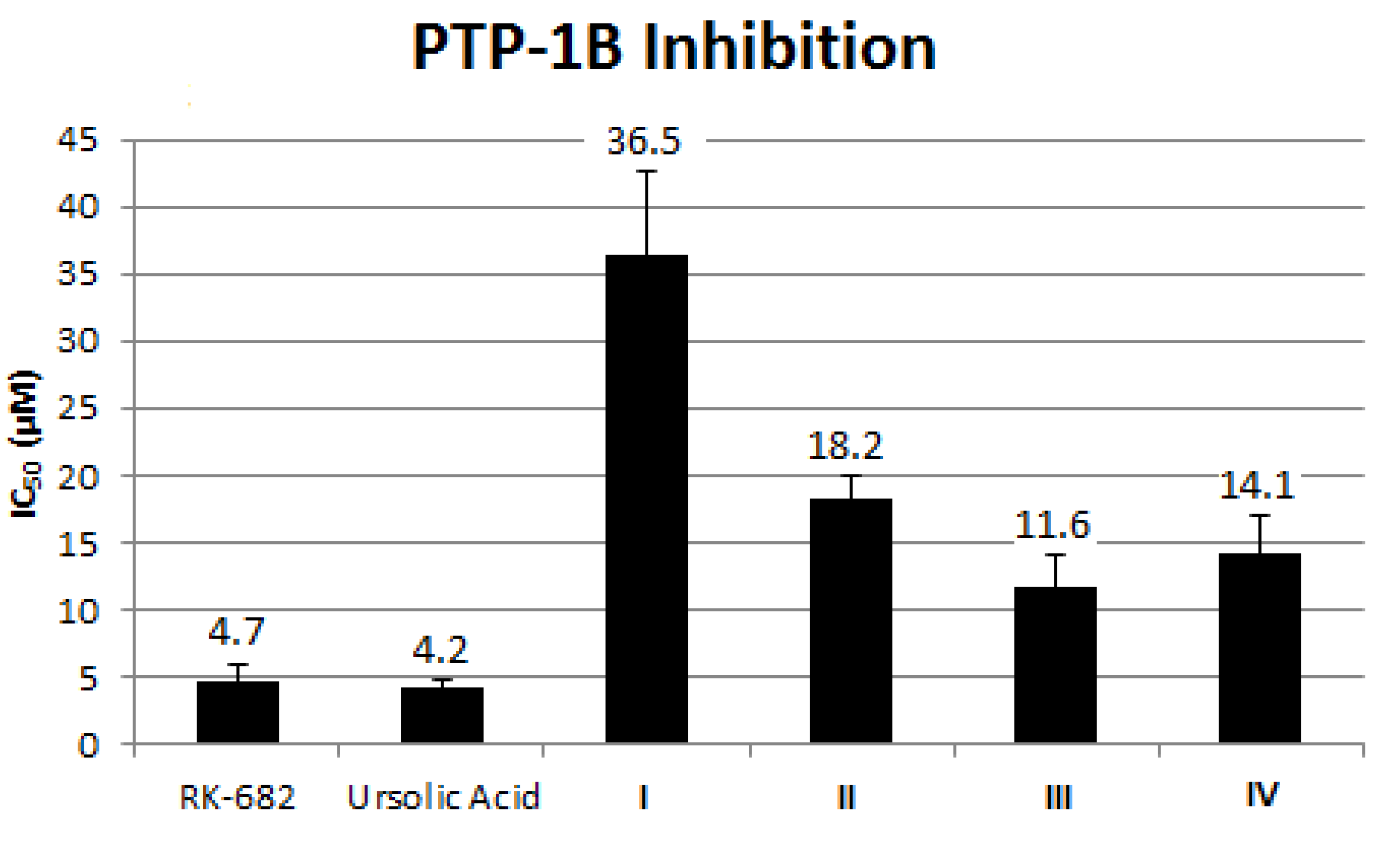
2.1.7. Effect of Alkaloid on PTP-1B Inhibition
2.2. Discussion
3. Experimental
3.1. Plant Material
3.2. Extraction and Fractionation
3.3. Isolation and Purification
3.4. Identification and Characterization of Alkaloids
3.5. Cell Culture
3.6. Cellular Viability
3.7. ORAC Assay
3.8. DPPH Assay
3.9. Intracellular ROS Measurement
3.11. PTP-1B Inhibition
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Van Bergen, M.A.; Snoeijer, W. Revision of Catharanthus G. Don. Series of Revisions of Apocynaceae XLI; Backhuys Publishers: Leiden, The Netherlands, 1996; pp. 32–35. [Google Scholar]
- Don, G. Catharanthus roseus. In Medicinal Plants of the World; Ross, I.A., Ed.; Human Press: Totowa, NJ, USA, 1999; pp. 109–118. [Google Scholar]
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac. J. Trop. Dis. 2012, 2, 239–250. [Google Scholar] [CrossRef]
- Ong, H.C.; Ahmad, N.; Milow, P. Traditional medicinal plants used by the temuan villagers in Kampung Tering, Negeri Sembilan, Malaysia. Ethno Med. 2011, 5, 169–173. [Google Scholar]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; de Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef]
- Letchuman, G.R.; Wan Nazaimoon, W.M.; Wan Mohamad, W.B.; Chandran, L.R.; Tee, G.H.; Jamaiyah, H.; Isa, M.R.; Zanariah, H.; Fatanah, I.; Ahmad Faudzi, Y. Prevalence of diabetes in the malaysian national health morbidity survey III 2006. Med. J. Malays. 2010, 65, 173–179. [Google Scholar]
- Kevin, L.Y.W.; Hussin, A.H.; Zhari, I.; Chin, J.H. Sub-acute oral toxicity study of methanol leaves extract of Catharanthus roseus in rats. J. Acute Dis. 2012, 1, 38–41. [Google Scholar] [CrossRef]
- Nammi, S.; Boini, M.K.; Lodagala, S.D.; Behara, R.B. The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complement. Altern. Med. 2003, 3, e2. [Google Scholar] [CrossRef] [Green Version]
- Ohadoma, S.C.; Michael, H.U. Effects of co-administration of methanol leaf extract of Catharanthus roseus on the hypoglycemic activity of metformin and glibenclamide in rats. Asian Pac. J. Trop. Med. 2011, 4, 475–477. [Google Scholar] [CrossRef]
- Gacche, R.N.; Dhole, N.A. Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: An attempt to standardize the botanicals for amelioration of diabetes complications. Food Chem. Toxicol. 2011, 49, 1806–1813. [Google Scholar] [CrossRef]
- Ganga, R.M.; Satyanarayana, S.; Eswar, K.K. Safety of Gliclazide with the aqueous extract of Vinca rosea on pharmacodynamic activity in normal and alloxan induced diabetic rats. J. Pharm. Res. 2012, 5, 1555–1558. [Google Scholar]
- Chattopadhyay, R.R. A comparative evaluation of some blood sugar lowering agents of plant origin. J. Ethnopharmacol. 1999, 67, 367–372. [Google Scholar] [CrossRef]
- Singh, S.F.; Vats, P.; Suri, S.; Shyam, R.; Kumria, M.M.; Ranganathan, S.; Sridharan, K. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2001, 76, 269–277. [Google Scholar] [CrossRef]
- Ferreres, F.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Sottomayor, M. New phenolic compounds and antioxidant potential of Catharanthus roseus. J. Agric. Food Chem. 2008, 56, 9967–9974. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Gorman, M.; Neuss, N.; Biemann, K. Vinca alkaloid X The structure of vindoline. J. Am. Chem. Soc. 1962, 84, 1058–1059. [Google Scholar] [CrossRef]
- El-Sayed, A.; Handy, G.A.; Cordell, G.A. Catharanthus alkaloids. XXXVIII. Confirming structural evidence and antineoplastic activity of the bisindole alkaloids leurosine-N'b-oxide (pleurosine), roseadine and vindolicine from Catharanthus roseus. J. Nat. Prod. 1983, 46, 517–527. [Google Scholar] [CrossRef]
- Atta ur, R.; Bashir, M.; Kaleem, S.; Fatima, T. 16-epi-19-S-vindolinine, an indoline alkaloid from Catharanthus roseus. Phytochemistry 1983, 22, 1021–1023. [Google Scholar] [CrossRef]
- Wenkert, E.; Cochran, D.W.; Hagaman, E.W.; Schell, F.M.; Neuss, N.; Katner, A.S. Carbon-13 nuclear magnetic resonance spectroscopy of naturally occuring substances. XIX. Aspidorsperma alkaloids. J. Am. Chem. Soc. 1973, 95, 4990–4995. [Google Scholar] [CrossRef]
- Bisby, R.H.; Brooke, R.; Navaratnam, S. Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay. Food Chem. 2008, 108, 1002–1007. [Google Scholar] [CrossRef]
- Nkhili, E.; Brat, P. Reexamination of the ORAC assay: Effect of metal ions. Anal. Bioanal. Chem. 2011, 400, 1451–1458. [Google Scholar]
- Pereira, D.M.; Faria, J.; Gaspar, L.; Ferreres, F.; Valentão, P.; Sottomayor, M.; Andrade, P.B. Exploiting Catharanthus roseus roots: Source of antioxidants. Food Chem. 2010, 121, 56–61. [Google Scholar] [CrossRef]
- Svoboda, G.A. The Alkaloids of Catharanthus roseus G. Don (Vinca rosea L.) in Cancer Chemotherapy. In Current Topics in Plant Science; Gunkel, J.E., Ed.; Academic Press: New York, NY, USA, 1969; pp. 303–335. [Google Scholar]
- Hohmeier, H.E.; Newgard, C.B. Cell lines derived from pancreatic islets. Mol. Cell. Endocrinol. 2004, 228, 121–128. [Google Scholar] [CrossRef]
- Akbar, S.; Bellary, S.; Griffiths, H.R. Dietary antioxidant interventions in type 2 diabetes patients: A meta-analysis. Br. J. Diabetes Vasc. Dis. 2011, 11, 62–68. [Google Scholar] [CrossRef]
- Bensellam, M.; Laybutt, D.R.; Jonas, J.C. The molecular mechanisms of pancreatic β-cell glucotoxicity: Recent findings and future research directions. Mol. Cell. Endocrinol. 2012, 364, 1–27. [Google Scholar] [CrossRef]
- Poitout, V.; Stout, L.E.; Armstrong, M.B.; Walseth, T.F.; Sorenson, R.L.; Robertson, R.P. Morphological and functional characterization of beta TC-6 cells—An insulin-secreting cell line derived from transgenic mice. Diabetes 1995, 44, 306–313. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Looi, C.Y.; Moharram, B.; Paybar, M.; Wong, Y.L.; Leong, K.H.; Mohamad, K.; Arya, A.; Wong, W.F.; Mustafa, M.R. Induction of apoptosis in melanoma A375 cells by a chloroform fraction of Centratherum anthelminticum (L.) seeds involves NF-kappaB, p53 and Bcl-2-controlled mitochondrial signaling pathways. BMC Complem. Altern. Med. 2013, 13, e166. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Paydar, M.; Wong, Y.L.; Moharram, B.A.; Wong, W.F.; Looi, C.Y. In vitro Anti-oxidant and anti-cancer activity of methanolic extract from Sanchezia speciosa leaves. Pak. J. Biol. Sci. 2013, 16, 1212–1215. [Google Scholar] [CrossRef]
- Arya, A.; Looi, C.Y.; Cheah, S.C.; Mustafa, M.R.; Mohd, M.A. Anti-diabetic effects of Centratherum anthelminticum seeds methanolic fraction on pancreatic cells, β-TC6 and its alleviating role in type 2 diabetic rats. J. Ethnopharmacol. 2012, 144, 22–32. [Google Scholar] [CrossRef]
- Lee, K.J.; Jeong, H.G. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol. Lett. 2007, 173, 80–87. [Google Scholar] [CrossRef]
- Loaiza, A.; Porras, O.H.; Barros, L.F. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J. Neurosci. 2003, 23, 7337–7342. [Google Scholar]
- Lund, I.K.; Andersen, H.S.; Iversen, L.F. Structure-based design of selective and potent inhibitors of protein-tyrosine phosphatase β. J. Biol. Chem. 2004, 279, 24226–24235. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.-C.; Mustafa, M.R.; Awang, K. Antidiabetic and Antioxidant Properties of Alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770-9784. https://doi.org/10.3390/molecules18089770
Tiong SH, Looi CY, Hazni H, Arya A, Paydar M, Wong WF, Cheah S-C, Mustafa MR, Awang K. Antidiabetic and Antioxidant Properties of Alkaloids from Catharanthus roseus (L.) G. Don. Molecules. 2013; 18(8):9770-9784. https://doi.org/10.3390/molecules18089770
Chicago/Turabian StyleTiong, Soon Huat, Chung Yeng Looi, Hazrina Hazni, Aditya Arya, Mohammadjavad Paydar, Won Fen Wong, Shiau-Chuen Cheah, Mohd Rais Mustafa, and Khalijah Awang. 2013. "Antidiabetic and Antioxidant Properties of Alkaloids from Catharanthus roseus (L.) G. Don" Molecules 18, no. 8: 9770-9784. https://doi.org/10.3390/molecules18089770





